Abstract
Purpose
To evaluate the significance of CT perfusion parameters predicting response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma (PDAC).
Materials and methods
Seventy patients with PDAC prospectively had CT perfusion acquisition incorporated into baseline multiphase staging CT. Twenty-eight who were naïve to therapy were retained for further investigation. Perfusion was performed 5–42.5 s after contrast, followed by parenchymal and portal venous phases. Blood flow (BF), blood volume (BV), and permeability surface area product (PS) were calculated using deconvolution algorithms. Patients were categorized as responders or non-responders per RECIST 1.1. Perfusion variables with AUC ≥ 0.70 in differentiating responders from non-responders were retained. Logistic regression was used to assess associations between baseline perfusion variables and response.
Results
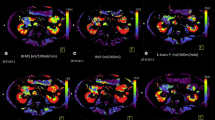
18 of 28 patients showed favorable response to therapy. Baseline heterogeneity variables in tumor max ROI were higher in non-responders than responders [median BF coefficient of variation (CV) 0.91 vs. 0.51 respectively, odds ratio (OR) 6.8 per one standard deviation (1-SD) increase, P = 0.047; median PS CV 1.6 vs. 0.68, OR 3.9 per 1-SD increase, P = 0.047; and median BV CV 0.75 vs. 0.54, OR = 4.0 per 1-SD increase, P = 0.047]. Baseline BV mean in tumor center was lower in non-responders than responders (median BV mean: 0.74 vs. 2.9 ml/100 g respectively, OR 0.28 per 1-SD increase, P = 0.047).
Conclusion
For patients with PDAC receiving neoadjuvant therapy, lower and more heterogeneous perfusion parameters correlated with an unfavorable response to therapy. Such quantitative information can be acquired utilizing a comprehensive protocol interleaving perfusion CT acquisition with standard of care multiphase CT scans using a single contrast injection, which could be used to identify surgical candidates and predict outcome.
Graphical abstract






Similar content being viewed by others
Data availability
All data generated or analyzed during the study are included in the published paper.
Abbreviations
- PDAC:
-
Pancreatic ductal adenocarcinoma
- ROI:
-
Region of interest
- AUC:
-
Area under the receiver operating characteristic curve
- IQR:
-
Interquartile range
- CV:
-
Coefficient of variation
- CT:
-
Computed tomography
- BR:
-
Borderline resectable
- LA:
-
Locally advanced
- BV:
-
Blood volume
- PS:
-
Permeability surface area product
- BF:
-
Blood flow
- TTP:
-
Time to peak concentration
- CA:
-
Carbohydrate antigen
- CTDIvol :
-
CT dose index
- SSDE:
-
Size specific dose index
References
Nelson DW, Chang S-C, Grunkemeier G, et al. Resectable Distal Pancreas Cancer: Time to Reconsider the Role of Upfront Surgery. Ann Surg Oncol. 2018;25(13):4012–4019.
Zhong J, Switchenko J, Behera M, et al. Chemotherapy with or Without Definitive Radiation Therapy in Inoperable Pancreatic Cancer. Ann Surg Oncol. 2018;25(4):1026–1033.
Anon. Cancer of the Pancreas - Cancer Stat Facts. SEER. Available at: http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed July 18, 2019.
Luberice K, Downs D, Sadowitz B, et al. Has survival improved following resection for pancreatic adenocarcinoma? The American Journal of Surgery. 2017;214(2):341–346.
Cassinotto C, Mouries A, Lafourcade J-P, et al. Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology. 2014;273(1):108–116.
Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261(1):12–17.
Michelakos T, Pergolini I, Castillo CF, et al. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment With FOLFIRINOX. Annals of Surgery. 2019;269(4):733–740.
Katz MHG, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118(23):5749–5756.
Klotz E, Haberland U, Glatting G, et al. Technical prerequisites and imaging protocols for CT perfusion imaging in oncology. Eur J Radiol. 2015. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0720048X15300176.
Prezzi D, Khan A, Goh V. Perfusion CT imaging of treatment response in oncology. Eur J Radiol. 2015;84(12):2380–2385.
Fukukura Y, Takumi K, Higashi M, et al. Contrast-enhanced CT and diffusion-weighted MR imaging: Performance as a prognostic factor in patients with pancreatic ductal adenocarcinoma. Eur J Radiol. 2014;83(4):612–619.
D’Onofrio M, Gallotti A, Mantovani W, et al. Perfusion CT can predict tumoral grading of pancreatic adenocarcinoma. Eur J Radiol. 2013;82(2):227–233.
Hamdy A, Ichikawa Y, Toyomasu Y, et al. Perfusion CT to Assess Response to Neoadjuvant Chemotherapy and Radiation Therapy in Pancreatic Ductal Adenocarcinoma: Initial Experience. Radiology. 2019:182561.
Konno Y, Hiraka T, Kanoto M, et al. Pancreatic perfusion imaging method that reduces radiation dose and maintains image quality by combining volumetric perfusion CT with multiphasic contrast enhanced-CT. Pancreatology. 2020. Available at: http://www.sciencedirect.com/science/article/pii/S1424390320306591. Accessed September 8, 2020.
O’Malley RB, Soloff EV, Coveler AL, et al. Feasibility of wide detector CT perfusion imaging performed during routine staging and restaging of pancreatic ductal adenocarcinoma. Abdom Radiol. 2021;46(5):1992–2002.
AAPM Task Group 23. AAPM Report No. 96 The Measurement, Reporting, and Management of Radiation Dose in CT. AAPM Report No. 96 The Measurement, Reporting, and Management of Radiation Dose in CT. 2008. Available at: https://www.aapm.org/pubs/reports/RPT_96.pdf. Accessed January 2, 2020.
AAPM Task Group 204. AAPM Report No. 204 Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations. Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations. 2011. Available at: https://www.aapm.org/pubs/reports/rpt_204.pdf. Accessed January 2, 2020.
Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? BJR. 2003;76(suppl_1):S36–S42.
Aslan S, Nural MS, Camlidag I, et al. Efficacy of perfusion CT in differentiating of pancreatic ductal adenocarcinoma from mass-forming chronic pancreatitis and characterization of isoattenuating pancreatic lesions. Abdom Radiol. 2019;44(2):593–603.
David W. Hosmer, Stanley Lemeshow. Applied Logistic Regression. 2nd ed. New York, NY: John Wiley and Sons; 2000.
Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811–818.
Kovač JD, DJurić-Stefanović A, Dugalić V, et al. CT perfusion and diffusion-weighted MR imaging of pancreatic adenocarcinoma: can we predict tumor grade using functional parameters? Acta Radiologica. 2018:028418511881220–9.
Perik TH, van Genugten EAJ, Aarntzen EHJG, et al. Quantitative CT perfusion imaging in patients with pancreatic cancer: a systematic review. Abdom Radiol. 2021. Available at: https://doi.org/10.1007/s00261-021-03190-w. Accessed July 9, 2021.
Caswell DR, Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Medicine. 2017;15(1):133.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94.
Ligero M, Garcia-Ruiz A, Viaplana C, et al. A CT-based Radiomics Signature is Associated with Response to Immune Checkpoint Inhibitors in Advanced Solid Tumors. Radiology. 2021;299(1):109–119.
Lubner MG, Smith AD, Sandrasegaran K, et al. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. RadioGraphics. 2017;37(5):1483–1503.
Sandrasegaran K, Lin Y, Asare-Sawiri M, et al. CT texture analysis of pancreatic cancer. Eur Radiol. 2019;29(3):1067–1073.
Borhani AA, Dewan R, Furlan A, et al. Assessment of Response to Neoadjuvant Therapy Using CT Texture Analysis in Patients With Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. American Journal of Roentgenology. 2020;214(2):362–369.
George E, Wortman JR, Fulwadhva UP, et al. Dual energy CT applications in pancreatic pathologies. Br J Radiol. 2017;90(1080):20170411.
Bao J, Liu A, Zhao C, et al. Correlation Between Dual-Energy Computed Tomography Single Scan and Computed Tomography Perfusion for Pancreatic Cancer Patients: Initial Experience. Journal of Computer Assisted Tomography. 2019:1.
Stiller W, Skornitzke S, Fritz F, et al. Correlation of Quantitative Dual-Energy Computed Tomography Iodine Maps and Abdominal Computed Tomography Perfusion Measurements: Are Single-Acquisition Dual-Energy Computed Tomography Iodine Maps More Than a Reduced-Dose Surrogate of Conventional Computed Tomography Perfusion? Investigative Radiology. 2015;50(10):703–708.
Schneeweiß S, Horger M, Grözinger A, et al. CT-perfusion measurements in pancreatic carcinoma with different kinetic models: Is there a chance for tumour grading based on functional parameters? Cancer Imaging. 2016;16(1):43.
Funding
This research was supported by GE Healthcare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
IRB approval was obtained for this prospective research.
Informed consent
All subjects signed written informed consent before participating.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Malley, R.B., Cox, D., Soloff, E.V. et al. CT perfusion as a potential biomarker for pancreatic ductal adenocarcinoma during routine staging and restaging. Abdom Radiol 47, 3770–3781 (2022). https://doi.org/10.1007/s00261-022-03638-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03638-7