Abstract
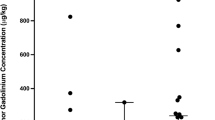
Gadofosveset is a Gd-based protein-binding blood pool agent with increased relaxivities and blood half-life compared with conventional Gd-based contrast agents (GBCAs). No experience exists about the use of gadofosveset as an extracellular agent. In this report we present the first clinical experience with gadofosveset in enhancing intracranial tumors. Ten patients with different intracranial tumors were examined with a standard dose (0.03 mmol/kg) of gadofosveset compared with a standard dose (0.1 mmol/kg) of conventional GBCA. As a result of its significantly higher relaxivity, gadofosveset could, despite its low dose, achieve a sufficient contrast enhancement. The visual rating of the intensity of enhancement and the contrast to noise ratios were comparable to conventional agents. The detection and delineation of more complex lesions was rated equal. In one nonenhancing low grade astrocytoma an enhancing nodule became visible only 5 h after gadofosvesest injection. As shown in this initial report, contrast-enhanced intracranial tumor imaging is possible with the protein-binding blood pool agent gadofosveset. The agent gives a significant tumor contrast in early postcontrast imaging comparable with conventional agents. As a result of its unique longer lasting contrast, the use of gadofosveset might enable a new approach to imaging mild or nonenhancing tumors.






Similar content being viewed by others
References
Brant-Zawadski M, Norman D, Newton TH (1984) Magnetic resonance imaging of the brain: the optimal screening technique. Radiology 152:71–77
Byrne TN (1994) Imaging of gliomas. Sem Oncol 21:162–171
Black PM (1991) Brain tumor. Part 2. N Engl J Med 324:1555–1564
Stack JP, Antoun NM, Jenkins JP, Metcalfe R, Isherwood I (1988) Gadolinium-DTPA as a contrast agent in magnetic resonance imaging of the brain. Neuroradiology 30:145–154
Hesselink JR, Healy ME, Press GA, Brahme FJ (1988) Benefits of Gd-DTPA for MR imaging of intracranial abnormalities. J Comput Assist Tomogr 12:266–274
Elster AD, Moody DM, Ball MR, Laster DW (1989) Is Gd-DTPA required for routine cranial MR imaging. Radiology 173:231–238
Sze G, Milano E, Johnson C, Heier L (1990) Detection of brain metastases: comparison of contrastenhanced MR with unenhanced MR and enhanced CT. AJNR Am J Neuroradiol 11:785–791
Neuwelt EA (2004) Mechanisms of disease: the blood-brain barrier. Neurosurgery 54:131–140
Bart J, Groen HJ, Hendrikse NH et al (2000) The blood-brain barrier and oncology: new insights into function and modulation. Cancer Treat Rev 26:449–462
Nikolaou K, Kramer H, Grosse C, Clevert D et al (2006) High-spatial-resolution multistation MR angiography with parallel imaging and blood pool contrast agent: initial experience. Radiology 241:861–872
Runge VM (2001) A review of contrast media research in 1999–2000. Invest Radiol 36:123–130
Brekenfeld C, Foert E, Hundt W et al (2001) Enhancement of cerebral diseases: how much contrast agent is enough? Comparison of 0.1, 0.2, and 0.3 mmol/kg gadoteridol at 0.2 T with 0.1 mmol/kg gadoteridol at 1.5 T. Invest Radiol 36:266–275
Huppertz A, Rohrer M (2004) Gadobutrol, a highly concentrated MR-imaging contrast agent: its physicochemical characteristics and the basis for its use in contrast-enhanced MR angiography and perfusion imaging. Eur Radiol Suppl 14([Suppl 5]):M12–M18
Essig M, Weber MA, von Tengg-Kobligk H, Knopp MV, Yuh WT, Giesel FL (2006) Contrast-enhanced magnetic resonance imaging of central nervous tumors: agents, mechanisms and applications. Top Magn Reson Imaging 17:89–106
Hartmann M, Wiethoff AJ, Hentrich HR, Rohrer M (2006) Initial imaging recommendations for Vasovist angiography. Eur Radiol Suppl 16([Suppl 2]):B15–B23
Grist TM, Korosec FR, Peters DC et al (1998) Steady-state and dynamic MR angiography with MS-325: initial experience in humans. Radiology 207:539–544
Perreault P, Edelmann MA, Baum RA et al (2003) MR angiography with gadofosveset trisodium for peripheral artery disease: phase II trial. Radiology 229:811–820
Goyen M, Edelmann M, Perreault P et al (2005) MR angiography of aortoiliac occlusive disease: a phase III study of the safety and effectiveness of the blood-pool contrast agent MS-325. Radiology 236:825–833
Eldredge HB, Spiller M, Chasse JM, Greeenwood MT, Caravan P (2006) Species dependence on plasma protein binding and relaxivityof the gadolinium-based MRI contrast agent MS-325. Invest Radiol 41:229–243
Adzamli K, Yablonski DA, Chicoine MR et al (2003) Albumin-binding MR blood pool agents as MRI contrast agents in an intracranial mouse glioma model. Magn Reson Med 49:586–590
Farooki A, Narra V, Brown J (2004) Gadofosveset EPIX/Schering. Curr Opin Investig Drugs 5:967–976
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 40:715–724
Maravilla KR, Maldjian JA, Schmalfuss IM, Kuhn MJ, Bowen BC, Wippold FJ 2nd, Runge VM, Knopp MV, Kremer S, Wolansky LJ, Anzalone N, Essig M, Gustafsson L (2006) Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology 240:389–400
Cowper SE, Boyer PJ (2006) Nephrogenic systemic fibrosis: an update. Curr Rheumatol Rep 8:151–157
Grobner T (2006) Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis. Nephrol Dial Transplant 21:1104–1108
Thomsen HS, Marckmann P, Logager VB (2008) Update on nephrogenic systemic fibrosis. Magn Reson Imaging Clin N Am 16:551–560
Frenzel T, Lengsfeld P, Schirmer H, Hutter J, Weinmann HJ (2008) Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol 43:817–828
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Essig, M., Rohrer, M., Giesel, F. et al. Human brain tumor imaging with a protein-binding MR contrast agent: initial experience. Eur Radiol 20, 218–226 (2010). https://doi.org/10.1007/s00330-009-1530-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1530-3