Abstract
Objectives
Validation of the feasibility and efficacy of volume perfusion computed tomography (VPCT) in the preoperative assessment of cerebral gliomas by applying a 128-slice CT covering the entire tumour.
Methods
Forty-six patients (25 men, 21 women; mean age 52.8 years) with cerebral gliomas were evaluated with VPCT. Two readers independently evaluated VPCT data, drawing volumes of interest (VOIs) around the tumour according to maximum intensity projection volumes, which were mapped automatically onto the cerebral blood volume (CBV), flow (CBF) and permeability (Ktrans) perfusion datasets. As control, a second VOI was placed in the contralateral healthy cortex. Correlation among perfusion parameters, tumour grade, hemisphere and VOIs was assessed. The diagnostic power of perfusion parameters was analysed by receiver operating characteristics curve analyses.
Results
VPCT was feasible in the assessment of the entire tumour extent. Mean values of Ktrans, CBV, CBF in high-grade gliomas were significantly higher compared with low-grade (p < 0.01). Ktrans demonstrated the highest diagnostic (97% sensitivity), positive (100%) and negative (94%) prognostic values.
Conclusions
VPCT was feasible in all subjects. All areas of different perfusion characteristics are depicted and quantified in colour-coded 3D maps. The derived parameters correlate well with tumour histopathology, differentiating low- from high-grade gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perfusion-weighted MRI (PWI) and CT perfusion (PCT) play an important role in the preoperative assessment of intra-axial brain tumours. The correlation among perfusion parameters, tumour grade and treatment response has already been established through the non-invasive dynamic measurement of regional cerebral blood flow (CBF) and volume (CBV) as well as permeability, a marker of blood–brain barrier disturbance [1–5]. Furthermore, perfusion imaging has demonstrated a potential to distinguish tumour recurrence from radionecrosis and to differentiate between cerebral tumour types, such as lymphomas and gliomas [1, 2].
In patients with intra-axial brain tumours, PCT renders essential information about tumour vascularisation and blood–brain barrier disruption. Quantification of tissue perfusion characteristics contributes to the prediction of tumour grade and plays an important role in prognosis, therapeutical management and assessment of treatment response [6–8].
With its high accessibility, short time and the ability to offer additional haemodynamic information, PCT is increasingly being used in neuroradiological imaging. Moreover, with the linear relation between density changes and tissue concentration of contrast medium along with the lack of susceptibility artefacts triggered by haemorrhage or mineral depositions, PCT outweighs the role of PWI in brain perfusion imaging.
The main limitation of currently available multi-detector row CT in the evaluation of cerebral perfusion is the relatively narrow range of brain tissue covered (up to 40 mm above the circle of Willis with 64-slice CT) [9]. Volume Perfusion CT (VPCT) using periodic spirals is not limited by the detector width and thus, dynamic data can be obtained allowing the complete evaluation of brain perfusion [10].
The aim of the present study was to evaluate the feasibility and efficacy of brain VPCT in the preoperative assessment of cerebral gliomas.
Materials & methods
The study was approved by our institutional review board and written informed consent was obtained from all patients or their next of kin. From September 2008 to December 2009, 46 consecutive patients with suspected cerebral gliomas were enrolled prospectively in our study. All subjects had not received any kind of treatment or biopsy at the time of examination. Before VPCT all patients underwent brain MRI and were diagnosed with a suspected glial tumour. In all cases, VPCT was followed by stereotactic biopsy or surgery in order to evaluate the histopathology of the tumour. All the histopathological specimens were examined by a board-certified neuropathologist and were graded according to WHO guidelines. According to their degree of malignancy, they were then classified into low-grade (I and II) and high-grade (III and IV) consistent with WHO classification. The above mentioned classification was also statistically proven (Table 1). Neuroradiological analysis was conducted blinded to patients’ clinical data and initial MRI findings. No information about the diseased hemisphere was given to the raters. VPCT data were analysed by two neuroradiologists with different levels of experience in neuro-perfusion imaging techniques (A.X and P.S with 2 and 10 years’ respectively).
VPCT imaging
Imaging was performed using 128-detector row CT (SOMATOM Definition AS+, Siemens, Forchheim, Germany) with adaptive 4D spiral mode. The technique is based on a constant periodic bidirectional table movement. Thus, entire organs larger than the detector width, and, as such, the brain, can be imaged at a temporal sampling of 1.5 s and time-resolved perfusion characteristics can be evaluated and quantified. The imaging parameters for the VPCT were 80 kV and 200mAs, 30 spiral images with an image volume of 96 mm on the Z-axis and a travel time of 1.5 s per spiral. Reconstructed images of 5 mm were obtained every 3 mm of the total imaging volume. Total CT data acquisition time was 45 s.
All injections of contrast medium were performed through an 18-gauge cannula placed in the cubital vein. The biphasic injection protocol consisted of 30 ml of highly iodinated contrast agent (Iomeprol 400, Bracco, Konstanz, Germany) injected at a rate of 5 ml/s followed by 20 ml contrast agent at a rate of 1 ml/s and finally 20 ml of saline chaser applied at a rate of 1 ml/s. Start delay was 4 s in all patients. There were no patients with cardiovascular disease or any condition that could affect the ejection fraction. The contrast material was always preheated to body temperature before injection. All patients positioned their head in a tiltable head holder and were fixed with an additional strap in order to prevent motion during CT data acquisition.
Data processing and analysis
Data were evaluated with commercially available 3D perfusion software (Syngo Volume Perfusion CT Neuro, Siemens, Forchheim, Germany). When necessary, acquired volumes were corrected for motion with a 3D rigid motion correction, based on skull movement. Signal-to-noise ratio was improved using a spatiotemporal filtering for all data (Adaptive 4D Noise Reduction) [11, 12].
Bone was automatically removed with a contour finder that identified the skull bone and an additional segmentation based on Hounsfield unit (HU) values for identifying brain parenchyma (voxels with HU values between 15 and 100 HU were included). These segmentation steps were performed on the average baseline volume from all time points without contrast material. Vessels were automatically detected on the time maximum intensity projection (tMIP) images. All voxels that were above a configured percentage of maximum enhancement were excluded from the calculation. By drawing a volume of interest (VOI) around the tumour, vessels could be defined separately for the tumour and the remaining healthy brain parenchyma. Absolute values of dynamic perfusion parameters were acquired and the whole tumour volume was recorded. Quantitative perfusion values for cerebral blood volume (CBV) and volume transfer coefficient (Ktrans) were obtained from a modified Patlak algorithm. Ktrans from the slope of the Patlak fit describes the portion of whole blood flow that is extracted into the extravascular space. The intercept of the fit describes the blood volume [1]. Cerebral blood flow (CBF) was determined with a deconvolution-based algorithm [13]. After parameter calculation, VOI was drawn in the MIP volume and then automatically mapped onto the result parameter volumes of CBV, Ktrans and CBF. The two neuroradiologists evaluated each 3D MIP image independently in order to delineate manually a VOI including the entire tumour extent, avoiding areas of necrosis (Figs. 1 and 2).
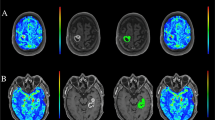
A 38-year-old man with the histopathological diagnosis of low-grade glioma WHO II. The contrast-enhanced maximum intensity projection (MIP) images in three planes, axial (a), coronal (b) and sagittal (c) depict the entire tumour extent, which is included in volume of interest (VOI 1) and show no enhancement within the tumour in the right insular lobe. A control VOI is drawn in the contralateral cortex
A 59-year-old woman with the histopathological diagnosis of glioblastoma multiforme WHO IV. The contrast-enhanced MIP images show enhancement of the solid tumour part in left hemisphere (frontoparietal region) with central necrosis. A VOI is drawn covering the entire tumour extent in three planes, axial (a), coronal (b) and sagittal (c). Control VOI is delineated in the contralateral cortex
A second VOI, cortically pronounced, was drawn around the contralateral healthy cortical tissue of the brain to obtain control perfusion values. Disputes between interpreters were decided by consensus.
Quantification of the tumour volume
The whole tumour volume, given in cm3, consisted of all voxels included in the delineated tumour VOI according to the 3D MIPs. The same rules applied to VOIs of the healthy hemisphere.
Statistical analysis
To analyse differences in perfusion parameters between high- and low-grade tumours as well as between tumour (diseased hemisphere) and contralateral cortical hemisphere (control hemisphere) two-way repeated measures ANOVAs were performed. The possible confounder age was included as a covariate. Perfusion parameters were analysed between high- and low-grade tumours, separately for control and tumour, by Welch’s t-tests, and between control and tumour by paired t-tests, separately for low- and high-grade tumours. Tumour VOIs were compared between high- and low-grade tumours by one-way ANOVA, including age as a covariate. Normality of perfusion parameters and of VOIs was checked by quantile-quantile plots. The significance level for all tests was set at α = 5%.
The diagnostic power of the studied perfusion parameters in terms of sensitivity, specificity, and positive and negative predictive value was analysed by receiver operating characteristic (ROC) curve analyses. Optimal cut-off values for classifying tumours as either low- or high-grade were determined according to the Youden criterion, which marks the point on an ROC curve where “sensitivity + specificity–1” is maximal.
All statistical analyses were performed with the free software R (version 2.8, www.r-project.org).
Results
Patients’ demographics and clinical characteristics are presented in Table 1. A significant difference (t-test: p < 0.01) in age was noted between the two subgroups. Age marked a possible confounder and was modelled as a co-variable in all comparisons between the two study groups. Distribution of sex was similar in both groups (Fisher’s exact test: p = 0.36). VPCT was feasible in all subjects and none of the patients experienced any adverse effects related to the study procedure. All studies could be evaluated without artefacts. The complete extent of the tumour was visible in all cases.
Influence of tumour grade on perfusion parameters
The results of the overall analysis of perfusion parameters (CBF, CBV and Ktrans) can be studied in Table 2. Each of these parameters is significantly influenced by the tumour grade (all p < 0.01), and each of them differs between the tumour and the control hemisphere (all p < 0.01). The potential confounder age did not influence any of these parameters. Moreover, there was a significant interaction between the factors “tumour grade” and “hemisphere”. This interaction means that the difference between low- and high-grade tumours might only exist in one hemisphere (most likely in the diseased one) or that it is at least of different strengths in the two hemispheres. Vice versa, the hemisphere effect might be different in low- and high-grade patients. Therefore, in the following subsections, the analysis is once split by tumour grade and once by hemisphere (either control or diseased).
Comparison of perfusion parameters between affected and normal cerebral parenchyma
The direct comparison of control and diseased hemispheres is shown in Table 3. While CBF is not significantly different between the two sides for low-grade tumours (p = 0.10), it is significantly higher in the diseased hemisphere for high-grade tumours (p < 0.01). For CBV and Ktrans, the difference between affected and unaffected hemispheres was found to be significant for both low- and high-grade tumours (all p < 0.01). However, for both parameters, the difference between affected and unaffected hemispheres appears to be stronger in the high-grade tumours.
Comparison of perfusion parameters between high-grade and low-grade gliomas
Regarding the results of the analysis split by hemisphere, the difference in parameters between tumour grades is only present in the diseased hemispheres (Table 4). A significant increase in all three perfusion parameters was noted in high-grade tumours compared with low-grade tumours.
Diagnostic value of perfusion parameters
Optimal cut-off values for each perfusion parameter as well as their preoperative classification accuracy are shown in Table 5. The most accurate diagnostic marker is Ktrans, which identifies 97% (sensitivity) of all histopathologically high-grade tumours. Moreover, all tumours classified as high-grade, with Ktrans above the determined cut-off value of 2.21 ml/100 ml/min, proved to be high-grade on histopathology (positive predictive value = 100%).
Quantification of the whole tumour volume
The whole tumour volume was assessed in all 46 patients. The average tumour size for low-grade and high-grade gliomas was 42.5 ± 36.2 cm3 and 52.5 ± 36.8cm3, respectively. No significant difference was noted through the comparison of tumour volumes between high-grade and low-grade gliomas (Table 2). In addition, tumour volumes were independent of the possible confounder age (p = 0.26).
Discussion
The 128-slice VPCT system with bidirectional table movement, which enables the multispiral acquisition of dynamic CT data, is not limited by the detector width and therefore, can be used to obtain perfusion data of large brain volumes. So far, whole brain CT perfusion has been applied only in the evaluation of ischaemic cerebrovascular disease [10, 14, 15].
In the current study, our objective was to validate VPCT as a novel feasible imaging tool, which allows the quantitative assessment of both microvascular density and capillary permeability in one setting for the preoperative grading of brain gliomas. Additionally, we attempted to explore whether VPCT maps enable the 3D assessment of glioma perfusion and the differentiation of high-grade from low-grade gliomas.
We report the application of VPCT in the preoperative assessment of a large cohort of patients with primary cerebral gliomas, overcoming the limitation of restricted coverage area of PCT. By applying VPCT, we have defined and quantified VOIs, delineating the whole tumour extent, instead of regions of interest (ROIs) that were restricted to a series of slices. Furthermore, we have obtained dynamic volume perfusion data of the whole tumour and we have been able to differentiate high-grade from low-grade gliomas according to absolute perfusion parameters.
The fixation of the head before imaging and the application of a highly standardised protocol have ensured the elimination of motion artefacts and therefore, the reliable post-processing and analysis of perfusion data.
The applied biphasic injection protocol was aimed at a constant and compact bolus of contrast agent, resulting in a plateau in the time-density curve (Fig. 3). Such an injection protocol has ensured a most reliable depiction of the delayed permeability, which characterises the immature endothelium of neovascularisation [16].
Dynamic CT perfusion imaging techniques are based on the quantification of tissue-related distribution of the contrast agent which acts as a tracer. The distribution of contrast medium after infusion is determined by the microvascularisation and diffusion across the endothelial membrane. VPCT, similar to PCT, allows the quantification of absolute values of physiological parameters including blood volume, blood flow, capillary permeability and leakage. These parameters present pathophysiological correlation with the microscopic changes that occur with tumour angiogenesis [17, 18]. The absolute perfusion parameters CBV, CBF and Ktrans correspond to histopathological microvascular density, which constitutes the gold standard for such an assessment because of its direct correlation with angiogenic factor expression, tumour growth and metastatic occurrence [19]. Tumour volume however, proved to be an unreliable marker for diagnosing the grade of cerebral gliomas.
Our data analysis suggests that perfusion parameters obtained with VPCT are quantified in a more reliable way, as the entire tumour extent is now included in the analysis and consequently, the perfusion values obtained are much more representative of the entire tumour and reproducible [20].
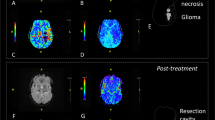
In view of the heterogeneous nature of cerebral gliomas, with varying degrees of cellular and nuclear pleomorphism, mitotic activity, vascular proliferation and necrosis [21, 22], VPCT could have the potential to significantly improve neurosurgical biopsy guidance, as the entire tumour is covered and all areas of different perfusion characteristics are included and depicted in colour-coded 3D brain perfusion maps (Fig. 4). After VPCT, neuroradiologists are able to locate the most hyperperfused malignant tumour site. Thus, this new perfusion technique could offer the ability to improve the diagnostic efficacy and also the accuracy of biopsy guidance in patients with cerebral gliomas. On the contrary, with the previous PCT method, the brain area to be perfused was restricted to 2–4 cm (depending on the detector width) and defined before the perfusion imaging, which means that a hyperperfused tumour part could be possibly missed, especially in multifocal tumours or those exceeding the fixed detector width.
Perfusion images of a 63-year-old man with the histopathological diagnosis of a high-grade glioma WHO IV in the left hemisphere. a Axial perfusion image shows CBV, b sagittal perfusion image depicts CBF and c axial perfusion image shows Ktrans. Note the rim-like notable elevation of the CBV and CBF (marked red) and the disruption of the blood–brain barrier depicted with the corresponding elevation of Ktrans (marked green), within the tumour tissue
Consistent with previously published data of PCT studies, significantly increased CBV and CBF values were also noted in our study in the subgroup of high-grade gliomas [1, 6–8, 23]. The already known histopathology of such tumours with vascular proliferation and vasodilated feeding arterioles supports these findings [6–8].
The increased vascular leakage has been considered as a surrogate marker of tumour neoangiogenesis and grade [23]. Our perfusion data, in accordance with previous PCT studies, support these histopathological findings, as high-grade gliomas demonstrated significantly higher Ktrans values in comparison to healthy parenchyma and low-grade gliomas [1, 6–8].
Histopathologically, contrary to high-grade gliomas low-grade gliomas are characterised by the absence of neovascularisation [2, 24]. However, monitoring to detect malignant transformation or active proliferation is crucial in order to estimate the malignant potential of such tumours. VPCT could represent a reliable method for the detailed monitoring of low-grade gliomas. Interestingly, we have found a statistically significant decrease in CBV and a significant increase in Ktrans in low-grade gliomas, compared with normal contralateral tissue. These findings have not been reported so far and may implicate a possible malignant transformation in some of the patients. Follow-up of this subgroup will be carried out.
In our VPCT study, we confirm the implication of our previous PCT published data, that high-grade gliomas can be differentiated from low-grade gliomas on the basis of CBV, CBF and Ktrans absolute values [1]. There are a number of PCT series suggesting the preoperative differentiation of cerebral gliomas; however, to our knowledge this is the first time in the literature that such a differentiation has been made by applying VPCT and thus, by acquiring dynamic perfusion parameters through the definition of tumour VOIs.
The clear increase in all three perfusion parameters in high-grade tumours in comparison to low-grade supports the studied perfusion parameters as valuable diagnostic markers for the classification of gliomas before histopathological diagnosis. Consistent with previously published data, the most accurate diagnostic marker in our study was Ktrans with the highest specificity of 100%; however, with a much lower threshold of 2.21 ml/100 ml/min in comparison to that already reported [7]. On the contrary, previous reports have shown that permeability and CBV parameters were of similar prognostic value and higher than CBF [6, 23]. Moreover, our data analysis has revealed that CBV and CBF values have comparable diagnostic accuracy with a sensitivity of 93% and 90%, respectively and a specificity of 94% for both parameters (Table 5).
In spite of the obvious advantages of VPCT over PCT and PWI, the radiation dose of 5.3 mSv per VPCT should be mentioned as a potential concern. The radiation dose of the Adaptive 4D Spiral is within the range of existing protocols, with 3 to 5 mSv covering 2–4 cm, but allows the coverage of the whole tumour [1, 25, 26]. The maximum imaging range of 96 mm proved to be sufficient in whole tumour-extent coverage in all patients. As far as spatial resolution of VPCT is concerned, further research is required in order to acquire much more detailed colour-coded anatomical information. A possible advance of VPCT in the future could be the evolution of commercially available 3D-perfusion software, which could enable the fusion of perfusion maps with high anatomical MRI data.
In conclusion, dynamic perfusion data, obtained from a single VPCT, enable the assessment of the entire tumour, revealing through the colour-coded perfusion maps the diverse perfusion characteristics of heterogeneous cerebral gliomas. VPCT is a feasible tool for the preoperative histopathological classification of cerebral gliomas, as the entire tumour is covered and all areas of different perfusion characteristics are depicted and quantified on colour-coded 3D perfusion maps. With perfusion imaging being progressively an indispensable component in the preoperative evaluation of cerebral tumours, we should stress the importance of the evolution of perfusion techniques and point out the necessity of integrating VPCT into the preoperative clinical routine. However, judicious application is required particularly in the case of low-grade gliomas. Further investigation will evaluate the role of VPCT in the daily neuro-oncology practice.
References
Schramm P, Xyda A, Klotz E et al (2010) Dynamic CT perfusion imaging of intra-axial brain tumours: Differentiation of high-grade gliomas from primary CNS Lymphomas. Eur Radiol 20:2482–2490
Roberts HC, Roberts TP, Brasch RC et al (2000) Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol 21:891–899
Law M, Yang S, Babb JS et al (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Gossmann A, Helbich TH, Kuriyama N et al (2002) Dynamic contrast-enhanced magnetic resonance imaging as surrogate marker of tumor response to anti-angiogenic therapy in a xenograft model of glioblastoma multiforme. J Magn Reson Imaging 15:233–240
Cao Y, Shen Z, Chenevert TL et al (2006) Estimate of vascular permeability and cerebral blood volume using Gd-DTPA contrast enhancement and dynamic T2*-weighted MRI. J Magn Reson Imaging 24:288–296
Jain R, Ellika SK, Scarpace L et al (2008) Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. AJNR Am J Neuradiol 29:694–700
Ding B, Ling HW, Chen KM et al (2006) Comparison of cerebral blood volume and permeability in preoperative grading of intracranial glioma using CT perfusion imaging. Neuroradiology 48:773–781
Cenic A, Nabavi DG, Craen RA et al (2000) A CT method to measure hemodynamics in brain tumors: Validation and application of cerebral blood flow maps. AJNR Am J Neuroradiol 21:462–470
Röther J, Jonetz-Mentzel L, Fiala A et al (2000) Hemodynamic assessment of acute stroke using dynamic single-slice computed tomographic perfusion imaging. Arch Neurol 57:1161–1166
Morhard D, Wirth CD, Fesl G et al (2010) Advantages of extended brain perfusion computed tomography: 9.6 cm coverage with time resolved computed tomography-angiography in comparison to standard stroke-computed tomography. Invest Radiol 45:363–369
Klotz E, Raupach R, Fichte H, et al (2009) Multiple spatial frequency band filtering of whole brain perfusion CT data: Dose reduction and improved spatial resolution [abstr]. In: European Congress of Radiology, B-392
Raupach R, Bruder H, Schmidt B, et al (2009) A novel spatiotemporal filter for artifact and noise reduction in CT [abstr]. In: European Congress of Radiology, B-518
Abels B, Klotz E, Tomandl BF et al (2010) Perfusion CT in acute ischemic stroke: A qualitative and quantitative comparison of deconvolution and maximum slope approach. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A2151
Murayama K, Katada K, Nakane M et al (2009) Whole-Brain Perfusion CT performed with a prototype 256-detector row CT System: initial experience. Radiology 250:202–211
Wittkamp G, Buerke B, Dziewas R et al (2010) Whole brain perfused blood volume CT: visualization of infarcted tissue compared to quantitative perfusion CT. Acad Radiol 17:427–432
Van Dijke CF, Brasch RC, Roberts TP et al (1996) Mammary carcinoma model: correlation of macromolecular contrast-enhanced MR imaging characterizations of tumor microvasculature and histologic capillary density. Radiology 198:813–818
Miles KA (2002) Functional computed tomography in oncology. Eur J Cancer 38:2079–2084
Miles KA, Charnsangavej C, Lee FT et al (2000) Application of CT in the investigation of angiogenesis in oncology. Acad Radiol 7:840–850
Weidner N (1995) Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 147:9–19
Ng QS, Goh V, Fichte H et al (2006) Lung cancer perfusion at multi-detector row CT: reproducibility of the whole tumor quantitative measurements. Radiology 239:547–553
Burger PC, Vogel FS, Green SB et al (1985) Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer 56:1106–1111
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Ellika SK, Jain R, Patel SC et al (2007) Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. AJNR Am J Neuroradiol 28:1981–1987
Daldrup H, Shames DM, Wendland M et al (1998) Correlation of dynamic contrast-enhanced MR imaging with histologic tumor grade: comparison of macromolecular and small-molecular contrast media. AJR Am J Roentgenol 171:941–949
Jain R, Scarpace L, Ellika S et al (2007) First-pass perfusion computed tomography: initial experience in differentiating recurrent brain tumors from radiation effects and radiation necrosis. Neurosurgery 61:786–787
Mnyusiwalla A, Aviv RI, Symons SP (2009) Radiation dose from multidetector row CT imaging for acute stroke. Neuroradiology 51:635–640
Acknowledgement
Ulrike Haberland is an employee of the CT division of Siemens Healthcare Sector (Forchheim, Germany) which manufactured the CT equipment used in this study. Ernst Klotz is a principal scientist of the CT division of Siemens Healthcare Sector (Forchheim, Germany) which manufactured the CT equipment used in this study. Gunter Erb is an employee of the Bracco Imaging (Konstanz, Germany) which manufactured the contrast agent used in this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Xyda, A., Haberland, U., Klotz, E. et al. Brain volume perfusion CT performed with 128-detector row CT system in patients with cerebral gliomas: A feasibility study. Eur Radiol 21, 1811–1819 (2011). https://doi.org/10.1007/s00330-011-2150-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2150-2