Abstract
The feasibility of large-core-needle magnetic resonance imaging (MRI)-guided breast biopsy at 3 T was assessed. Thirty-one suspicious breast lesions shown only by MRI were detected in 30 patients. Biopsy procedures were performed in a closed-bore 3-T clinical MR system on a dedicated phased-array breast coil with a commercially available add-on stereotactic biopsy device. Tissue sampling was technically successful in 29/31 (94%) lesions. Median lesion size (n = 29) was 9 mm. Histopathological analysis showed 19 benign lesions (66%) and one inconclusive biopsy result (3%). At follow-up of these lesions, 15 lesions showed no malignancy, no information was available in three patients and two lesions turned out to be malignant (one lesion at surgical excision 1 month after biopsy and one lesion at a second biopsy because of a more malignant enhancement curve at 12-months follow-up MRI). Nine biopsy results showed a malignant lesion (31%) which were all surgically removed. No complications occurred. MRI-guided biopsy at 3 T is a safe and effective method for breast biopsy in lesions that are occult on mammography and ultrasound. Follow-up MRI at 6 months after the biopsy should be performed in case of a benign biopsy result.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) of the breast is a promising diagnostic technique with a high sensitivity and a reasonable specificity for the detection of invasive breast tumors [1, 2]. Approximately 10% of patients with a malignant breast lesion detected on mammography has an additional malignancy that is mammographically and ultrasonographically occult [3, 4]. When these additional suspicious breast lesions are detected on MR images, histopathological analysis is required. MRI-guided tissue sampling of these “MRI-only lesions” can be accomplished by means of wire localization and surgical excision, vacuum-assisted biopsy, or by large-core-needle biopsy (LCNB) [5].
LCNB has several advantages over wire localization, including decreased invasiveness, morbidity, and costs [6, 7]. MRI-guided breast biopsy performed at 1.5 T, using a 14-gauge MRI-compatible needle, has been reported to have 98% diagnostic accuracy [8]. Some authors do not recommend MRI-guided core biopsy for lesions <10 mm, because the results are often inconclusive [6, 9]. Nevertheless, the demand for biopsy of suspect sub-centimeter MRI-only lesions is high.
MRI systems operating at high magnetic fields strengths (e.g., 3 T) become increasingly available in the clinical setting. The possibility of acquiring high-resolution images in a short period of time, nominates 3-T breast imaging as a promising alternative to 1.5-T imaging for diagnostic purposes. The possibility of decreasing MR data acquisition time while retaining an acceptable signal-to-noise ratio (SNR) and spatial resolution makes high-field MRI appealing for interventional purposes as well. However, if breast biopsies were to be performed at 3 T, the susceptibility artifact of the needle would become larger and could complicate accurate tissue sampling. This prompted us to assess the feasibility of 3-T MRI-guided LCNB of suspicious nonpalpable breast lesions.
Materials and methods
Patient characteristics
Thirty-one women (mean age: 48 years; range: 29–78) with 32 suspicious breast lesions which were only detected by MRI (MRI-only) were examined between September 2005 and February 2008. Indications for diagnostic breast MRI were problem solving (n = 11), increased risk of breast cancer (n = 7), preoperative staging (n = 3), work-up of a nonpalpable breast lesion (n = 5), tumor-positive axillary lymph node with negative mammography (n = 1), bloody nipple discharge (n = 2) and unknown (n = 2). Patients were referred for MRI-guided biopsy from ten different hospitals. Of the 32 lesions, 18 lesions were characterized as BI-RADS-MRI III, nine lesions as BI-RADS-MRI IV and four as a BI-RADS-MRI V lesion. The BI-RADS-MRI classification of one lesion was unknown. No approval by the ethics board or informed consent was obtained because the MRI-guided breast biopsies were not performed in a study setting, but in the clinical setting. In our country, patients do not have to give informed consent for a clinical procedure.
MRI-guided biopsy procedure and equipment
Second-look ultrasound was recommended in all patients; if the lesion could be retrieved on ultrasound images, ultrasound-guided biopsy was performed; if the lesion was occult on ultrasound images, MRI-guided biopsy was performed. MRI-guided biopsies were performed on a 3-T clinical MR system (Achieva, Philips Healthcare, Best, The Netherlands). Patients were placed in the prone position in a dedicated phased-array bilateral breast coil, with the affected breast in a dedicated breast coil which had an add-on biopsy device with a medial and lateral compression plate (Fig. 1) (MRI Devices, Würzburg, Germany). The biopsy system comprised a gadolinium-filled guiding marker tube that can be positioned in the feet-head and anterior-posterior direction along a centimeter scale and angulated around the feet-head axis from +45° to -45° degrees. First, fat-suppressed high-resolution T1-weighted gradient-echo images were acquired after the administration of 0.1 mmol/kg gadolinium (Magnevist, Bayer-Schering, Germany) to verify the position of the lesion. The administration of the contrast agent was delayed as long as possible during the procedure to avoid vanishing of the target lesion. The main parameters of this MR sequence are listed in Table 1. After correct positioning of the marker tube, the table top was moved out of the bore and local anesthesia (lidocaine 1%) was administered subcutaneously. The marker tube was replaced by a 14-gauge sterile biopsy needle surrounded by a 13-gauge needle holder (Somatex, Teltow, Germany). The needle and sheath were horizontally inserted in the breast and advanced towards the lesion. The position of the needle and sheath were confirmed by using the fat-suppressed high-resolution T1-weighted gradient-echo sequence. After correct placement of the biopsy needle was confirmed, the 14-gauge biopsy gun (Somatex, Teltow, Germany) was inserted through the sheath and three to five tissue samples were obtained.
Stereotactic system for MRI-guided preoperative wire-localization and breast biopsy. This system consists of a breast coil, which can be used for diagnostic MRI of the breast, and an add-on stereotactic device. The breast is compressed by two compression plates. The system allows angulation of the needle
Data collection
Histopathological analysis of the lesions was performed and lesions were classified in two categories: benign or malignant. In case of a benign lesion, the images of the biopsy procedure were re-evaluated to determine whether the tissue samples were representative. If the samples were considered not representative, a second biopsy or surgical excision was performed, based on the level of suspicion of malignancy and patient preference. Malignant lesions were surgically removed.
Results
Of the 32 suspicious MRI-only lesions, one lesion was seen on second-look ultrasound, and was therefore sampled under ultrasound guidance. In all other cases, MRI-guided biopsy was performed. In two cases, MRI-guided biopsy was not feasible: in one patient a 9-mm lesion was located directly posterior to the mammilla and in another patient a 9-mm lesion was located directly anterior to the thoracic wall (Fig. 2). No adequate compression could be achieved in the region in which the lesions were located, which made introduction of the biopsy needle not feasible in both cases. In the other 28 patients with 29 lesions, the 3-T MRI-guided LCNB was considered to be technically successful. The size of the needle artifact was 9.5 mm. A typical example of the acquired images during the procedure is shown in Fig. 3. None of the procedures had to be interrupted or stopped. No severe side effects were observed.
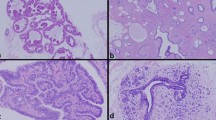
The ‘MRI-only’ lesion size ranged from 3 to 45 mm with a median lesion size of 9 mm. Histopathological analysis of the biopsy samples resulted in 19/29 (66%) benign lesions (two fibroadenomas, three fibrocystic changes, six hyperplasias/adenoses, five normal fibroglandular tissues, one intramammary lymph node, one lobular carcinoma in situ (LCIS) and one papilloma) (Table 2). The results of one biopsy procedure were inconclusive (1/29, 3%). This lesion was surgically removed, histopathological analysis showed no malignancy. Follow-up was heterogeneous (Fig. 4) due to large number of referring hospitals. In summary, follow-up of the 20 non-malignant biopsies showed no malignancy in 15 cases and revealed two malignant lesions: one LCIS lesion was surgically removed 1 month after the biopsy and additional ductal carcinoma in situ (DCIS) and a small invasive ductal component were demonstrated during pathological analysis of the lump. The second case showed a more malignant kinetic enhancement curve at 12-months follow-up MRI. The lesions was sampled again and pathology showed an invasive lobular carcinoma. No follow-up information was available in three patients.
Nine lesions (9/29, 31%) showed malignant pathological features in the sampled tissue: two ductal carcinomas in situ, two invasive lobular carcinomas (apart from the lesion described above), two invasive ductal carcinomas and three adenocarcinomas (Table 2). All malignant lesions were surgically removed: modified radical mastectomy (n = 3), breast conserving surgery (n = 3), unknown type of surgery (n = 3).
Discussion
We report our preliminary experience in MRI-guided large-core-needle breast biopsies using a 3-T closed-bore clinical MRI system. In our patient series, MRI-guided LCNB was technically successful in 93% of patients (28/30). Two lesions could not be sampled since both lesions were located in a difficultly accessible location in the breast. In the most proximal and the most distal parts of the breast, adequate compression is difficult to achieve, which makes introduction of the biopsy needle not feasible. We advise to pursue MRI-guided breast biopsies even in these areas of the breast. If tissue sampling turns out not to be feasible, follow-up MRI is advised (to assess growth or change in the dynamic enhancement curve) or surgical excision of the lesions depending on the level of suspicion of malignancy. To increase the diagnostic accuracy of tissue sampling of small breast tumors, vacuum-assisted biopsy systems have been proposed and are increasingly introduced in clinical practice. Vacuum-assisted biopsy has great potential in (MRI-guided) breast biopsy. When vacuum-assisted breast biopsy is performed, more tissue is sampled compared with the amount of tissue removed with LCNB. This would probably outweigh the negative effect of the larger needle artifact caused by the use of a larger biopsy needle in vacuum-assisted biopsy. However, vacuum-assisted biopsy is not available in all institutions yet and, to our knowledge, no study has directly compared the diagnostic accuracy of LCNB with a vacuum-assisted biopsy system in a randomized controlled trial.
We found 19 benign (66%), one inconclusive biopsy result (3%) and nine (31%) malignant lesions. These numbers correspond to the 35%, 34% and 25% malignant lesions reported in comparable studies [8–10]. The studies by Kuhl et al. [8] and Fisher et al. [9] reported 98% (77/78) of the biopsy samples to be representative with the use of a 14-gauge needle [8] and 89% (25/28) using an 18- to 22-gauge biopsy needle [9]. The 97% (28/29) representative results we found using a 14-gauge core biopsy are comparable with the results described by Kuhl et al. [8]. In our study, two benign lesions turned out to be malignant at follow-up. The second lesion was a lobular carcinoma in situ, which was surgically removed. One LCIS lesion was surgically removed, and additional DCIS and a small (<4 mm) invasive ductal carcinoma were found at histopathological examination of the lump. The other lesion showed a more malignant kinetic enhancement curve at 12-months follow-up MRI. Because of the change in lesion characteristics, the lesion was sampled again and an invasive lobular carcinoma was found. Given the change in enhancement characteristics and the relatively long period of follow-up, it remains unclear whether the lobular carcinoma developed during follow-up or should be considered as a missed malignancy. For this reason the MRI BI-RADS lexicon committee recommend a 6-month breast-MRI follow-up after MRI-guided biopsy.
Currently, only one study has been published on breast imaging at high field strength: Kuhl et al. [8] reported a prospective intra-individual comparative study performed on 37 patients with 53 enhancing breast lesions who underwent contrast-enhanced breast MRI at 1.5 T and 3 T. The high resolution of the 3-T images allowed depiction of lesion morphology with unprecedented accuracy [11], suggesting a promising role for 3-T breast MRI. In this patient series, we used 14-gauge titanium biopsy needles. Large-core needles (14-gauge) are recommended for percutaneous biopsy of breast lesions to ensure sampling of sufficient tissue [12]. However, larger needles cause larger susceptibility artifacts on MR images. Furthermore, susceptibility artifacts increase with increasing field strength. This can cause a substantial signal void around the 14-gauge biopsy needle when performing MRI-guided breast biopsy at 3 T, especially when the biopsy needle is placed perpendicular to the main magnetic field, as is usually the case in MRI-guided breast biopsy [9]. Our study showed that a signal void of 9.5 mm can be achieved at 3 T. To our knowledge, no reports on MRI-guided breast biopsies at 3 T are available. The extent of the signal void of comparable studies were 4 mm (1.5 T, 14-gauge needle) [8], 5 mm (0.5 T, 16-gauge needle) [9] and 10 mm (ultra-fast scan protocol) [9], which is smaller than the signal void we found at 3 T, as was expected. However, the 9.5-mm signal void did not reduce the accuracy of the tissue sampling. The previously mentioned studies reported a diagnostic accuracy of 98% (58/59) [8]. Fisher et al. [9] did not report the diagnostic accuracy of their patient series.
In conclusion, MRI-guided 14-gauge core-needle biopsy at 3 T is a safe and effective method for breast biopsy in lesions that are occult on mammography and ultrasound. In the case of benign biopsy results, a 6-month follow-up MRI should be performed.
References
Berg WA, Gutierrez L, NessAiver MS et al (2004) Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 233:830–849
Bluemke DA, Gatsonis CA, Chen MH et al (2004) Magnetic resonance imaging of the breast prior to biopsy. JAMA 292:2735–2742
Fischer U, Kopka L, Grabbe E (1999) Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 213:881–888
Malur S, Wurdinger S, Moritz A, Michels W, Schneider A (2001) Comparison of written reports of mammography, sonography and magnetic resonance mammography for preoperative evaluation of breast lesions, with special emphasis on magnetic resonance mammography. Breast Cancer Res 3:55–60
Smith LF, Henry-Tillman R, Mancino AT et al (2001) Magnetic resonance imaging-guided core needle biopsy and needle localized excision of occult breast lesions. Am J Surg 182:414–418
Helbich TH (2001) Localization and biopsy of breast lesions by magnetic resonance imaging guidance. J Magn Reson Imaging 13:903–911
Liberman L, Fahs MC, Dershaw DD et al (1995) Impact of stereotaxic core breast biopsy on cost of diagnosis. Radiology 195:633–637
Kuhl CK, Morakkabati N, Leutner CC, Schmiedel A, Wardelmann E, Schild HH (2001) MR imaging–guided large-core (14-gauge) needle biopsy of small lesions visible at breast MR imaging alone. Radiology 220:31–39
Fischer U, Kopka L, Grabbe E (1998) Magnetic resonance guided localization and biopsy of suspicious breast lesions. Top Magn Reson Imaging 9:44–59
van den Bosch MA, Daniel BL, Pal S et al (2006) MRI-guided needle localization of suspicious breast lesions: results of a freehand technique. Eur Radiol 16:1811–1817
Kuhl CK, Jost P, Morakkabati N, Zivanovic O, Schild HH, Gieseke J (2006) Contrast-enhanced MR imaging of the breast at 3.0 and 1.5 T in the same patients: initial experience. Radiology 239:666–676
Liberman L, Dershaw DD, Rosen PP, Abramson AF, Deutch BM, Hann LE (1994) Stereotaxic 14-gauge breast biopsy: how many core biopsy specimens are needed? Radiology 192:793–795
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Peters, N.H.G.M., Meeuwis, C., Bakker, C.J.G. et al. Feasibility of MRI-guided large-core-needle biopsy of suspiscious breast lesions at 3 T. Eur Radiol 19, 1639–1644 (2009). https://doi.org/10.1007/s00330-009-1310-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1310-0