Abstract
Carrion decomposition has a significant impact on soil chemical profiles. However, soil nutrient research associated with animal carcasses in Antarctica has been relatively scarce, and the effect of penguin carrion decomposition on soil chemical composition is largely unknown. We aimed to determine Antarctica’s soil chemistry profiles associated with penguin carrion. Soil samples were collected from a penguin rookery near King Sejong Station, Barton Peninsula, King George Island, Antarctica. Dry combustion methods were used to identify soil nitrogen and sulfur, while ammonia, nitrate, and phosphate were determined colorimetrically using a spectrophotometer. In addition, total carbon, pH, electrical conductivity, soil moisture, and soil porosity were also determined. Overall, soil chemical properties were not significantly different between the stages of decomposition and the sampling locations. These findings suggest that nutrients from penguin carrion disperse and leach in limited quantities into the soil, probably due to the active scavenging activities by vertebrate scavengers and the slower decomposition rate resulting from cold temperatures in the Antarctic region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctica is home to over 20 million breeding pairs of penguins (British antarctic survey). Despite this, there is currently no published data on the amount of penguin carrion produced in this region. Carrion is described as a heterotrophic biomass, decaying organic matter, or necromass derived from animals or other consumers (Barton et al. 2019). During decomposition, carrion-derived nutrients are channeled into underground pathways by bacteria and fungi that break down large and complex organic molecules from different animal tissues (Bornemissza 1956; Carter and Tibbett 2008).
The nutrient releases from carrion contribute to hot spots that directly and indirectly affect soil conditions in situ (Bornemissza 1956). Nitrogen (N), phosphorus (P), and sodium (Na) concentrations increase at the carrion decomposition site (Benninger et al. 2008; Parmenter and MacMahon 2009). Meanwhile, soil moisture, ammonium, and pH fluctuate underneath the carrion during the early stage of decomposition (Keenan et al. 2018; Quaggiotto et al. 2019). During the advanced and dry stages of decomposition, an increase in levels of phosphate (PO43−) (Quaggiotto et al. 2019) and nitrate (NO3−) (Keenan et al. 2018) were observed.
The rate at which decomposition products enter the soil plays an important role in the changes observed in microorganisms and vegetation community structures (Breton 2013). This rate depends on the existing vegetation community, soil types, and climatic conditions. Bacterial growth and insect activities are slowed or inhibited at lower temperatures, thus delaying the decomposition process (Goff 2009).
Antarctica is known to have extreme climate conditions that significantly affect soil properties and distribution (Bockheim and McLeod 2015). The soil predominately consists of sandy soil and single-grain textures, reflecting limited soil weathering (Balks et al. 2013). Soils in ice-free continental Antarctica areas generally lack organic matter (Kim et al. 2012). On the other hand, the ornithogenic soil of the Antarctic coastal areas is enriched with nutrients for terrestrial ecosystems surrounding penguin rookeries (Ugolini 1970).
Penguins in Antarctica had the greatest impact on soil properties, leading to the development of ornithogenic soils (Cannone et al. 2008; Schaefer et al. 2008; Simas et al. 2008). Penguin guano plays a crucial role in the formation and enrichment of soil in polar habitats. It contains nutrients, such as carbon, nitrogen, and phosphorus, which contribute to the growth and maintenance of ecosystems in nutrient-poor areas, like Antarctica (Shatova et al. 2016; Rodrigues et al. 2021). Similarly, the decomposition of carrion releases nutrients like carbon, nitrogen, and phosphorus into the soil, which helps to boost microbial activity and nutrient cycling (Heine and Speir 1989; Zhu et al. 2009). The plant growth, nutrient fluxes, and invertebrate food webs in soil have all been greatly impacted by ornithogenic nutrient enrichment from guano, feathers, eggs, and carcasses (Sanchez-Pinero and Polis 2000; Callaham et al. 2012; Zhu et al. 2012).
The decomposition products serve as resource impulses incorporated into the surrounding habitat, leading to changes in soil arthropod species composition and their functions (Mondor et al. 2012). Although literature has been published on soil chemistry and the decomposition process in the temperate and tropical regions, there are no studies on the relationship between penguin carrion and soil nutrient dynamics in Antarctica. The only decomposition studies in Antarctica which involved dead remains were conducted in 2015 by (Nędzarek et al. 2015), who reported that nitrogen and phosphorus released from the fish remains may form important chemical cues for scavengers. However, there are still no penguin carrion-related studies conducted in Antarctica until now. Therefore, the present study aims to fill the knowledge gap by examining the soil chemistry profiles associated with penguin carrion in Antarctica.
Methods
Sample collection
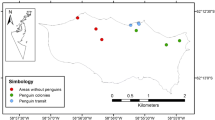
Soils were collected from 30th November 2019 until 5th January 2020 at Narebski Point, antarctic specially protected area (ASPA) 171), Barton Peninsula, King George Island, Antarctica (62° 13ʹ S, 58° 47ʹ W, 10 m a.s.l.) (Fig. 1). Before fieldwork, written permission to enter ASPA 171 was obtained from the Korea Polar Research Institute (KOPRI). Daily weather data for the study period was taken from the weather station in ASPA 171. The sample collection was based on haphazard sampling, which involves the collection of samples dependent on their availability during sampling time. Therefore, approximately 30 g of the soil of dry (n = 17) and fresh (n = 2) decomposing penguin carrion were collected using a trowel from beneath and around (5 meters away) the penguin carcasses (Fig. 2), which consisted of two different species, namely Pygoscelis papua (Forster, 1781) and Pygoscelis antarcticus (Forster, 1781). The fresh stage (Fig. 3A) of penguin decomposition is characterized by freshly killed penguins (mostly by predators such as seals or skuas), where a large part of soft tissues and internal organs remain intact. In contrast, the dry decay stage (Fig. 3B) of the decomposition process was defined as the skeletonization stage of the penguin carrion, where only bones and feathers were left visible. Thirty grams of control soil (n = 3) was also collected from an area approximately 500 m away (Fig. 2) from ASPA 171 devoid of penguin populations. All soils were brought back to King Sejong station and kept in the – 20 ℃ freezer. The soils were then transported back to Malaysia in an ice cooler box with permission to import to the country and subsequently to the Institute of Medical Molecular Biotechnology (IMMB), Faculty of Medicine, Universiti Teknologi MARA, and immediately stored in a – 20 ℃ freezer there upon arrival.
Soil analyses
Soil particle analysis was identified using the simplified method by (Kettler; Doran; Gilbert 2001). Later, the textural class of soil was determined based on the United States Department of Agricultural (USDA) soil textural classification (Soil Survey Division Staff 1951). Soil moisture and soil porosity were determined gravimetrically by drying samples (~ 10 g) at 45 °C for 72 h (soil moisture) and 60 °C for 72 h (soil porosity) (Johnson 1962; Flint and Flint 2002). Soil porosity is calculated from the bulk density (ρb) and particle density (ρp) and is defined as one minus the solid fraction of a sample (porosity = 1 – (ρb/ρp)). Soil pH and electrical conductivity (EC) were measured using a digitally calibrated pH probe (Eutech, United States of America (USA)) and a portable EC probe (Eutech, USA) in a 1:5 soil: water suspension. Total soil nitrogen (N) and sulfur (S) were identified using the dry combustion (Matejovic 1997) method, which finely grounds samples and then measures using a Unicube organic elemental analyzer (Elementar, Germany). Total organic carbon (TOC) and total carbon (TC) were measured using high-temperature platinum-catalyzed combustion with Shimadzu instruments (Japan). The concentration of ammonia (NH3) was measured using Nessler and US EPA method 350.2 (EPA-NPDES 1974). For this purpose, a 1:5 soil: water suspension was mixed with Mineral Stabilizer. Then, a Polyvinyl Alcohol Dispersing Agent was added to accelerate the color formation in the reaction of the Nessler Reagent with ammonia. The concentration was measured at 425-nm wavelength in a spectrophotometer (Molecular Devices, USA). On the other hand, nitrate (NO3¯) concentration was measured using the cadmium reduction method (American Public Health Association 1992). In this method, NitraVer 5 Nitrate reagent Powder Pillow was mixed with 1:5 soil: water suspension. The mixture was then measured at 500-nm wavelength in a spectrophotometer, as mentioned above. Finally, phosphate (PO43−) was measured colorimetrically based on the reaction with ammonium molybdate. Phosphorus was extracted from the soil using Bray 2 (Bray, R.H. and Kurtz 2009) solution as an extractant, and the absorbance of the compound was measured at 882 nm in a spectrophotometer as mentioned above.
Statistical analysis
Non-parametric analyses were conducted following Shapiro–Wilk normality testing. All data were analyzed using the GraphPad 9.0 statistical program. Statistical results with p < 0.05 are considered significantly different. Kruskal–Wallis tests were conducted to test the differences between soil chemical profiles from the sample groups. The samples were grouped into seven groups based on penguin species (i.e., Chinstrap Penguin or Gentoo Penguin), soil sample location (around or beneath penguin carrion), and stage of decomposition (fresh or dry). The seven groups were Gentoo dry around (GDA), Gentoo dry beneath (GDB), Gentoo fresh around (GFA), Gentoo fresh beneath (GFB), Chinstrap dry around (CDA), Chinstrap dry beneath (CDB), and control soil.
Results
Weather data
The average daily temperature (℃) during the sampling was (0.84 ± 1.60 ℃), with a minimum of − 2.19 ℃ and a maximum of 5.01 ℃ (Supplementary Table 1).
Soil particle analysis, pH, and EC
According to the USDA soil triangle, the soil samples from the sampling site vary in particle size. They have been classified as sandy loam, sandy clay loam, sandy clay, sand, loamy sand, and clay.
Table 1 shows the Kruskal–Wallis H statistic test results, including mean and standard deviations for the control soil, CDA, CDB, GDA, GDB, GFA, and GFB. Meanwhile, Fig. 4 shows an overview of soil (A) pH, (B) electrical conductivity (mS/cm), (C) moisture (%), (D) porosity (%), (E) ammonium (μg/mL), (F) nitrate (μg/mL), (G) phosphate (μg/mL), (H) nitrogen (%), (I) sulfur (%), and (J) organic carbon (%). No significant differences in pH levels were observed, as shown by a Kruskal–Wallis H statistic of 7.23 and a p-value of 0.300. The examination of electrical conductivity (EC) indicates that there are no statistically significant differences between the groups. This is supported by a Kruskal–Wallis H statistic of 9.83 and a p-value of 0.132. The groups that exhibited an alkaline soil, together with the control soils, demonstrated the highest pH value of 5.800 ± 0.100. Additionally, the GDB group exhibited the greatest electrical conductivity (EC) value of 364.175 ± 264.324.
Soil moisture and porosity
The moisture and porosity levels across the groups showed no significant variations, as indicated by the Kruskal–Wallis H statistics of 3.63 and 3.17, respectively. Furthermore, the p-values corresponding to these statistics were greater than the significance level of 0.05, confirming the absence of any meaningful differences. The data presented in Table 1 demonstrate that the porosity percentages of all samples were almost identical, ranging from 0.233 to 0.238%. In some locations, CDA soil had the highest average moisture content of 1.905 ± 1.200, while GFA soil had the lowest of 0.775 ± 0.431.
Ammonia (NH3), nitrate (NO3 ¯), and phosphate (PO4 3−)
The study found no statistical significance on ammonia concentration across the groups (Kruskal–Wallis H statistic of 8.45 and a p-value of 0.133). Similarly, no statistically significant variations were seen in nitrate levels (Kruskal–Wallis H statistic of 3.67 and a p-value of 0.598). Also, there were no statistically significant variations seen in phosphate levels between the groups, as indicated by a Kruskal–Wallis H statistic of 11.28 and a p-value of 0.080. Due to the small sample size, the study was unable to perform both nitrate and ammonia analyses, resulting in no observable results in GFA.
Organic carbon (OC), nitrogen (N), and sulfur (S)
Total organic carbon (TOC) levels do not differ significantly between the groups (Kruskal–Wallis H statistic of 4.99 and a p-value of 0.545). The nitrogen analysis also reveals no statistically significant differences among the groups (Kruskal–Wallis H statistic 1.48 and a p-value of 0.961). Similarly, hydrogen levels do not exhibit significant differences between groups, as indicated by a Kruskal–Wallis H statistic of 1.72 and a p-value of 0.943. Meanwhile, sulfur does not show significant differences among most groups, with a Kruskal–Wallis H statistic of 5.82 and a p-value of 0.444.
Discussion
In general, the physicochemical responses of soils associated with the decomposition of penguin carrion in ASPA 171 can vary greatly. This study showed the uneven distribution of soil chemical profiles between soil sampling locations (i.e., beneath, around, control) and the decomposition stages (i.e., fresh, dry) of penguin carrion. The control soil, which was collected 500 m from the penguin carrion, had the lowest total C, N, and PO43− percentage, the lowest EC, the highest moisture, and the lowest porosity. The findings are potentially explained by the chemical disturbance to the soil with the presence of the decomposing penguin carrion, which can impact the growth of soil vegetation by changing the soil’s moisture retention ability and air content (Ritz et al. 2008). In addition, the microbial activity associated with the decomposition of the penguin carrion could influence the moisture content beneath and around the soils associated with the penguin carrion. These results are in line with a previous study (Carter et al. 2010), which stated that soil moisture is affected by the soil microbial activity that acts as the primary decomposer in the soil. Furthermore, moisture availability can affect microbial motility, the diffusion of nutrients and waste, and the activity of extracellular enzymes (Carter et al. 2010).
Carrion is known to have dramatic localized effects on soil properties (Melis et al. 2007), including impacts on pH and nutrient content due to the direct leakage of fluids and the transfer of carrion tissues to the soil by invertebrates, such as arthropods (Barton et al. 2013). This study observed insignificant changes in the soil pH collected from beneath and around the penguin carrion. Decreased soil pH during carrion decomposition was reported in previous studies (Towne 2000; Aitkenhead-Peterson et al. 2012), whereas others have noticed an increase (Benninger et al. 2008; Meyer et al. 2013; Keenan et al. 2018; Szelecz et al. 2018) and still, several authors found no significant change (Cobaugh et al. 2015; Fancher et al. 2017). Even though the changes in pH during the decomposition process vary, the soil pH is important in controlling the soil chemistry reactions and a key driver of bacterial community structure (Lauber et al. 2009), influencing the composition of the soil invertebrate decomposer community.
Nutrients diffuse from the carrion into the soil, causing pH, EC, and nutrient concentrations to change (Mondor et al. 2012). An increase in EC has been reported in cadaver decomposition island (CDI) studies examining human remains (Barton et al. 2020). In this study, soil with carrion is observed to have a higher EC than control soil. These results agreed with those observed in earlier studies where EC increased during active and advanced decomposition and returned to the initial conditions during the late skeletal phase (Keenan et al. 2018).
The soil of fresh carrion had a higher percentage of S and N than that of dry carrion in this study. Carrion decomposition in the soil is an important source of nutrients, especially nitrogen (N), which may affect the turnover of the soil organic N pool, such as free amino acids (FAAs) (Potocka and Krzemińska 2018). However, N did not remain in the environment in these forms for long indicating a higher amount of N found in the soil of fresh carrion (Cammack et al. 2015). Denitrifying bacteria will later reduce nitrites and nitrates and release N back into the atmosphere as N2, continuing the N cycle (Janet I. Sprent 1987; F. J. Stevenson and Cole 1999). Meanwhile, a high concentration of S from fresh carrion soil is contributed by the sulfur found in the carrion proteins and other soft tissues (Parmenter and Macmahon 2009).
When soil nutrients deplete during carrion decomposition, microbes metabolize substrates using organic carbon fermentation pathways instead of aerobic oxygen pathways (Cammack et al. 2015) leading to a higher C percentage in fresh carrion soil than in dry carrion and control soils in this study. Another possible explanation is that once an animal dies, microbes, and other scavengers break down its complex carbon-containing molecules into simpler building blocks that both microbes and larger scavengers utilize, which are then released back into the atmosphere as waste CO2, thus perpetuating the C cycle (Stevenson and Cole 1999; Carter et al. 2007).
In this study, PO43− concentration was higher than NH3 and NO3¯. A high PO43− concentration was related to the P cycle, which occurred on a more protracted temporal scale (F. J. Stevenson and Cole 1999). P is a vital component of DNA and RNA (Watson and Crick 1953), the phospholipid bilayer of cells (Nagle and Tristram 2000), and the production of adenosine triphosphate (Mildvan 2006). P moves through the food web through consumption and the action of decomposers, with sequestered P then being metabolized out of larger organic molecules and eventually released back into the environment as PO43− (Parmenter and Macmahon 2009). In addition, the high concentration of phosphorus found in this study could be attributed to the formation of soil in the Antarctica region. Phosphorus is a key component of soil formation and its presence, mainly from the ornithogenic soil, has a significant impact on the distribution and cycling of soil phosphorus. This, in turn, affects the composition and formation of Antarctic soils (Bate et al. 2008; Nizamutdinov et al. 2021).
An increase in soil nutrient concentrations associated with the decomposition of penguin carrion was observed in this study, following previous carrion decomposition studies from temperate (Benninger et al. 2008; Aitkenhead-Peterson et al. 2012; Fancher et al. 2017; Szelecz et al. 2018; Barton et al. 2020; Woelber-Kastner et al. 2021) and tropical regions (Yong et al. 2019). However, this study indicates no significant difference in the soil chemical profiles between control soil and soil beneath and around penguin carrion. A possible explanation for these results is Antarctica’s weather and low biodiversity. Antarctica is inhospitable to most terrestrial organisms due to its cold temperatures, strong winds, short vegetation season, and limited ice-free areas (Potocka and Krzemińska 2018). As a result, biodiversity in ice-free regions is extremely low, with only two native higher plants and fauna dominated by macro-invertebrates and two species of macro-arthropods, Belgica antarctica (Jacobs, 1900) and Parochlus steinenii (Gercke 1889) (Frenot et al. 2005; Hughes and Pertierra 2016). It is worth noting that insects that feed on dead organisms play a significant role in breaking down carrion, which results in the release of concentrated nutrients into the soil (Payne 1965; Pechal et al. 2014). This finding is consistent with previous research, which has shown that arthropods, vertebrate detritivores, scavengers, and their predators are primarily responsible for dispersing nutrients away from the center of carrion (Payne et al. 1968; Putman 1978; Braack 1987; DeVault et al. 2003; Parmenter and Macmahon 2009). In addition, a previous study reported that the temperature directly impacted scavenging insect assemblages and carrion decomposition (Farwig et al. 2014).
This finding further supports a previous study that concluded that the decomposition process is dramatically reduced when the rate of bacteria proliferation slows down (Woolen 2019). Additionally, through our observation during the sampling, skuas (Stercorarius sp.) are attracted to the fresh penguin carrion by scavenging on the internal organs and flesh. Skuas’ feeding behavior rapidly removes nutrient-rich tissue from the carrion, altering their nutrient composition and impacting decomposition. This scavenging activity may limit the deposition of the carrion nutrients into the soil and has a significant impact on the nutrient cycle in Antarctica. The skuas compete and limit the food resources of other scavengers, including arthropods, nematodes, fungi, and bacteria (Zaini et al. 2023). This competition impacts the rate and extent of nutrient extraction and dispersion. A carrion’s soft tissues and organs contribute up to 50% of nutrient inputs from animal decomposition (Barton et al. 2019). It is also believed that the diversity and scavenging rate of vertebrate scavenging species have important implications on how nutrients move within terrestrial systems (Schlichting et al. 2018).
Besides, the microbial ecology in Antarctica is diverse, which affects the distribution of nutrients in the area. The presence of the microbial community depends on the response of the environment (Santamans et al. 2017). Therefore, when carrion is present, it changes the prevalence of different bacterial types, leading to a change in the composition of the soil microbiota. This, in turn, perpetuates the uneven dispersion of nutrients in the area.
The variation in the levels of nutrients found in carrion soil observed in this study can be attributed to the challenging environmental conditions in Antarctica. The soil in this region is characterized by a lack of nutrients, high salt concentration, low organic carbon, low soil moisture, and low average annual temperature, which creates a difficult ecological environment for all living organisms, affecting the distribution of nutrient levels (Diaz et al. 2021; Varliero et al. 2024). However, in comparison to the tropical and temperate climates, both regions offer a more conducive environment for supporting the soil ecosystem. This is mainly due to the presence of higher water availability, temperatures, and abundant soil nutrients, which collectively contribute to a greater diversity of bacterial species (Varliero et al. 2024), which affects the carrion decomposition process. Therefore, the difference in nutrient distribution observed in this study can be attributed to environmental factors, particularly in comparison to temperate and tropical climates, which offer more favorable circumstances for sustaining the soil ecology.
The process of carrion decomposition in Antarctica is influenced by soil microorganisms and soil chemistry, which are closely related to the mineralogy and microchemistry of the soil. The significance of microbial communities in modifying the soil environment and exerting influence on soil properties is underscored by their impact on soil formation in Antarctica (Krauze et al. 2021) during the process of carrion decomposition. Technological advancements such as the utilization of scanning electron microscopy (SEM) and X-ray fluorescence (XRF) techniques have the potential to enhance our understanding of soil. Scanning electron microscopy (SEM) is able to visualize soil samples with great resolution, providing insights into the physical composition and shape of soil particles, microorganisms, and organic substances (Ali et al. 2023), which provide a valuable insight into various aspects of the soil, including mineralogy, microchemistry, and microbial interactions.
Conclusion
The overview of this investigation is depicted in Fig. 5. This study aims to determine the soil chemistry profiles associated with penguin carrion in ASPA 171, King George Island, Antarctic Peninsula. This investigation showed no significant differences in soil chemical profiles between penguin species (i.e., Chinstrap Penguin or Gentoo Penguin), soil sample location (around or beneath penguin carrion), stage of decomposition (fresh or dry), or control soil. These results suggested that the low temperature slowed the rate of carrion decomposition. Furthermore, intense vertebrate scavenger activities on the fresh penguin carrion impeded the transfer of nutrients from the penguin carrion to the soil. Further investigation and experimentation with a higher number of samples, different carrion species, and documentation throughout the season are highly recommended. Additionally, a relationship between necrobiome communities and soil chemistry profiles should be examined better to understand Antarctica’s nutrient cycling and carrion ecology.
References
Aitkenhead-Peterson JA, Owings CG, Alexander MB et al (2012) Mapping the lateral extent of human cadaver decomposition with soil chemistry. Forensic Sci Int 216:127–134
Ali A, Zhang N, Santos RM (2023) Mineral characterization using scanning electron microscopy (SEM): a review of the fundamentals, advancements, and research directions. Appl Sci 13:12600
American Public Health Association (1992) APHA Method 4500-H pH Value: Standard Methods for the Examination of Water and Wastewater. Standard Methods for the Examination of Water and Wastewater 552: 4500
Balks MR, López-Martínez J, Goryachkin SV et al (2013) Windows on Antarctic soil-landscape relationships: comparison across selected regions of Antarctica. Geol Soc Spec Publ 381:397–410
Barton PS, Cunningham SA, Lindenmayer DB, Manning AD (2013) The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 171:761–772
Barton PS, Evans MJ, Foster CN et al (2019) Towards quantifying carrion biomass in ecosystems. Trends Ecol Evol 34:950–961
Barton PS, Reboldi A, Dawson BM et al (2020) Soil chemical markers distinguishing human and pig decomposition islands: a preliminary study. Forensic Sci Med Pathol 16:605–612
Bate DB, Barrett JE, Poage MA, Virginia RA (2008) Soil phosphorus cycling in an Antarctic polar desert. Geoderma 144:21–31
Benninger LA, Carter DO, Forbes SL (2008) The biochemical alteration of soil beneath a decomposing carcass. Forensic Sci Int 180:70–75
Bockheim JG, McLeod M (2015) The soils of Antarctica. Springer, Cham
Bornemissza GF (1956) An analysis of arthropod succession in carrion and the effect of its decomposiion on the soil fauna. Aust J Zool 5:1–12
Braack LEO (1987) Community dynamics of carrion-attendant arthropods in tropical african woodland. Oecologia 72:402–409
Bray RH, Kurtz LT (2009) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Breton H (2013) Determining the impact of carrion decomposition on soil microbial activity levels and community composition Doctor of Philosophy in Applied Bioscience
British Antarctic Survey Antarctic Penguins, 2017
Callaham MA, Butt KR, Lowe CN (2012) Stable isotope evidence for marine-derived avian inputs of nitrogen into soil, vegetation, and earthworms on the isle of Rum, Scotland, UK. Eur J Soil Biol 52:78–83
Cammack JA, Pimsler ML, Crippen TL, Tomberlin JK (2015) Chemical ecology of vertebrate carrion. Carrion ecology evolution and their applications, 1st edn. CRC Press, pp 187–211
Cannone N, Wagner D, Hubberten HW, Guglielmin M (2008) Biotic and abiotic factors influencing soil properties across a latitudinal gradient in Victoria Land, Antarctica. Geoderma 144:50–65
Carter D, Tibbett M (2008) Cadaver decomposition and soil. Soil analysis in forensic taphonomy, 1st edn. CRC Press, pp 29–51
Carter DO, Yellowlees D, Tibbett M (2007) Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 94(1):12–24
Carter DO, Yellowlees D, Tibbett M (2010) Moisture can be the dominant environmental parameter governing cadaver decomposition in soil. Forensic Sci Int 200:60–66
Cobaugh KL, Schaeffer SM, DeBruyn JM (2015) Functional and structural succession of soil microbial communities below decomposing human cadavers. PLoS ONE 10:1–20
DeVault TL, Rhodes OE Jr, Shivik JA (2003) Scavenging by vertebrates : and evolutionary on an important perspectives in terrestrial transfer energy pathway ecosystems. Oikos 102:225–234
Diaz MA, Gardner CB, Welch SA et al (2021) Geochemical zones and environmental gradients for soils from the central transantarctic mountains, Antarctica. Biogeosciences 18:1629–1644
EPA-NPDES (1974) Method 350.2: Nitrogen, ammonia (colorimetric, titrimetric, potentiometric distillation procedure). 5–9
Fancher JP, Aitkenhead-Peterson JA, Farris T et al (2017) An evaluation of soil chemistry in human cadaver decomposition islands: potential for estimating postmortem interval (PMI). Forensic Sci Int 279:130–139
Farwig N, Brandl R, Siemann S et al (2014) Decomposition rate of carrion is dependent on composition not abundance of the assemblages of insect scavengers. Oecologia 175:1291–1300
Flint LE, Flint AL (2002) Porosity. In: Methods of Soil Analysis, Part 4: Physical Methods. Soil Science Society of America, pp 241–254
Frenot Y, Chown SL, Whinam J et al (2005) Biological invasions in the Antarctic: extent, impacts and implications. Biol Rev Camb Philos Soc 80:45–72
Goff ML (2009) Early post-mortem changes and stages of decomposition in exposed cadavers. Exp Appl Acarol 49:21–36
Heine JC, Speir TW (1989) Ornithogenic soils of the cape bird Adelie penguin rookeries, Antarctica. Polar Biol 10:207–212
Hughes KA, Pertierra LR (2016) Evaluation of non-native species policy development and implementation within the Antarctic treaty area. Biol Conserv 200:149–159
Johnson AI (1962) Methods of measuring Soil Moisture in the Field. GEological Survey Water-Supply Paper 1619-U 112:11–32.
Keenan SW, Schaeffer SM, Jin VL, DeBruyn JM (2018) Mortality hotspots: nitrogen cycling in forest soils during vertebrate decomposition. Soil Biol Biochem 121:165–176
Kettler TA, Doran JW, Gilbert TL (2001) Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci Soc Am J 852:849–852
Kim OS, Chae N, Lim HS et al (2012) Bacterial diversity in ornithogenic soils compared to mineral soils on King George Island, Antarctica. J Microbiol 50:1081–1085
Krauze P, Wagner D, Yang S, Spinola D, Kühn P (2021) Influence of prokaryotic microorganisms on initial soil formation along a glacier forefield on King George Island, maritime Antarctica. Sci Rep 11(1). https://doi.org/10.1038/s41598-021-92205-z
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
Matejovic I (1997) Communications in soil science and plant analysis determination of carbon and nitrogen in samples of various soils by the dry combustion determination of carbon and nitrogen in samples of various soils by the dry combustion. Commun Soil Sci Plantanal 28:1499–1511
Melis C, Selva N, Teurlings I et al (2007) Soil and vegetation nutrient response to bison carcasses in Białowieża Primeval Forest, Poland. Ecol Res 22:807–813
Meyer J, Anderson B, Carter DO (2013) Seasonal variation of carcass decomposition and gravesoil chemistry in a cold (Dfa) climate. J Forensic Sci 58(5):1175–1182
Mildvan AS (2006) The role of metals in enzyme-catalyzed substitutions at each of the phosphorus atoms of ATP. Adv Enzymol Relat Areas Mol Biol 49:103–126
Mondor E, Tremblay M, Tomberlin J et al (2012) The ecology of carrion decomposition. Nat Educ 3:21
Nagle JF, Tristram-Nagle S (2000) Structure of lipid bilayers. In Biochimica et Biophysica Acta - Reviews on Biomembranes 1469(3). https://doi.org/10.1016/S0304-4157(00)00016-2
Nędzarek A, Tórz A, Rakusa-Suszczewski S, Bonisławska M (2015) Nitrogen and phosphorus release during fish decomposition and implications for the ecosystem of maritime Antarctica. Polar Biol 38:733–740
Nizamutdinov T, Andreev M, Abakumov E (2021) The role of the ornithogenic factor in soil formation on the Antarctic oasis territory Bunger Hills (East Antarctica). Eurasian J Soil Sci 10:308–319
Parmenter RR, Macmahon JA (2009) Carrion decomposition and nutrient cycling in a semiarid shrub-steppe ecosystem. Ecol Monogr 79:637–661
Payne JA (1965) A summer carrion study of the baby pig Sus Scrofa Linnaeus. Ecology 46:592–602
Payne JA, King EW, Beinhart G (1968) Arthropod succession and decomposition of buried pigs. Nature 219:1180–1181
Pechal JL, Benbow ME, Crippen TL et al (2014) Delayed insect access alters carrion decomposition and necrophagous insect community assembly. Ecosphere 5:1–21
Potocka M, Krzemińska E (2018) Trichocera maculipennis (Diptera)-an invasive species in Maritime Antarctica. PeerJ 6:e5408
Putman RJ (1978) Patterns of carbon dioxide evolution from decaying carrion decomposition of small mammal carrion in temperate systems 1. Oikos 31(1):47–57
Quaggiotto MM, Evans MJ, Higgins A et al (2019) Dynamic soil nutrient and moisture changes under decomposing vertebrate carcasses. Biogeochemistry 146:71–82
Ritz K, Dawson L, Miller D (2008) Criminal and Environmental Soil Forensics. Springer
Rodrigues WF, de Oliveira FS, Schaefer CE, Leite MG, Pavinato PS (2021) Phosphatization under birds’ activity: ornithogenesis at different scales on Antarctic soilscapes. Geoderma 391:114950
Sanchez-Pinero F, Polis GA (2000) Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81:3117–3132
Santamans AC, Boluda R, Picazo A et al (2017) Soil features in rookeries of Antarctic penguins reveal sea to land biotransport of chemical pollutants. PLoS ONE 12:e0181901
Schaefer CEGR, Simas FNB, Gilkes RJ et al (2008) Micromorphology and microchemistry of selected cryosols from maritime Antarctica. Geoderma 144:104–115
Schlichting PE, Love CN, Webster SC, Beasley JC (2018) Efficiency and composition of vertebrate scavengers at the land-water interface in the chernobyl exclusion zone. Food Webs 18:e00107
Shatova O, Wing SR, Gault-Ringold M et al (2016) Seabird guano enhances phytoplankton production in the Southern Ocean. J Exp Mar Biol Ecol 483:74–87
Simas FNB, Schaefer CEGR, Filho MRA et al (2008) Genesis, properties and classification of cryosols from Admiralty Bay, maritime Antarctica. Geoderma 144:116–122
Soil Survey Division Staff (1951) U.S Department of Agriculture Handbook 18. In: United States Department of Agriculture
Sprent JI (1987) The ecology of the nitrogen cycle. Cambridge University Press, Cambridge, p 151
Stevenson FJ, Cole MA (1999) Cycles of Soils: Carbon, Nitrogen, Phosphorus, Sulphur, Micronutrients, 2nd edn. Wiley, Hoboken
Szelecz I, Koenig I, Seppey CVW et al (2018) Soil chemistry changes beneath decomposing cadavers over a one-year period. Forensic Sci Int 286:155–165
Towne EG (2000) Prairie vegetation and soil nutrient responses to ungulate carcasses. Oecologia 122(2):232–239
Ugolini FC (1970) Antarctic soils and their ecology. Antarctic ecology, 2nd edn. Academic press London, London, pp 673–692
Varliero G, Lebre PH, Adams B et al (2024) Biogeographic survey of soil bacterial communities across Antarctica. Microbiome 12:1–22
Watson JD, Crick FHC (1953) Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 171(4356) https://doi.org/10.1038/171737a0
Woelber-Kastner BK, Frey SD, Howard DR, Hall CL (2021) Insect reproductive behaviors are important mediators of carrion nutrient release into soil. Sci Rep 11:1–9
Woolen K (2019) Chilled to the Bone: An Analysis on the Effects of Cold Temperatures and Weather Conditions Altering the Decomposition Process in Pig (Sus Scrofa) Remains. Illinois State University
Yong SK, Jalaludin NH, Brau E et al (2019) Changes in soil nutrients (ammonia, phosphate and nitrate) associated with rat carcass decomposition under tropical climatic conditions. Soil Res 57:482–488
Zaini NA, Low VL, Gebrelassie SS et al (2023) Arthropods, nematodes, fungi, and bacteria associated with penguin carrion in Barton Peninsula, King George Island, Antarctica. Polar Biol 47:41–52
Zhu R, Liu Y, Ma E et al (2009) Nutrient compositions and potential greenhouse gas production in penguin guano, ornithogenic soils and seal colony soils in coastal Antarctica. Antarct Sci 21:427–438
Zhu R, Chen Q, Ding W, Xu H (2012) Impact of seabird activity on nitrous oxide and methane fluxes from High Arctic tundra in Svalbard, Norway. J Geophys Res Biogeosci 117:1–16
Acknowledgements
This research is supported by the Sultan Mizan Antarctic Research Foundation (100-IRMI/PRI 16/6/2 (037/2019)). We thank Muhamad Zulhilmi bin Ramlee for the guidance on total carbon measurement. We also express our sincere thanks to Abby Kimpton Jones for dedicating her time to meticulously proofreading this manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed to the different parts of this manuscript as follows: N.A.Z. contributed to methodology, investigation, formal analysis, visualization, and writing of the original draft. S.S.I. contributed to methodology, resources, and writing, reviewing, and editing of the manuscript. V.L.L. contributed to writing, reviewing, and editing of the manuscript. M.H.M. contributed to writing, reviewing, and editing of the manuscript. J.H. contributed to writing, reviewing, and editing of the manuscript. W.Y.L. contributed to writing, reviewing, and editing of the manuscript. C.C.H. contributed to conceptualization, methodology, validation, and writing, reviewing, and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaini, N.A., Ismail, S.S., Low, V.L. et al. Soil chemical properties associated with penguin carrion in Barton Peninsula, King George Island, Antarctica. Polar Biol (2024). https://doi.org/10.1007/s00300-024-03264-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00300-024-03264-7