Abstract
Objective
To explore and to compare the magnitude and spatial pattern of in vivo femorotibial cartilage deformation in healthy and in osteoarthritic (OA) knees.
Methods
One knee each in 30 women (age: 55 ± 6 years; BMI: 28 ± 2.4 kg/m2; 11 healthy and 19 with radiographic femorotibial OA) was examined at 3Tesla using a coronal fat-suppressed gradient echo SPGR sequence. Regional and subregional femorotibial cartilage thickness was determined under unloaded and loaded conditions, with 50% body weight being applied to the knee in 20° knee flexion during imaging.
Results
Cartilage became significantly (p < 0.05) thinner during loading in the medial tibia (−2.7%), the weight-bearing medial femur (−4.1%) and in the lateral tibia (−1.8%), but not in the lateral femur (+0.1%). The magnitude of deformation in the medial tibia and femur tended to be greater in osteoarthritic knees than in healthy knees. The subregional pattern of cartilage deformation was similar for the different stages of radiographic OA.
Conclusion
Osteoarthritic cartilage tended to display greater deformation upon loading than healthy cartilage, suggesting that knee OA affects the mechanical properties of cartilage. The pattern of in vivo deformation indicated that cartilage loss in OA progression is mechanically driven.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is an important health concern and among the top 10 leading conditions in Europe with respect to its socio-economic burden to society [1]. The knee joint is the largest joint in the human body and is the most commonly affected site in OA [2]. Amongst other structural changes, OA is characterised by loss of cartilage thickness [3] and MR imaging has previously been validated and shown to provide reliable measures of cartilage thickness [4, 5] and of cartilage loss in knee OA [6]. Further, this methodology has been previously used to determine the magnitude of in vivo cartilage deformation of the patella [7–9] and of the femorotibial joint [10] by comparing cartilage thickness before and after loading.
Histochemical analyses have suggested that OA cartilage has a lower proteoglycan and collagen content [11, 12] and a reduced collagen fibril connectivity and fibril orientation than healthy cartilage [13, 14]. Further, it was shown that OA cartilage suffers from greater permeability and elevated water concentrations, potentially leading to cartilage swelling [15, 16]. OA cartilage also displayed a diminished compressive modulus [17, 18] and greater deformation upon loading [16, 19] in ex vivo studies, suggesting that OA cartilage is less stiff and has a lower resistance upon loading, due to its altered macro-molecular composition.
In vivo studies using dual-orthogonal fluoroscopy and MR imaging-based cartilage 3D modelling have not only evaluated the cartilage thickness distribution in joints, but also cartilage-to-cartilage contact areas and deformation magnitudes during gait in healthy knees [20–24]. These studies reported greater deformation in the medial than in the lateral femorotibial compartment and that peak deformation occurred in central, weight-bearing areas, where no meniscal coverage was present [25]. These findings have suggested that a regional difference exists in femorotibial load distribution in healthy knees with regions of high vs. low mechanical loading. No previous study, however, has compared the magnitude and the pattern of in vivo cartilage deformation between healthy and osteoarthritic knees using quantitative data from MR imaging. The aim of this investigation therefore was to use an MR imaging-compatible in vivo loading device to study the subregional pattern of cartilage deformation in knees with and without signs of radiographic OA i.e. osteophyte (OP) growth and affection of the joint space width (JSW) [26, 27].
We tested the hypothesis that (1) the magnitude of deformation differs between osteoarthritic and healthy knees, suggesting differences in cartilage material properties and their response to mechanical loading, and that (2) the regional pattern of in vivo cartilage deformation coincides with the longitudinal pattern of cartilage loss observed in OA [28], suggesting that the patterns of rates of cartilage loss are mechanically driven.
Materials & methods
Study participants
Thirty female participants were recruited (age: 55.1 ± 6.0 years, BMI: 28.0 ± 2.4 kg/m2; Table 1) by newspaper advertisements from the region of San Francisco, Ca. General exclusion criteria for this study were MR imaging contraindications, a history of knee disease (other than OA), knee surgery (including meniscus surgery), and intra-articular steroid injections during the last 6 months. Inclusion criteria for the healthy cohort were: No evidence of radiographic OA (Kellgren Lawrence grading [KLG] 0 [no OPs and no change in JSW]) and no pain, stiffness or functional disabilities (when assessed by the WOMAC [Western Ontario and McMaster Universities] index for knee osteoarthritis [29]) during the past year (Table1). Inclusion criteria into the OA cohort were: Radiographic evidence of OA in the medial compartment in at least one knee (KLG2 [definite OPs, but unimpaired JSW] or KLG3 [definite OPs and moderate diminution of JSW] [26, 27]), and frequent (most days of a month during the past year) knee pain, aching, or stiffness. The limb axis was evaluated by measuring the knee angle from a fixed-flexion radiograph as previously described by Kraus et al. and Moreland et al. [30, 31] (Table 1). The KLG and knee angle readings were performed by a musculoskeletal radiologist (T.L.). Exclusion criteria were a medial joint space width (JSW) <2 mm, and the medial JSW greater than the lateral JSW. The study was approved by the Community of Human Research of the University of California, San Francisco (Approval number: H6513-32254-01; approval date: 17th April 2008) and written consent was obtained from all individuals before participation in this study.
Eleven individuals (37%) had no signs of radiographic OA (KLG0) whereas 19 had signs of medial femorotibial OA: 10 females (33%) were classified as KLG2 and 9 (30%) as KLG3 (Table 1). The dominant knee was studied in the healthy cohort (KLG0), and the more severely affected of both knees in the OA cohort (KLG2/3).
MRI and in vivo loading protocol
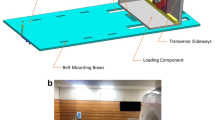
All 30 female participants were placed in a wheelchair after arrival at the MRI centre in order to avoid “external” loading of the limb for a period of 45 min prior to the scanning procedure. Although muscle activity cannot be avoided in this (or any other) physiological position, it simulated a common situation of “physical rest” of the participants, as taken during daily life. After setup of the participants (supine positioning, 20° knee flexion, foot fixation on footplate, 3D localisation and coil adjustment) the “unloaded” images were acquired (for time schedule see Fig. 1a). A load equivalent to 50% of the participant’s body weight (simulating loading conditions in the static standing position on both legs [32–34]) was applied via a loading apparatus to the chosen knee for 45 min while acquiring the “loaded” image (Fig. 1b+c). This was achieved via a non-elastic cable that ran below the loading table and was attached to a sliding foot plate on the one end, and, to non-ferromagnetic weights (through a pulley mechanism) on the other end. The load was applied to the loading cable, with only the subject’s imaged lower extremity being in contact with the foot plate. During loading a small superiorly directed translation typically took place. However, the new location was identified with a localizer scan and the knee was returned to the isocenter. “Loaded” and “unloaded” MR image protocol was as follows: 3D Localizer, Asset Calibration, 3-D double oblique coronal SPGR, T1 rho map, T2 map, sagittal and coronal fast spin echo (FSE). For image analysis we used the 3-D coronal fat-suppressed gradient echo SPGR sequence with 1.5-mm slice thickness (no inter-slice gap) and an in-plane resolution of 0.42 mm x 0.83 mm (interpolated to 0.31 mm x 0.31 mm; repetition time 24 ms; echo time 6.8 ms; flip angle 18°; matrix 384 x 192 interpolated to 512 x 512; field of view = 16 cm, number of excitations = 2, acquisition time = 4:44 min), using a 3-Tesla MRI (Signa HDx, General Electrics, Waukesha, WI, USA) and an 8-channel phased array transmit-receive knee coil (Invivo, Orlando, FL, USA).
a Time schedule for unloaded and loaded image acquisition. b Schematic illustration of the loading apparatus, applying 50% of the participant’s body-weight to the knee. c Picture of a test person in the MR magnet with the loading apparatus (image B and C taken with friendly permission from Souza et al. [50])
Cartilage thickness measurement
Quality control of the MR images and femorotibial cartilage segmentation were performed at the imaging centre of Chondrometrics GmbH, Ainring, Germany. Cartilage thickness was evaluated in the 4 femorotibial cartilage regions by manual delineation in each section of the medial and lateral tibia (MT and LT) and of the medial and lateral central femoral condyle (cMF and cLF) using a previously described methodology [4, 6, 35]. A region of interest (ROI) was set based on clearly defined anatomical landmarks, extending from the intercondylar notch anteriorly to 60% of the slices to the posterior end of the femoral condyles. With regard to performing a paired analysis of several (i.e. loaded vs. non-loaded) acquisitions, corresponding slices (which were only used in context of segmentation, but not when computing cartilage thickness outcomes) were identified by the reader [4]. The readers were blinded to the time sequence of the MR image acquisitions, and to the radiographic status of the participants.
For subregional cartilage thickness evaluation the medial and lateral tibial cartilage (MT/LT) was divided into central (cMT/cLT)), external (eMT/eLT)), internal (iMT/iLT), anterior (aMT/aLT) and posterior (pMT/pLT) subregions, with cMT and cLT covering exactly 20% of the MT/LT area of subchondral bone, respectively (Fig. 2). The central medial and lateral femoral cartilage (cMF/cLF) was divided into central (ccMF/ccLF), external (ecMF/ecLF), and internal (icMF/icLF) subregions, each covering 33% of the cartilage area. The test-retest reproducibility and sensitivity to change in cartilage thickness in OA of this algorithm has been demonstrated previously [6].
View on the 4 femorotibial cartilage plates (medial and lateral tibia [MT, LT] and on the medial and lateral central femur [cMF, cLF]) and on the 16 femorotibial cartilage subregions (internal, external, central, anterior and posterior in MT, LT, cMF, cLF) in a a schematic transversal plane and in b a MR image coronal plane
To evaluate whether the reproducibility of cartilage thickness measurements was similar under loaded compared to unloaded conditions as described previously in Wirth et al. [36] test-retest data was obtained in 4 (of the 30) study participants with the loading device applied as described above. The root mean square (RMS) CV% (coefficient of variation) for the 4 participants was determined in 4 cartilage regions and in 16 cartilage subregions. These were 2.6% for the medial tibia, 4.0% for the medial central femur, 3.0% for the lateral tibia and 1.9% the lateral central femur. The precision errors were on average 3.3% across the 20 regions/subregions under loaded conditions versus 2.6% under non-weight-bearing conditions across these 20 regions/subregions (1.4% medial tibia, 2.3% medial central femur, 1.7% lateral tibia and 2.2% lateral central femur) according to Wirth et al. [36].
Statistical analysis
All statistical analyses were performed using PASW version 18.0 (IBM, Chicago, IL, USA). To test for differences in magnitude of cartilage thickness under loading and unloading and for differences between healthy and OA affected knees, a two-sided paired t-test was used. Results were considered significant at p < 0.05.
Results
Demographic data, anatomical knee axis, knee symptoms and unloaded cartilage thickness values of the studied individuals are shown in Table 1. Of the 30 study participants, 12 had neutral alignment (4 KLG0, 5 KLG2, 3 KLG3), 14 varus alignment (4 KLG0, 4 KLG2, 6 KLG3), and 4 had valgus alignment (3 KLG0, 1 KLG2, 0 KLG3). There was no significant correlation between the knee axis with the ratio of deformation observed between the medial (MT + cMF) and lateral (LT + cLF) femorotibial compartment (r = 0.052, p = 0.78).
Femorotibial loading significantly reduced cartilage thickness across all 30 knees (independent of KL grades) in MT (−2.7%; p < 0.000), cMF (−4.1%; p < 0.000) and LT (−1.8%; p = 0.003), but not in cLF (+0.1%; p = 0.92) (Table 2). The subregion with the greatest percentage deformation was the central subregion in cMF (−6.3%, p < 0.000), followed by the external (−5.2%, p < 0.000) and central (−5.0%, p < 0.000) subregions of MT, and by the internal subregions of cMF (−3.9%, p < 0.000) and LT (−3.5%, p < 0.000) (Table 2).
Comparison of magnitude of deformation between healthy and OA knees
A trend towards greater deformation was observed in knees with medial radiographic OA versus healthy knees in the medial tibia (−3.09% in OA versus −2.08% in healthy; p = 0.47) and in the medial central, weight-bearing femur: (−4.79% in OA versus −2.84% in healthy; p = 0.17). In the lateral tibia, no difference was observed between the healthy and the osteoarthritic knees: (−1.70% in OA versus −1.64% in healthy; p = 1.00). In the lateral central, weight-bearing femur some deformation occurred in osteoarthritic knees (−0.81%) but none in the healthy knees (+1.83%; difference p = 0.10) (see Table 2 and Fig. 3). There were no significant differences when comparing KLG2 and KLG3.
Comparison of the regional and subregional pattern of deformation between healthy and OA knees
The relative pattern of deformation amongst the 4 femorotibial cartilage plates (cMF, MT, cLF, LT) did not differ between the healthy and the OA knees. Independent of radiographic disease status, the medial central, weight-bearing femur displayed the greatest deformation and the lateral central, weight-bearing femur displayed the smallest deformation upon loading (cMF > MT > LT > cLF). The subregional pattern of deformation was also very similar between the healthy knee and knees with medial radiographic OA, with the central subregion of cMF displaying relatively the greatest changes during loading (Fig. 4).
Subregional magnitude in loss of cartilage thickness due to loading or due to annual progression observed in OA (data summarised from the meta-analysis of Eckstein and co-workers [28]). Data are given as percentage change from the baseline value
Discussion
This study has investigated, for the first time, the magnitude and the subregional pattern of in vivo cartilage deformation in healthy knees versus knees with radiographic OA, using an MR imaging-compatible loading device. We found that static loading reduced cartilage thickness significantly in the medial tibia and femur and in the lateral tibia, and that knees affected by OA displayed a trend towards greater deformation compared with knees without radiographic OA. The subregional pattern of deformation, however, was very similar between healthy and osteoarthritic knees.
Previous studies tested cartilage deformation under loading ex vivo [11, 12, 16, 18, 19] without appropriately accounting for in vivo femorotibial loading conditions (networking of inner cartilage, cartilage-to-cartilage or cartilage-to-subchondral bone interactions). The strength of this investigation is the use of a novel MR imaging-compatible loading device for in vivo femorotibial loading. This setting permits the magnitude and pattern of cartilage deformation to be determined and the mechanical properties of cartilage to be (indirectly) evaluated at different stages of knee OA (KLG0, 2, 3) under in vivo conditions, without the use of invasive methods.
Limitations of this study are the small sample size in each KLG group, which limits the power by which differences in deformation (between healthy and OA knees) can be ascertained statistically and that only females were studied and therefore the results cannot be automatically assumed to apply in men. Previous studies, however, did not find significant differences in (patellar) cartilage deformation between men and women under loading [9, 37] and therefore we believe that these findings are likely to also hold for men. Another limitation is that only relatively small static loads can be applied during imaging, as motion artefacts increase with higher loads because of the participants’ movements. Nevertheless, the effects seen in this study are in principal agreement with ex vivo results on the material properties of OA cartilage (see below) but further investigations need to be performed in larger sample sizes and/or varied loading conditions. It must also be kept in mind that cartilage deformation during in vivo loading is determined not only by the mechanical properties of the cartilage but also by the load magnitude and distribution. However, the loads were applied as a proportion of the body weight in this study, and we found no differences in the subregional pattern of deformation between healthy and OA knees.
Previous studies reported that cartilage matrix synthesis and degradation are in a state of imbalance in OA [38, 39] and that cartilage composition is altered when, for instance, measured by T2 mapping [40, 41]. Other studies reported greater deformation of osteoarthritic cartilage compared with healthy cartilage in ex vivo models [11, 12, 16, 18, 19]. Changes in macro-molecular composition have been suggested to account for the altered load response of osteoarthritic cartilage [42]. The trend towards greater deformation in OA versus healthy knees was only apparent in the medial (but not in the lateral) femorotibial compartment, which is interesting, as OA participants were selected based on the presence of medial femorotibial OA (medial osteophytes or joint space narrowing). We therefore speculate that only the compartment displaying radiographic OA may be affected by a decline in cartilage mechanical properties, but not the contra-lateral compartment. Although the increased medial deformation may alternatively be explained by more varus malalignment (and hence greater medial versus lateral loading) in the OA participants, measurement of the knee angle revealed similar angles across healthy and OA knees, and—more importantly—the knee angle did not exhibit a correlation with the medial versus lateral femorotibial compartment deformation ratio across the 30 participants.
The largest deformation was observed in the central aspect of the medial femorotibial compartment (medial tibia and weight-bearing femur) and this observation was consistent in both healthy and OA knees. Once OA is present because of an individual predisposition [2], deformations occurring in these regions may lead to changes in cartilage composition, to altered cartilage mechanical properties and if persisting to loss in cartilage tissue [42]. The subregional pattern of cartilage deformation observed here was very similar to that observed with regard to the subregional pattern of cartilage (thickness) loss in MR imaging-based longitudinal studies of knee OA [43–46]. This agreement of spatial patterns suggests that the subregions encountering the greatest magnitude of deformation (in OA and healthy knees) are the same as those that also encounter the greatest rates of cartilage loss in knee OA, and that progression of cartilage loss in OA may thus be mechanically driven.
Changes in cartilage composition and mechanical properties are likely to be present before the onset of radiographic changes. Potentially, the mechanical properties of the cartilage may be tested using in vivo loading by MR imaging for scientific and diagnostic purposes, in order to detect a potential decline in the mechanical properties of the cartilage in vivo, before the onset of radiographic knee OA. Other methods such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) or T1 rho have been used to directly evaluate cartilage compositional measures (and their changes during early OA) [47–49] and the technique proposed here provides the opportunity to evaluate the relationship between compositional changes (as determined by these parametric imaging techniques) and the functional properties (deformation, i.e. as determined in this study).
Conclusion
The results of this study indicate that the cartilage in the medial femorotibial compartment (medial tibia and weight-bearing femur) of knees with medial radiographic OA displays a trend towards greater in vivo deformation than that observed in healthy knees. The subregional pattern of deformation, however, displayed great similarities in healthy and OA knees. The pattern of in vivo deformation closely matched that of cartilage loss observed in longitudinal studies of OA progression, indicating that cartilage loss in OA progression is at least partly driven by mechanical factors.
References
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367:1747–1757
Englund M (2010) The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res Clin Rheumatol 24:39–46
Eckstein F, Maschek S, Wirth W et al (2009) One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis 68:674–679
Eckstein F, Hudelmaier M, Wirth W et al (2006) Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis 65:433–441
Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C (2006) Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage 14(Suppl A):A46–A75
Wirth W, Hellio Le Graverand MP, Wyman BT et al (2009) Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage 17:291–297
Eckstein F, Tieschky M, Faber SC, Haubner M, Kolem H, Englmeier KH, Reiser M (1998) Effect of physical exercise on cartilage volume and thickness in vivo: MR imaging study. Radiology 207:243–248
Eckstein F, Tieschky M, Faber S, Englmeier KH, Reiser M (1999) Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat Embryol Berl 200:419–424
Eckstein F, Lemberger B, Stammberger T, Englmeier KH, Reiser M (2000) Patellar cartilage deformation in vivo after static versus dynamic loading. J Biomech 33:819–825
Eckstein F, Lemberger B, Gratzke C, Hudelmaier M, Glaser C, Englmeier KH, Reiser M (2005) In vivo cartilage deformation after different types of activity and its dependence on physical training status. Ann Rheum Dis 64:291–295
Guilak F, Ratcliffe A, Lane N, Rosenwasser MP, Mow VC (1994) Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res 12:474–484
Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ (2000) Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports 10:186–198
Bi X, Yang X, Bostrom MP et al (2007) Fourier transform infrared imaging and MR microscopy studies detect compositional and structural changes in cartilage in a rabbit model of osteoarthritis. Anal Bioanal Chem 387:1601–1612
Bi X, Li G, Doty SB, Camacho NP (2005) A novel method for determination of collagen orientation in cartilage by Fourier transform infrared imaging spectroscopy (FT-IRIS). Osteoarthritis Cartilage 13:1050–1058
Saarakkala S, Julkunen P, Kiviranta P, Makitalo J, Jurvelin JS, Korhonen RK (2010) Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage 18:73–81
Bank RA, Soudry M, Maroudas A, Mizrahi J, TeKoppele JM (2000) The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum 43:2202–2210
Kiviranta P, Lammentausta E, Toyras J, Kiviranta I, Jurvelin JS (2008) Indentation diagnostics of cartilage degeneration. Osteoarthritis Cartilage 16:796–804
Milentijevic D, Torzilli PA (2005) Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. J Biomech 38:493–502
Maroudas AI (1976) Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature 260:808–809
DeFrate LE, Sun H, Gill TJ, Rubash HE, Li G (2004) In vivo tibiofemoral contact analysis using 3D MRI-based knee models. J Biomech 37:1499–1504
Li G, Park SE, DeFrate LE, Schutzer ME, Ji L, Gill TJ, Rubash HE (2005) The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin Biomech Bristol Avon 20:736–744
Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE (2005) In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. Am J Sports Med 33:102–107
Komistek RD, Dennis DA, Mahfouz M (2003) In vivo fluoroscopic analysis of the normal human knee. Clin Orthop Relat Res 410:69–81
Kozanek M, Hosseini A, Liu F, Van de Velde SK, Gill TJ, Rubash HE, Li G (2009) Tibiofemoral kinematics and condylar motion during the stance phase of gait. J Biomech 42:1877–1884
Liu F, Kozanek M, Hosseini A, Van de Velde SK, Gill TJ, Rubash HE, Li G (2010) In vivo tibiofemoral cartilage deformation during the stance phase of gait. J Biomech 43:658–665
JH KELLGREN, Lawrence JS (1957) Radiological assessment of rheumatoid arthritis. Ann Rheum Dis 16:485–493
Altman RD, Hochberg M, Murphy WA Jr, Wolfe F, Lequesne M (1995) Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage 3(Suppl A):3–70
Eckstein F, Guermazi A, Roemer FW (2009) Quantitative MR imaging of cartilage and trabecular bone in osteoarthritis. Radiol Clin North Am 47:655–673
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Kraus VB, Vail TP, Worrell T, McDaniel G (2005) A comparative assessment of alignment angle of the knee by radiographic and physical examination methods. Arthritis Rheum 52:1730–1735
Moreland JR, Bassett LW, Hanker GJ (1987) Radiographic analysis of the axial alignment of the lower extremity. J Bone Joint Surg Am 69:745–749
Herberhold C, Faber S, Stammberger T et al (1999) In situ measurement of articular cartilage deformation in intact femoropatellar joints under static loading. J Biomech 32:1287–1295
Nishii T, Kuroda K, Matsuoka Y, Sahara T, Yoshikawa H (2008) Change in knee cartilage T2 in response to mechanical loading. J Magn Reson Imaging 28:175–180
Mayerhoefer ME, Welsch GH, Mamisch TC et al (2010) The in vivo effects of unloading and compression on T1-Gd (dGEMRIC) relaxation times in healthy articular knee cartilage at 3.0 Tesla. Eur Radiol 20:443–449
Eckstein F, Ateshian G, Burgkart R et al (2006) Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthritis Cartilage 14:974–983
Wirth W, Eckstein F (2008) A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging 27:737–744
Hudelmaier M, Glaser C, Hohe J, Englmeier KH, Reiser M, Putz R, Eckstein F (2001) Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum 44:2556–2561
Buckwalter JA, Mankin HJ, Grodzinsky AJ (2005) Articular cartilage and osteoarthritis. Instr Course Lect 54:465–480
Lorenzo P, Bayliss MT, Heinegard D (2004) Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol 23:381–391
Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S (2004) T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 232:592–598
Mosher TJ, Dardzinski BJ, Smith MB (2000) Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2–preliminary findings at 3T. Radiology 214:259–266
Saarakkala S, Julkunen P, Kiviranta P, Makitalo J, Jurvelin JS, Korhonen RK (2010) Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage 18:73–81
Eckstein F, Wirth W, Hudelmaier M et al (2008) Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum 59:1563–1570
Le Graverand MP, Buck RJ, Wyman BT et al (2010) Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls: a multicentre study using 3.0 Tesla MRI and Lyon-Schuss radiography. Ann Rheum Dis 69:155–162
Wirth W, Hellio Le Graverand MP, Wyman BT et al (2009) Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage 17:291–297
Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio Le Graverand-Gastineau MP, Keen H, Roemer FW (2008) Imaging in osteoarthritis. Rheum Dis Clin North Am 34:645–687
Mosher TJ, Dardzinski BJ (2004) Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8:355–368
Burstein D, Gray M, Mosher T, Dardzinski B (2009) Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am 47:675–686
Eckstein F, Burstein D, Link TM (2006) Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed 19:822–854
Souza RB, Stehling C, Wyman BT et al (2010) The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage 18(12):1557–1563
Acknowledgements
This work was funded by Pfizer Inc.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Cotofana, S., Eckstein, F., Wirth, W. et al. In vivo measures of cartilage deformation: patterns in healthy and osteoarthritic female knees using 3T MR imaging. Eur Radiol 21, 1127–1135 (2011). https://doi.org/10.1007/s00330-011-2057-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2057-y