Abstract
Oxygen-sensitive 3He-MRI was studied for the detection of differences in intrapulmonary oxygen partial pressure (pO2) between patients with normal lung transplants and those with bronchiolitis obliterans syndrome (BOS). Using software developed in-house, oxygen-sensitive 3He-MRI datasets from patients with normal lung grafts (n = 8) and with BOS (n = 6) were evaluated quantitatively. Datasets were acqiured on a 1.5-T system using a spoiled gradient echo pulse sequence. Underlying diseases were pulmonary emphysema (n = 10 datasets) and fibrosis (n = 4). BOS status was verified by pulmonary function tests. Additionally, 3He-MRI was assessed blindedly for ventilation defects. Median intrapulmonary pO2 in patients with normal lung grafts was 146 mbar compared with 108 mbar in patients with BOS. Homogeneity of pO2 distribution was greater in normal grafts (standard deviation pO2 34 versus 43 mbar). Median oxygen decrease rate during breath hold was higher in unaffected patients (−1.75 mbar/s versus −0.38 mbar/s). Normal grafts showed fewer ventilation defects (5% versus 28%, medians). Oxygen-sensitive 3He-MRI appears capable of demonstrating differences of intrapulmonary pO2 between normal lung grafts and grafts affected by BOS. Oxygen-sensitive 3He-MRI may add helpful regional information to other diagnostic techniques for the assessment and follow-up of lung transplant recipients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
3He-MRI is a novel technique for functional imaging of the lung. A variety of different pulse sequence types have been described, including spin density, diffusion-weighted, dynamic ventilation and oxygen-sensitive imaging [1–4]. Spin density and dynamic imaging have been applied to lung transplant recipients with the aim to improve non-invasive image-based diagnostics of obliterative bronchiolitis (OB) [5, 6]. In a follow-up study, spin density imaging detected OB earlier than pulmonary function tests in two out of five cases [7].
Pulmonary function testing (PFT) is widely used as the standard diagnostic tool for the follow-up of lung transplant recipients. Transbronchial lung biopsies can confirm clinical suspicion of bronchiolitis obliterans syndrome (BOS). Both approaches have inherent disadvantages. PFT remains a global screen in which functional impairment of lung regions affected by OB may be compensated for by healthy regions [8]. Bronchoscopy with transbronchial biopsy is invasive. Due to the inhomogeneous distribution of OB, foci of disease can be missed [9]. Computed tomography (CT), the standard imaging method in lung transplant recipients, is only moderately sensitive in the detection of OB [10].
3He-MRI is a sensitive method to detect ventilatory disturbances in a variety of lung diseases [11–14]. Patients with a clinical diagnosis of BOS show an increased incidence of ventilation defects when compared with patients with a normal lung graft [7]. Since in OB ventilation becomes impaired by obliteration of small airways, impaired pulmonary gas exchange may reduce local alveolar oxygen partial pressure (pO2) even before a lung region shows signal loss due to attenuated 3He intake. Oxygen-sensitive 3He-MRI has been validated as a noninvasive method to measure the regional distribution of intrapulmonary pO2 [15, 16].
In oxygen-sensitive 3He-MRI the T1-shortening effect of molecular oxygen is used to calculate the pO2 in a respective volume of interest by signal intensity changes in repeated imaging during a single breath-hold. Intrapulmonary pO2 is the result of a local steady-state balance between alveolar ventilation and perfusion of the pulmonary capillary bed, i.e. a regional \( {\mathop V\limits^\prime }{_{A} } \mathord{\left/ {\vphantom {{_{A} } Q}} \right. \kern-\nulldelimiterspace} Q \) ratio. Since BO is an airway disease, ventilation becomes impaired in affected regions of the lung. Perfusion will adapt to these changes depending on the responsiveness of hypoxic pulmonary vasoconstriction. In a state of local alveolar hypoventilation, the resultant decrease of intrapulmonary pO2 is expected to be compensated for, at least partially, by adaptive changes in perfusion. Nevertheless, the distribution of \({\mathop V\limits^\prime }_{A} /Q\) ratios and hence, intrapulmonary pO2 values, would be expected to widen. Even more so, since BO itself is distributed rather inhomogeneously.
The present study investigates whether 3He-MRI is capable of differentiating BOS from normal lung grafts, based on the measurements of the intrapulmonary pO2 measured by oxygen-sensitive 3He-MRI. This might help to better delineate the role of functional 3He-MRI in the early detection of OB.
Materials and methods
Patients
Approval of the University’s Ethics Committee and written informed patient consent were obtained for follow-up after lung transplantation including functional 3He-MRI.
The cohort of this retrospective evaluation consisted of 12 patients (three female, nine male) aged between 39 and 61 years (median, 51). There were four double-lung recipients and eight single-lung recipients. The underlying disease necessitating lung transplantation was terminal emphysema in eight cases (including two cases with α-1-antitrypsin deficiency) and idiopathic pulmonary fibrosis in the remaining four (including two with acute interstitial pneumonia). Two of these patients contributed two datasets during follow-up, so a total of 14 datasets from these 12 patients were used for postprocessing. Of these datasets, eight were acquired in normal grafts, and six in presence of a clinical diagnosis of BOS. The cohort was selected from a group of 23 consecutively scanned lung transplant recipients during a period of 26 months.
Conventional follow-up and diagnosis of BOS were performed according to current recommendations of the International Society for Heart and Lung Transplantation, including serial pulmonary function testing (PFT) [17]. BOS was diagnosed on grounds of PFT deterioration if the ratio between current forced expiratory volume in 1 s (FEV1) and the best post-operative FEV1 became smaller than 0.81.
Inclusion criterion for this observational series was an oxygen-sensitive MRI dataset of diagnostic quality. Image series with low signal-to-noise ratio (SNR ≤ 3) due to low gas intake, very advanced states of BOS and prematurely aborted breath-holding were excluded from the study.
3He-Gas
Polarization, storage, transport and application of 3He have been described in detail elsewhere [18, 19]. We used 3He polarized to 40–45% by metastability exchange optical pumping. The 3He gas was administered to the patients using a computer-controlled application device. The device was connected to an intensive care-type ventilator (Servo 900 C, Siemens Medical Solutions, Erlangen, Germany) and operating in the pressure-support mode, in order to allow a comfortable spontaneous breathing pattern. For imaging approximately 300 ml of hyperpolarized 3He were administered within a single breath to the patient.
Imaging
All measurements were performed on a 1.5-T clinical scanner (Magnetom Vision, Siemens Medical Solutions, Erlangen, Germany) tuned to the Larmor frequency of 3He (48.5 MHz). The scanner was equipped with a broadband amplifier and a dedicated certified double resonant (1H and 3He) bird cage coil (Fraunhofer Institut, St. Ingbert, Germany) for transmission and reception of radio frequency pulses. We used a spoiled gradient echo pulse sequence oriented coronally with the following parameters: repetition time 11 ms, echo time 4.2 ms, flip angle < 10°. Omission of a slice selection gradient lead to “single slice” projection images. In plane resolution was 3.95 × 2.5 mm at an acquisition matrix of 81 × 128 and a field of view of 320 mm. A double-acquisition technique allowed for mathematical separation of the effects of radio frequency pulses and T1 relaxation of 3He due to the presence of molecular oxygen. This included two imaging series, consisting of eight images, with each series acquired during inspiratory breath hold. In both series, all parameters had to be kept constant (including inspired gas and 3He volumes) except the inter-scan interval. This parameter was set to 1.005 s in the first, and 3, 4, or 5 s in the second imaging series, the latter depending on the patients’ capability to hold their breath [15].
During the same imaging session, 3He spin density images were acquired using the same scanner and coil. This was done during inspiratory breath-hold using a two-dimensional spoiled gradient echo pulse sequence. Images were oriented coronally and provided a slice thickness of 10–15 mm with an interslice gap of 2–5 mm (repetition time 11 ms, echo time 4.2 ms, flip angle 10°, centric reordering, in-plane resolution 4.2 × 2.7 mm, matrix 81 × 128, field of view 340 mm, sinc interpolation to a 256 matrix).
Image evaluation
Images were transferred to a commercially available IBM-compatible PC. An in-house developed software based on PV-Wave (Visual Numerics, Boulder, Colo.) was used for post-processing [20, 21]. The software is able to read image files in the DICOM standard and automatically excludes regions with a SNR of 3.0 or less. Filtering in the image domain reduced remaining noise. The software calculates the intrapulmonary pO2 following the physical relationship between noise-corrected signal intensity (A) decay in two series of n images each, taken at different interscan delays τ1 and τ2, and pO2:
Results are displayed in colour-coded maps. In order to account for remaining image noise, a 2 × 2 matrix of image pixels was averaged giving the final 64 × 64 matrix, to be analysed for pO2. The resultant in-plane resolution was 5 × 5 mm. The software calculates mean pO2 and its standard deviation as well as the pO2 decrease rate during the period of apnea while the imaging process takes place (RO2). In case of unilateral lung transplants only the grafts underwent evaluation.
Spin density images were assessed for ventilation defects by two observers with several years practice in interpreting 3He-images. They were blinded to patient history except for transplant status (single, right, left, double). The total volume of ventilation defects was estimated as percentage of total lung volume and expressed as means of both observers’ results.
Statistical analysis
Data analysis was limited to descriptive statistics and graphic displays using box-whisker plots; significance testing, as well as the calculation of an interobserver agreement in the assessment of ventilation defects, was omitted due to the small study sample. Descriptive comparisons were made of mean intrapulmonary pO2, intraindividual pO2 standard deviation in order to describe homogeneity of its distribution and for mean decrease rate of pO2 during apnea.
A systematic error towards lower pO2 in image sets with poor SNR, which could be due to the influence of noise on the post-processing results, was studied by regression analysis of SNR and pO2 data.
Results
Median intrapulmonary pO2 in grafts of patients with diagnosed BOS was 108 mbar (range, min 79 mbar, max 131 mbar, see also Table 1), compared with an intrapulmonary pO2 of 146 mbar (median; range, min 108 mbar, max 179 mbar) in normal grafts. There was an overall trend towards lower intrapulmonary pO2 levels in BOS patients shown by Fig. 1. In BOS patients, intrapulmonary pO2 had a wider distribution, indicating a greater degree of ventilation-perfusion inhomogeneity (Fig. 2). Median standard deviation was 43 mbar in BOS patients versus 34 mbar in those with normal lung grafts. RO2, i.e. pO2 decrease rate during breath-holding, was less steep in BOS patients (median, −0.38 mbar/s breath-hold) than in unaffected patients (median, −1.75 mbar/s breathhold) (Fig. 3). As the number of study participants is low, Table 2 lists results for assumptions of Gaussian and non-Gaussian distribution.
Distribution of standard deviations of intrapulmonary pO2 (box-whisker plot). Standard deviation is larger in patients suffering from BOS, reflecting a greater inhomogeneity of intra-pulmonary pO2 distribution in this disease [plot depicting median, 25th and 75th percentiles (box), and minimum and maximum values (whiskers) of intra-individual pO2 standard deviations]
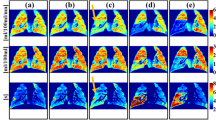
Colour-coded maps showed a normal mean intrapulmonary pO2 level with a quite homogeneous distribution in normal lung grafts, but lower and inhomogeneously distributed pO2 levels in patients suffering from BOS (Fig. 4). Accordingly, ventilation defects were smaller in normal grafts. Patients with normal grafts presented with a median ventilation deficit of 5% (min 4%, max 15%) versus BOS patients with 28% (min 10%, max 88%).
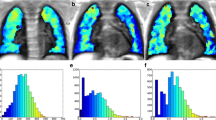
a Intrapulmonary pO2-map of a patient with a normal lung graft of a 42-year-old female after right-sided lung transplantation for terminal emphysema. b A pO2 map of the same patient after development of BOS, with a decline of FEV1 of 30% of her best post-operative FEV1. c pO2 histogram of the same patient at normal graft function, and d after development of BOS. The diminished and more inhomogeneous intrapulmonary pO2 after BOS development can be well appreciated in the colour-coded maps as well as in the respective pO2 histograms
Regression analysis did not detect any systematic linear relationship between SNR data and pO2 measurements (r = 0.21, Fig. 5). A systematic error towards lower pO2 in lung images displaying poor SNR, like those affected by BOS, was therefore not substantiated.
Discussion
In 12 recipients of unilateral or bilateral lung transplants, oxygen-sensitive 3He-MRI was added to post-transplant follow-up studies. In a total of 14 datasets, those from patients with normal lung grafts were compared with those from patients diagnosed with BOS. Analysis of intrapulmonary pO2 data of the grafts demonstrated overall lower and more inhomogeneously distributed intrapulmonary pO2 in patients diagnosed with BOS when compared with patients with an unaffected graft.
As a first step toward the goal of earlier non-invasive diagnosis of BOS, the technique and findings of this study confirm the predicted reduction of mean intrapulmonary pO2 and greater inhomogeneity of its distribution in clinically manifest BOS. The study showed that the pO2 decrease rate during apnea (RO2) was smaller in BOS grafts than in clinically unaffected transplants. Patients not affected by BOS showed results similar to those determined in healthy volunteer subjects (mean ± SD, 153 ± 11 mbar) reflecting a normal ventilation and oxygen exchange [22].
A major limitation of the technique is demonstrated in the pO2 maps of Fig. 4. One of the intrinsic disadvantages is that oxygen sensitive 3He-MRI is critically dependent on alveolar entry of sufficient amounts of hyperpolarized 3He gas. When a lung area contains no signal due to hypoventilation, no pO2 can be determined. However, such areas are easily delineated in spin density MR images of the lung as ventilation defects. It has been demonstrated in previous studies that patients affected by BOS show more and larger ventilation defects than non-affected patients [7, 23].
There is a variety of other conditions that may cause reduction and inhomogeneity of intrapulmonary pO2 distribution. Such abnormalities are known to occur in acute pulmonary embolism [24–27], in chronic thromboembolic pulmonary hypertension, as well as in pulmonary fibrosis or emphysema [1, 28, 29].
A pO2 analysis based on images with low SNR may theoretically introduce a systematical measurement error because of the magnitude reconstruction of the MR images [30]. To study the relevance of this bias, image SNR and resultant pO2 were correlated. We found a nonsignificant correlation with a coefficient r = 0.21, which does not definitively exclude SNR dependency of the pO2 measurements, but indicates at least minor importance.
There are several limitations to the present study. First, the small and relatively inhomogeneous study sample, with incomplete independence of compared groups, allows only a rather conservative interpretation of the results, and calls for further confirmation in larger cohorts. Technically, the pulse sequence applied for pO2 measurement is a two-dimensional sequence without slice selectivity. Such projection images are robust against diffusion of 3He polarization out of, or into, an imaged slice during the measurement interval, but they are inherently of restricted spatial resolution. First steps have been taken to develop and validate three-dimensional imagimg sequences, which provide marked spatial resolution and are, at the same time, robust against molecular diffusion effects [31, 32]. Finally, SNR remains a pivotal issue in oxygen-sensitive 3He MRI. The gas used in the present study was polarized to a grade of 40–45%. Meanwhile, new gas polarizers have been developed which achieve polarization grades of up to 60%. Higher polarization is expected to improve accuracy of 3He-MRI based pO2 measurements as the error produced by image noise is decreased.
Currently, a non-invasive, sensitive and specific method to detect BO in lung transplant recipients is lacking [8–10]. Nevertheless, early detection of the disease is crucial for timely adaptation of the immunosuppressant regimen, in order to preserve as much graft function as possible [33–35]. Although 3He-MRI may not prove to offer sufficient sensitivity and specificity to serve as a single diagnostic tool for BO detection, it may become of diagnostic use when combined with other imaging methods [36], e.g. to exclude infectious causes for deterioration of respiratory function [37]. Also, the regional information derived from 3He-MRI may provide a road map for transbronchial biopsies, in order to target the most severely affected areas at the least possible discomfort for the patient.
Conclusion
Oxygen sensitive 3He-MRI appears capable to differentiate pO2 level grades, distribution and uptake between normal lung grafts and those affected by BOS in lung transplant recipients. Intrapulmonary pO2 determined by oxygen sensitive 3He-MRI, and its spatial and temporal distribution analysis might become a valuable supplement to current methods for the assessment and follow-up of lung transplant recipients.
References
Eberle B, Markstaller K, Lill J, Deninger A, Schreiber W, Mayer E, Kauczor H-U, Weiler N (2000) Oxygen-sensitive 3He magnetic resonance imaging of the lungs in patients after unilateral lung transplantation. Am J Resp Crit Care Med 161:A718
Kauczor H-U, Hanke A, Van Beek EJ (2002) Assessment of lung ventilation by MR imaging: current status and future perspectives. Eur Radiol 12:1962–1970
Ley S, Zaporozhan J, Morbach A, Eberle B, Gast KK, Heussel C-P, Biedermann A, Mayer E, Schmiedeskamp J, Stepniak A, Schreiber WG, Kauczor H-U (2004) Functional evaluation of emphysema using diffusion-weighted 3helium-magnetic resonance imaging, high-resolution computed tomography, and lung function tests. Invest Radiol 39(7):427–434
Schreiber WG, Weiler N, Kauczor HU, Markstaller K, Eberle B, Hast J, Surkau R, Grossmann T, Deninger A, Hanisch G, Otten EW, Thelen M (2000) Ultraschnelle MRT der Lungenventilation mittels hochpolarisiertem Helium-3 [Ultrafast MRI of lung ventilation using hyperpolarized helium-3]. Fortschr Roentgenstr 172(2):129–133
Gast KK, Puderbach M, Rodriguez I, Eberle B, Markstaller K, Knitz F, Schmiedeskamp J, Weiler N, Schreiber W, Mayer E, Thelen M, Kauczor H (2003) Distribution of Ventilation in Lung transplant recipients: evaluation by Dynamic 3He-MRI with lung motion correction. Invest Radiol 38:341–348
Gast KK, Viallon M, Eberle B, Lill J, Puderbach M, Hanke A, Schmiedeskamp J, Kauczor H-U (2002) MR Imaging in lung transplant recipients using hyperpolarized 3He: comparison with CT. J Magn Reson Imag 15:268–274
Gast KK, Zaporozhan J, Ley S, Biedermann A, Knitz F, Eberle B, Schmiedeskamp J, Heussel CP, Mayer E, Schreiber WG, Thelen M, Kauczor HU (2004) 3He-MRI in follow-up of lung transplant recipients. Eur Radiol 14:78–85
Ruppel GL (1997) Spirometry. Respir Care Clin N Am 3:155–181
Kramer MR, Stoehr C, Whang JL, Berry GJ, Sibley R, Marshall SE, Patterson GM, Starnes VA, Theodore J (1993) The diagnosis of obliterative bronchiolitis after heart-lung and lung transplantation: low yield of transbronchial lung biopsy. J Heart Lung Transplant 12(4):675–681
Lee ES, Gotway MB, Reddy GP, Golden JA, Keith FM, Webb WR (2000) Early bronchiolitis obliterans following lung transplantation: accuracy of expiratory thin-section CT for diagnosis. Radiology 216:472–477
Altes T, Powers P, Knight-Scott J, Rakes G, Platts-Mills T, de Lange E, Alford B, Mugler JI, Brookeman J (2001) Hyperpolarized 3He MR Lung Ventilation Imaging in Asthmatics: Preliminary Findings. J Magn Reson Imag 13:378–384
van Beek E, Hill C, Woodhouse N, Fichele S, Fleming S, Howe B, Bott S, Wild J, Taylor C (2007) Assessment of lung disease in children with cystic fibrosis using hyperpolarized 3-Helium MRI: comparison with Shwachman score, Chrispin-Norman score and spirometry. Eur Radiol 17:1018–1024
Salerno M, de Lange E, Altes T, Truwit J, Brookeman J, Mugler J (2002) Emphysema: hyperpolarized Helium3 diffusion MR imaging of the lungs compared with spirometric indexes—initial experience. Radiology 222:252–260
van Beek EJ, Wild JM, Kauczor HU, Schreiber W, Mugler JPr, de Lange EE (2004) Functional MRI of the lung using hyperpolarized 3-helium gas. J Magn Reson Imaging 20(4):540–554
Eberle B, Weiler N, Markstaller K, Kauczor H, Deninger A, Ebert M, Grossmann T, Heil W, Lauer LO, Roberts TP, Schreiber WG, Surkau R, Dick WF, Otten EW, Thelen M (1999) Analysis of intrapulmonary O(2) concentration by MR imaging of inhaled hyperpolarized helium-3. J Appl Physiol 87(6):2043–2052
Fischer MC, Kadlecek S, Yu J, Ishii M, Emami K, Vahdat V, Lipson DA, Rizi RR (2005) Measurements of regional alveolar oxygen pressure using hyperpolarized 3He MRI. Acad Radiol 12(11):1430–1439
Cooper JD, Billingham M, Egan T, Hertz M, Higenbottam T, Lynch J, Mauer J, Paradis I, Patterson GA et al (1993) A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts: International Society for Heart and Lung Transplantation. J Heart Lung Transplant 12:713–716
Gast KK, Ley S, Zaporozhan J, Puderbach M, Eberle B, Biedermann A, Knitz F, Schmiedeskamp J, Weiler N, Schreiber W, Mayer E, Heussel C, Thelen M, Kauczor H (2003) Reformatierungen als Lösungsansatz für die Problematik der unterschiedlichen Schichtführung beim Vergleich von 3He-MRT und HR-CT der Lunge [Reformation as proposed solution for the problem of sectioning different levels with 3He-MRT and HR-CT of the chest]. Fortschr Röntgenstr 175:786–790
Wild JM, Schmiedeskamp J, Paley MN, Filbir F, Fichele S, Kasuboski L, Knitz F, Woodhouse N, Swift A, Heil W, Mill GH, Wolf M, Griffiths PD, Otten E, van Beek EJ (2002) MR imaging of the lungs with hyperpolarized helium-3 gas transported by air. Phys Med Biol 47:185–190
Lehmann F, Eberle B, Markstaller K, Gast KK, Schmiedeskamp J, Blümler P, Kauczor HU, Schreiber WG (2004) Ein Auswerteprogramm zur quantitativen Analyse von Messungen des alveolären Sauerstoffpartialdrucks (pAO2) mit der sauerstoffsensitiven 3He-MR-Tomographie [A software program for quantitative analysis of alveolar oxygen partial pressure (pAO2) with oxygen-sensitive 3He-MRI]. Fortschr Röntgenstr 176(10):1390–1398
Schreiber WG, Morbach AE, Stavngaard T, Gast KK, Herweling A, Sogaard LV, Windirsch M, Schmiedeskamp J, Heussel C, Kauczor H (2005) Assessment of lung microstructure with magnetic resonance imaging of hyperpolarized Helium-3. Respir Physiol Neurobiol 148(1–2):23–42
Deninger AJ, Eberle B, Ebert M, Grossmann T, Hanisch G, Heil W, Kauczor HU, Markstaller K, Otten E, Schreiber W, Surkau R, Weiler N (2000) (3)he-MRI-based measurements of intrapulmonary p(O2) and its time course during apnea in healthy volunteers: first results, reproducibility, and technical limitations. NMR Biomed 13(4):194–201
McAdams HP, Palmer SM, Donnelly LF, Charles HC, Tapson VF, MacFall JR (1999) Hyperpolarized 3He-enhanced MR imaging of lung transplant recipients: preliminary results. Am J Roentgenol 173:955–959
Fischer MC, Kadlecek S, Yu J, Ishii M, Emami K, Vahdat V, Lipson DA, Rizi RR (2005) Measurements of regional alveolar oxygen pressure using hyperpolarized 3He MRI. Acad Radiol 12(11):1430–1439
Rizi RR, Baumgardner J, Ishii M, Spector ZZ, Edvinsson J, Jalali A, Yu J, Itkin M, Lipson DA, Gefter W (2004) Determination of regional VA/Q by hyperpolarized 3He MRI. Magn Reson Med 52(1):65–72
Eberle B, Markstaller K, Stepniak A, Viallon M, Kauczor H-U (2002) 3Helium-MRI-Based Assessment of regional gas exchange impairment during experimental pulmonary artery occlusion. Anesthesiology 96:A1309
Kadlecek S, Rizi RR (2005) New diagnostic tests for pulmonary emboli. Acad Radiol 12(2):133–135
Hopkins S, Levin D, Emami K, Kadlecek S, Yu J, Ishii M, Rizi RR (2007) Advances in magnetic resonance imaging of lung physiology. J Appl Physiol 102(3):1244–1254
Markstaller K, Gast K, Herweling A, Schmiedeskamp J, Mayer E, Kauczor H, Eberle B (2003) Detektion regionaler Gasaustauschstörungen bei chronischer thrombembolischer pulmonaler Hypertonie (CTEPH) mittels 3Helium-MRT. Abstractband Deutscher Anästhesiecongress: 194
Henkelman RM (1985) Measurement of signal intensities in the presence of noise in MR images. Med Phys 12(2):232–233
Gast KK, Schreiber WG, Herweling A, Lehmann F, Erdös G, Schmiedeskamp J, Kauczor H-U, Eberle B (2005) Two-dimensional and three-dimensional oxygen mapping by 3He-MRI - validation in a lung phantom. Eur Radiol 15(9):1915–1922
Wild J, Fichele S, Woodhouse N, Paley M, Kasuboski L, van Beek E (2005) 3D volume-localized pO2 measurement in the human lung with 3He MRI. Magn Reson Med 53:1055–1064
Estenne M, Hertz MI (2002) Bronchiolitis obliterans after human lung transplantation. Review. Am J Respir Crit Care Med 166:440–444
Frost AE, Keller CA, Noon GP, Short HD, Cagle PT (1995) Outcome of the native lung after single lung transplant. Multiorgan Transplant Group. Chest 107(4):981–984
Izbicki G, Shitrit D, Aravot D, Sulkes J, Saute M, Sahar G, Kramer M (2002) Improved survival after lung transplantation in patients treated with tacrolimus/mycophenolate mofetil as compared with cyclosporine/azathioprine. Transplant Proc 34:3258–3259
Zaporozhan J, Ley S, Gast KK, Schmiedeskamp J, Biedermann A, Eberle B, Kauczor HU (2004) Functional analysis in single-lung transplant recipients: a comparative study of high-resolution CT, 3He-MRI, and pulmonary function tests. Chest 125(1):173–181
Collins J, Muller NL, Kazerooni EA, Paciocco G (2000) CT findings of pneumonia after lung transplantation. AJR Am J Roentgenol 175:811–818
Acknowledgements
The study was supported by the German Research Society (DFG, FOR 474) as well as in part by the research grant “Physiological 3He Imaging of the Lung” Nycomed Amersham plc, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 2.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc/2.0/.
About this article
Cite this article
Gast, K.K., Biedermann, A., Herweling, A. et al. Oxygen-sensitive 3He-MRI in bronchiolitis obliterans after lung transplantation. Eur Radiol 18, 530–537 (2008). https://doi.org/10.1007/s00330-007-0778-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-007-0778-8