Abstract
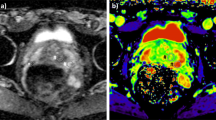
Evaluation of the accuracy of descriptive and physiological parameters calculated from signal intensity–time curves using T1-weighted dynamic contrast enhanced MRI (DCE MRI) to differentiate prostate cancers from the peripheral gland. Twenty-seven patients with prostate cancers were examined with DCE MRI prior radical prostatectomy. Regions of interest were defined in tumors and non-affected areas in the peripheral zone. Dynamic data were parameterized in amplitude and exchange rate constant (k ep) using a two-compartment model. Additionally, relative slope during 26, 39, 52 and 65 s, areas under the curve (AUC) and time to start of signal intensity increase (t lag) were determined. Vessel density (VD) of excised prostates was quantified in tumor areas using a CD34 stain. The parameter slope52 showed 20% higher values (P<0.001) in tumors than in the peripheral gland and compared with the other parameters the largest area under the ROC curve (0.81). The minimum total error rate was attained at a cut-point of 0.021, yielding a sample value of sensitivity and specificity of 70% and 88%, respectively, and a bias-corrected sum of sensitivity and specificity of 1.54. In addition, amplitude (P<0.001), k ep (P=0.03) and AUC (P<0.001) were significantly higher in tumors. t lag did not discriminate carcinomas from glandular tissue. VD was higher in tumors than in the non-affected peripheral prostate (P=0.05). However, none of the dynamic parameters in carcinomas showed a significant correlation with VD or Gleason score. Although pharmacokinetic modeling in DCE MRI showed potential to discriminate prostate cancers from peripheral prostate tissue, descriptive parameters of the early signal enhancement after contrast media injection reached higher sensitivity and specificity.






Similar content being viewed by others
References
Sarma AV, Schottenfeld D (2002) Prostate cancer incidence, mortality, and survival trends in the United States: 1981–2001. Semin Urol Oncol 20:3–9
Perrotti M, Han KR, Epstein RE, Kennedy EC, Rabbani F, Badani K, Pantuck AJ, Weiss RE, Cummings KB (1999) Prospective evaluation of endorectal magnetic resonance imaging to detect tumor foci in men with prior negative prostastic biopsy: a pilot study. J Urol 162:1314–1317
Jager GJ, Severens JL, Thornbury JR, de La Rosette JJ, Ruijs SH, Barentsz JO (2000) Prostate cancer staging: should MR imaging be used? A decision analytic approach. Radiology 215:445–451
Kurhanewicz J, Vigneron DB, Males RG, Swanson MG, Yu KK, Hricak H (2000) The prostate: MR imaging and spectroscopy. Present and future. Radiol Clin N Am 38:115–138
Schnall MD, Imai Y, Tomaszewski J, Pollack HM, Lenkinski RE, Kressel HY (1991) Prostate cancer: local staging with endorectal surface coil MRI. Radiology 178:797–802
Jager GJ, Barentsz JO, de la Rosette JJMCH, Rosenbusch G (1994) Preliminary results of endorectal surface coil magnetic resonance imaging for local staging of prostate cancer. Radiology 34:129–133
Chelsky MJ, Schnall MD, Seidmon EJ, Pollack HM (1993) Use of endorectal surface coil magnetic resonance imaging for local staging of prostate cancer. J Urol 150:391–395
Engelbrecht MR, Jager GJ, Laheij RJ, Verbeek AL, van Lier HJ, Barentsz JO (2002) Local staging of prostate cancer using magnetic resonance imaging: a meta-analysis. Eur Radiol 12:2294–2302
Brown G, Macvicar DA, Ayton V, Husband JE (1995) The role of intravenous contrast enhancement in magnetic resonance imaging of prostatic carcinoma. Clin Radiol 50:601–606
Padhani AR, Gapinski CJ, Macvicar DA, Parker GJ, Suckling J, Revell PB, Leach MO, Dearnaley DP, Husband JE (2000) Dynamic contrast enhanced MRI of prostate cancer: correlation with morphology and tumour stage, histological grade and PSA. Clin Radiol 55:99–109
Barentsz JO, Engelbrecht M, Jager GJ, Witjes JA, de LaRosette J, van Der Sanden BP, Huisman HJ, Heerschap A (1999) Fast dynamic gadolinium-enhanced MR imaging of urinary bladder and prostate cancer. J Magn Reson Imaging 10:295–304
Preziosi P, Orlacchio A, Giambattista G, Renzi P, Bortolotti L, Fabiano A, Cruciani E, Pasqualetti P (2003) Enhancement pattern of prostate cancer in dynamic MRI. Eur Radiol 13:925–930
Turnbull LW, Buckley DL, Turnbull LS, Liney GP, Knowles AJ (1999) Differentiation of prostatic carcinoma and benign prostatic hyperplasia: correlation between dynamic Gd-DTPA-enhanced MR imaging and histopathology. J Magn Reson Imaging 9:311–316
Huisman HJ, Engelbrecht MR, Barentsz JO (2001) Accurate estimation of pharmacokinetic contrast-enhanced dynamic MRI parameters of the prostate. J Magn Reson Imaging 13:607–614
Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ (1991) Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr 15:621–628
Port RE, Knopp MV, Hoffmann U, Milker-Zabel S, Brix G (1999) Multicompartment analysis of gadolinium chelate kinetics: blood-tissue exchange in mammary tumours as monitored by dynamic MR imaging. J Magn Reson Imaging 10:233–241
Hoffmann U, Brix G, Knopp MV, Hess T, Lorenz WJ (1995) Pharmacokinetic mapping of the breast: a new method for dynamic MR mammography. Magn Reson Med 33:506–514
Knopp MV, Weiss E, Sinn HP, Mattern J, Junkermann H, Radeleff J, Magener A, Brix G, Delorme S, Zuna I, van Kaick G (1999) Pathophysiologic basis of contrast enhancement in breast tumours. J Magn Reson Imaging 10:260–266
Hawighorst H, Knapstein PG, Weikel W, Knopp MV, Zuna I, Knof A, Brix G, Schaeffer U, Wilkens C, Schoenberg SO, Essig M, Vaupel P, van Kaick G (1997) Angiogenesis of uterine cervical carcinoma: characterization by pharmacokinetic magnetic resonance parameters and histological microvessel density with correlation to lymphatic involvement. Cancer Res 57:4777–4786
Moehler TM, Hawighorst H, Neben K, Egerer G, Hillengass J, Max R, Benner A, Ho AD, van Kaick G, Goldschmidt H (2001) Bone marrow microcirculation analysis in multiple myeloma by contrast-enhanced dynamic magnetic resonance imaging. Int J Cancer 93:862–868
Schlemmer HP, Merkle J, Grobholz R, Jaeger T, Michel MS, Werner A, Rabe A, van Kaick G (2004) Contrast enhanced dynamic MR imaging for the assessment of microvessel density in prostate cancer. Eur Radiol 14:309–317
Kiessling F, Lichy M, Grobholz R, Farhan N, Heilmann M, Michel MS, Trojan L, Werner A, Rabe J, Delorme S, Kauczor HU, Schlemmer HP (2003) Detection of prostate carcinomas with T1-weighted dynamic contrast-enhanced MRI. Value of two-compartment model. Radiologe 43:474–480
True LD (1994) Surgical pathology examination of the prostate gland. Practice survey by American society of clinical pathologists. Am J Clin Pathol 102:572–579
Gleason DF, Mellinger GT (1974) Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 111:58–64
Grobholz R, Bohrer MH, Siegsmund M, Jünemann K-P, Bleyl U, Woenckhaus M (2000) Correlation between neovascularisation and neuroendocrine differentiation in prostatic carcinoma. Pathol Res Pract 196:277–284
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Abel U, Berger J, Weber E (1984) MARKERTEST—Verfahren und Programme zur statistischen Validierung biologischer Marker. EDV Biol Med 15:117–125
Abel U, Berger J (1986) Comparison of resubstitution, data splitting, the bootstrap, and the jackknife as methods for estimating the validity indices of new marker tests. Biometrical J 28:899–908
Tofts PS, Brix G, Buckley DL, Evelhoch JL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Kiessling F, Krix M, Heilmann M, Vosseler S, Lichy M, Fink C, Farhan N, Kleinschmidt K, Schad L, Fusenig NE, Delorme S (2003) Comparing dynamic parameters of tumour vascularization in nude mice revealed by MRI and contrast enhanced intermittent power Doppler sonography. Invest Radiol 38:516–524
Rouviere O, Raudrant A, Ecochard R, Colin-Pangaud C, Pasquiou C, Bouvier R, Marechal JM, Lyonnet D (2003) Characterization of time-enhancement curves of benign and malignant prostate tissue at dynamic MR imaging. Eur Radiol 13:931–942
Jager GJ, Ruijter ETG, vd Ka CA et al (1997) Dynamic turbo-FLASH substraction technique for contrast-enhanced MR imaging of the prostate: correlation with histopathology. Radiology 203:645–652
Engelbrecht MR, Huisman HJ, Laheij RJ, Jager GJ, van Leenders GJ, Hulsbergen-Van De Kaa CA, de la Rosette JJ, Blickman JG, Barentsz JO (2003) Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology 229:248–254
Acknowledgements
The work was supported by a grant of the Tumorzentrum Heidelberg/Mannheim. We thank Alexandra Kappeler for excellent technical assistance and are indebted to J. König for the SAS macro AUROC for comparison of ROC curves according to the method by DeLong et al.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiessling, F., Lichy, M., Grobholz, R. et al. Simple models improve the discrimination of prostate cancers from the peripheral gland by T1-weighted dynamic MRI. Eur Radiol 14, 1793–1801 (2004). https://doi.org/10.1007/s00330-004-2386-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2386-1