Abstract
Mysis segerstralei is distributed over a wide geographic area and in habitats with a variety of salinity conditions, including marine and brackish waters around Svalbard. The species has seldom been found in freshwater lakes, and the discovery of M. segerstralei in Lake Pulmankijärvi at the border between Northeastern Norway and Finland, may represent the single known freshwater occurrences in western Europe. Svalbard lake systems are characterized by very low water temperatures, long-term ice cover, and low levels of nutrients. Food is thus limited, and chironomids generally dominate the stomach contents in Arctic charr, the only freshwater fish species on Svalbard. Based on several surveys in more than 30 of Svalbard lakes over many decades, M. segerstralei has only been found as food for Arctic charr in Lake Vårfluesjøen. In a later fishery survey, we studied the diet of Arctic charr in this lake. The stomach contents from Arctic charr sampled in the profundal habitats were dominated by M. segerstralei, but the species was also among the most frequent prey items in the littoral and pelagic habitats. This unexpected occurrence of M. segerstralei demonstrates the high importance of mysids even in a low-productive, High Arctic lake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Mysis relicta species group consists of several species living in the northern Holarctic, with M. relicta, M. salemaai, and M. segerstralei in Northern Europe and Russia, and M. segerstralei and M. diluviana in North America and on the High Arctic islands (Audzijonyte and Väinölä 2005). In southern Fennoscandia, the Mysis species’ have a natural occurrence in lakes below the marine limit, both in deep, cold, and relatively shallow lakes (Enckell 1980; Hessen and Kjellberg 1995). According to Audzijonyte and Väinölä (2005), M. segerstralei has a circumpolar distribution along the Arctic coasts and islands of Eurasia and North America (Audzijonyte and Väinölä 2006; Spikkeland et al. 2016). Thus, the species seems to be distributed over a wide geographic area and in habitats with a variety of salinity conditions, mainly close to and within river estuaries, and have seldom been found in freshwater lakes (Audzijonyte and Väinölä 2005). The finding of M. segerstralei in Lake Pulmankijärvi, at the border between Northeastern Norway and Finland, as far as we are aware, may represent the single known freshwater occurrence in western Europe (Spikkeland et al. 2016), and despite surveys in a set of similar lakes in Northern Norway, additional findings have never been reported (Holmquist 1959; Väinölä 1993). M. segerstralei is the only Mysis species documented from any of the Arctic islands, including Svalbard, where it was found in marine waters in the Hinlopen Strait, between the islands Nord-Austlandet and Spitsbergen (Audzijonyte and Väinölä 2005, 2006). Olofsson (2018) described a population of mysids, probably M. segerstralei, from a lagoon (“Der Reliktsee”) at the Van Mijenfjorden (77.88 N 16.78 E), and further, Holmquist (1959) referred to another sample of mysids from Olofsson’s samples in a small lagoon near Nordenskiöldsbreen, which probably also was M. segerstralei. Except from an observation of M. segerstralei in the stomachs of three Atlantic cod (Gadus morhua) captured in a meromictic lake (Torskevatnet; “Cod lake” in English) on Nordaustlandet, Svalbard in 1997 (pers. comm. M-A. Svenning), we have not been aware of any registrations of M. segerstralei in Svalbard freshwater lakes. Further, Walseng et al. (2018) who conducted a survey of freshwater invertebrates in 75 ponds and lakes on Svalbard in 2014 and 2015, did not discover mysids in any of the sampled locations.
Mysis species are important components in the diet of Arctic charr in several locations, including many of the southernmost charr lakes in Sweden and lakes in Finland. In the lakes of the Russian Republic of Karelia, M. relicta is, along with the European smelt (Osmerus eperlanus), a key prey species for large-sized piscivorous members of the Salvelinus alpinus complex that coexist with other fish species and several glacial relicts (Hammar 2014). In the large reservoir Limingen in Mid-Norway, M. relicta was first observed in Arctic charr stomachs in 1974 after an introduction of the species and has become an important part of the diet (Gregersen 2006). In the profundal zone of this reservoir, M. relicta dominated (50–60%) the Arctic charr diet (Knudsen et al. 2019). It is interesting to note that M. segerstralei is an intermediate host for the acanthocephalan Echinorhynchus bothniensis in Lake Pulmankijärvi (Aura et al. 2015) and the most heavily infected fish species were Arctic charr and a benthic-feeding morph of whitefish Coregonus lavaretus, indicating that both these species fed to a large extent on M. segerstralei.
From surveys in more than 30 Svalbard lakes from 1962 to 2020, chironomids have dominated the stomach contents of sampled Arctic charr, together with smaller amounts of caddisflies (Apatania zonella), nematodes, oligochaetes, and a few zooplankton species (Gullestad 1973; Hammar 1982, 2023; Svenning 1992, 1993; Aas 2007; Svenning et al. 2007, Svenning and Bergane 2020; Borgstrøm et al. 2015; Bergane 2018). Since larger charr are cannibalistic, smaller charr are frequently found as prey in resident charr larger than 20–25 cm (Svenning and Borgstrøm 1995; Hammar 2000; Svenning et al. 2007). In Lake Vårfluesjøen, northwestern Spitsbergen, however, M. segerstralei was registered as prey of Arctic charr for the first time in the late 1990s. In connection with a fishery survey in Vårfluesjøen in 2005, we had the opportunity to study the diet of Arctic charr by gillnet sampling of Arctic charr from both the littoral, profundal, and pelagic habitats.
Material and methods
Study site
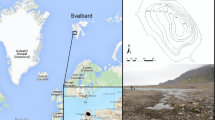
The Svalbard Archipelago consists of four large islands, Edgeøya, Barentsøya, Spitsbergen, and Nordaustlandet in addition to several smaller islands (Fig. 1). Lake Vårfluesjøen (79.7oN) is located 10 m.a.s.l. on the northwestern part of the largest island, Spitsbergen, on the eastern side of the Wood fjord (Fig. 1). The name of the lake comes from Dege (1938), who gave it the German name ‘Köcherfliegen-See’, which in English means ‘Caddis-fly Lake’. The surface area is 1.8 km2 and the maximum depth is 33 m. The water flow in the 1 km long outlet river is relatively stable/high during summer (July–September), i.e., migratory Arctic charr can easily descend and ascend this passage from early July to late September. As for most lake systems on Svalbard, there is no water flow in the outlet river during winter, i.e., from early October to late June (Svenning and Gullestad 2002). Vårfluesjøen receives substantial drainage from glaciers during summer, and water transparency in the ice-free period varies between 10 and 30 cm. Vårfluesjøen is oligotrophic and a typical cold monomictic lake with a complete mixing of the water column in summer, with water temperatures varying in the range 4–6 °C (Svenning 2015). The lake freezes over in late September, or early October, while ice cover usually disappears around mid-to-late July, corresponding to an ice-free period of ca. 3 months. The ice thickness gradually builds up to a maximum of 1.8–2 m (Svenning 2015).
We observed a rich bird community at the lake during the fieldwork in 2005, of which the Brent goose (Branta bernicla) dominated, with daily visits of flocks counting 20–40 individuals around the lake. Three other species, the red-throated loon (Gavia stellata), the black guillemot (Cepphus grylle), and the Arctic tern (Sterna paradisaea) were seen fishing in the lake. The bird activity may result in an increased nutrient and food supply to the lake by excrements, mainly from the numerous geese. Arctic charr is the only fish species in Lake Vårfluesjøen, forming both resident and anadromous individuals (Svenning 1993).
The origin of M. segerstralei in lake Vårfluesjøen may be related to the postglacial history of the lake, similar to the occurrence of this species in lakes on Victoria Island in the Canadian Arctic, where M. segerstralei occurs in several lakes situated from 11 to 93 m a. s. l. (Johnson 1964; Audzijonyte and Väinölä 2005). The marine limit in this area seems to be around 120–150 m above the present sea level (Andrews 1973). One of these lakes, the Greiner Lake, today situated 15 m above sea level, with a maximum depth of 11 m and a maximum summer temperature of approximately 8 °C, has risen from the sea within the last 1300 years (Johnson 1962), while Victoria Lake, is situated 11 m a. s. l., and is probably even younger than Greiner Lake. Accordingly, M. segerstralei originates from the marine environment, and is a late immigrant to these lakes from salt water, and a true marine relict. Likewise, the establishment of M. segerstralei in lake Vårfluesjøen has most probably taken place when the lake was still more or less at sea level, but thousands of years earlier than the establishment in the lakes on Victoria Island. Another crustacean, Gammaracanthus loricatus, an important component in food of resident Arctic charr in the lake Jensenvatn on the island Danskøya, in the northwestern corner of Spitsbergen, is likewise an ancient marine-glacial relict (Svenning 1992).
Sampling
For studying the diet of Arctic charr in the lake, fish sampling was performed by gillnetting in the littoral, pelagic, and profundal zones during the period 12–28 August 2005. Multi-mesh gillnets with panels of bar mesh sizes 8, 10, 12,5, 15, 18,5, 22, 26, 33, 39, and 45 mm were used. The gillnets set in the littoral and profundal zones were 40 m long and 1.5 m in height, while those set from the surface in the pelagic zone were 40 m long and 3 m in height. The gillnets were set in two depth zones in the profundal zone, at 10–12 m and 20–28 m. The nets were set in the afternoon and pulled or checked the day after. Catch per unit effort (CPUE) is presented as the number of fish per 100 m2 net per 24 h.
A total of nearly 3200 ascending anadromous Arctic charr captured in a trap placed in the outlet river from July 17 to August 28, were marked and released above the trap (Skogstad and Skogstad 2006). Resident charr was classified according to morphology and body coloration. Further, since all ascending fish were marked, all untagged fish older than four years were defined as resident charr. In total, 197 resident Arctic charr in the length range of 72–241 mm were captured and killed for stomach content sampling (Fig. 2).
All fish were measured for fork length (mm) and mass (g) and classified according to sex, maturity, and color of flesh. Stomachs and oesophagus’ were cut loose and preserved in 96% ethanol with all contents. Sagittal otoliths used for aging were removed from the fish and preserved in 96% ethanol with a small amount of glycerol.
Diet analysis
The stomach fullness was estimated on a percentage scale. Food items were identified and their relative contribution to stomach fullness was estimated. The average degree of fullness for each prey item was expressed as a percentage of the average degree of total fullness in the sample. Percentage occurrences, Fi = (Ni/N) · 100, and prey-specific abundances, Pi = (ΣSi/ΣSt) × 100, were calculated, where Ni is the number of fish with prey i in their stomach, N is the total number of fish with stomach contents, Si is the stomach fullness for prey i, and St the total stomach fullness of the fish (only stomachs with content included). By plotting prey-specific abundance (Pi) against the frequency of occurrence (Fi), we visualized the different categories of individual specialization and the general importance of the different food item categories in the diet (Amundsen et al. 1996). Some of the stomach contents consisted of small fragments of prey items, most likely mainly from chironomid pupae, but also from caddisfly larvae, as well as small pebbles from caddisfly cases, which we decided to categorize as unidentified.
Results
During the 2 weeks of gillnet sampling, the CPUE was higher in the littoral zone (1.70) than in the profundal (0.14) and pelagic (0.03) zones (Table 1). The diet of the resident Arctic charr consisted of Mysis segerstralei, larvae and pupae of Apatania zonella and chironomids, small Arctic charr, copepods (Cyclops abyssorum), and some undetermined fragments (Figs. 3, 4).
M. segerstralei dominated the diet of resident Arctic charr sampled in the profundal zone, with a frequency of occurrence and prey-specific abundance of 92.2 and 98.5%, respectively (Figs. 3, 4), and also amounted to a significant proportion of prey items found in charr captured in the littoral and pelagic zone. The length of the M. segerstralei found in stomach contents was in the range 11–15 mm. Prey-specific abundance and frequency of occurrence of M. segerstralei are high both at depth 10–12 m and 20–28 m in the profundal zone (Fig. 5).
The diet of Arctic charr captured in the littoral zone was dominated by A. zonella (Figs. 3, 4), with both a high frequency of occurrence (66.7%) and specific volume percent (98.7%). M. segerstralei was the second most abundant prey in this habitat. Only one individual from the littoral sample had eaten an Arctic charr (Fig. 3). This cannibal was 207 mm, and the length of the preyed individual was 23 mm, i.e., a young of the year.
In the pelagic habitat, where only 12 fishes were caught, a relatively high percentage of the stomach content consisted of unidentified insect fragments, most probably digested pupae of chironomids. Both M. segerstralei, A. zonella, and copepods (C. abyssorum) made up a significant part of the stomach contents as well (Figs. 3, 4).
Discussion
As expected from the origin of the name of Lake Vårfluesjøen, A. zonella larvae and pupae dominated the diet of resident charr captured in the littoral zone. In charr captured in the profundal zone, however, the stomach contents were totally dominated by M. segerstralei, while in the few fish caught in the pelagic zone, both A. zonella pupae and M. segerstralei were important stomach items. The dominance of M. segerstralei in the diet, as well as the scarcity of chironomids as prey items, deviates from all other diet studies of Arctic charr captured in both littoral, pelagic and profundal habitats in lakes on Svalbard (Gullestad 1973; Hammar 1982, 2023; Svenning 1992, 1993; Aas 2007; Svenning et al. 2007, Svenning and Bergane 2020; Borgstrøm et al. 2015; Bergane 2018).
There is little information about interactions between M. segerstralei and freshwater fish, but the closely related M. relicta is a typical opportunistic feeder, capable of utilizing a variety of food resources. This species may play an important role in structuring limnetic food webs, feeding on detritus, diatoms, phytoplankton, rotifers, ostracods, copepods, Cladocera, and even the amphipod Pontoporeia, small mysids and pollen (Grossnickle 1982; Hessen and Kjellberg 1995; Rudstam et al. 1999; Johannsson et al. 2011). Mysis may thus be a competitor to Arctic charr and other zooplankton-feeding fish, as concluded in a study from Lake Breiter, located in north-eastern Germany, one of the few lakes in Germany where M. relicta is registered. In this lake, Mysis was an important food item of two sympatric populations of European cisco, however, Mysis preyed on the same food organisms as the ciscoes, indicating a potential competition between the zooplantivore fish and Mysis (Scharf et al. 2008).
Primary production is generally low in Arctic lakes (Hobbie 1984), and very low in Vårfluesjøen and other lakes in the High Arctic, due to the short summer season with low temperatures and low nutrient concentrations, combined with high glacial silt contents and low light transmission (Svenning 2015). Accordingly, the food basis for prey species of M. segerstralei is far from optimal in such lakes, and both copepods and cladocerans may more easily be preyed down to a low level.
Seabird excrement (droppings) at Vårfluesjøen, especially from the relatively numerous Brent goose, may supply nutrients for primary production and food to several of the invertebrates in the lake. These droppings give rise to much detritus which also may be utilized directly by Mysis (Stockwell et al. 2020), as well by prey species of Mysis, such as copepods and Cladocera. The excrement may also be an important food source for A. zonella, and explain the high occurrence of this species in diet of littoral charr in Lake Vårfluesjøen, compared to most other lake systems on Svalbard (Svenning et al. 2007).
Large crustaceans, including Mysis species, are very attractive prey for fish due to their relatively large size (Nilsson 1967; Aass 1969), and the effect of fish predation is noticeably demonstrated in lakes in North America, where Lepidurus arcticus and the anostracan Polyartemia forcipata occur mainly in fishless lakes (Butler et al. 1980). A typical example is described by Nilsson (1967) after the introduction of Arctic charr to a small lake in northern Sweden inhabited by P. forcipata. This species immediately became the most important prey, and already the first season after the introduction of Arctic charr, it was replaced by the smaller Bythothrephes longimanus which similarly disappeared in the third season, rendering a zooplankton community dominated by small Bosmina spp. Likewise, in northeastern Greenland, L. arcticus occurred in all studied fishless lakes but was either absent or present in very low densities in lakes with fish (Jeppesen et al. 2001). Thus, when exposed to increased fish predation, for instance due to the invasion of new fish species, large crustacean species may quickly disappear in arctic lakes.
The presence and significance of M. segerstralei as a prey item for resident Arctic charr in Lake Vårfluesjøen is most probably a result of relatively low predation pressure, as most of the Vårfluesjøen charr older than 5 years are anadromous, leaving the lake in beginning of the summer season, and have their most important food consumption and growth in the sea (Svenning 1993). In 2005, nearly 3200 Arctic charr (0.1 to 5.5 kg) ascended the lake in July–August after the sea residence (Skogstad and Skogstad 2006). This means that this large migrant population was feeding in the sea during most of the ice-free period, and neither seem to feed at all during their freshwater residency (Rikardsen et al. 2003). Thus, resident Arctic charr, mainly in length groups 11–20 cm, seem more or less to be the only fish feeding on M. segerstralei in Lake Vårfluesjøen.
The low light intensity in deeper areas in Lake Vårfluesjøen, combined with less than 20–30 cm transparency due to the glacier melting, probably also contributes to reduced predation pressure on M. segerstralei. Arctic charr in Svalbard lakes feed throughout the winter period, in total darkness (Svenning et al. 2007), indicating that M. segerstralei might be eaten during the ice-covered period as well. During the ice-free period, there are small differences in water transparency between day and night at this high latitude, and predation pressure in the pelagic habitat would be nearly the same throughout the 24-h daily period. In Lake Pulmankijärvi, there is a high fish abundance including several species which feed on M. segerstralei, but still this species is important in the diet of both Arctic charr and benthic whitefish, suggesting that M. segerstralei tolerates high fish predation (Aura et al. 2015; Eloranta et al. 2015), but also suggesting that the food basis for Mysis in such more productive lakes is high.
The resident charr in Lake Vårfluesjøen occurs at a higher density in the littoral habitat than in the pelagic and profundal habitats, however, the largest resident individuals were captured mainly in the profundal habitat at depths from 10–12 m to 20–28 m (Fig. 5), which is probably an effect of the presence of M. segerstralei in this habitat. In some lakes in mid-Norway, M. relicta was likewise important for profundal Arctic charr, and a low occurrence in the diet of pelagic charr (Koksvik et al. 1995). In deep temperate lakes, the summer temperature may be warm in the upper layers, but due to temperature stratification, the deeper layers are still cold, in contrast to Arctic lakes such as Lake Vårfluesjøen, where the water temperatures in summer and autumn are low (4–6 °C) throughout the whole water column (Svenning 2015). The difference in temperature regimes between temperate and Arctic lakes may be decisive for the habitat use of young charr, with young fish staying in the cold profundal zone in temperate lakes (Bjøru and Sandlund 1995; Klemetsen et al. 2002), while they stay in the ‘supralittoral’ (less than 30–40 cm depth) of Arctic and shallow high alpine lakes (Svenning and Borgstrøm 1995; Vik 2002).
The smaller mean size of individuals of Arctic charr captured by multi-mesh gillnets in the littoral zone of Lake Vårfluesjøen compared to the fish captured in the profundal and pelagic zones is also an indication that the smallest fish prefer the shallowest habitats, probably due to an antipredator response defined by the size of prey fish and size of the cannibals (Svenning and Borgstrøm 1995; Vik 2002). With increasing size, the resident charr in Lake Vårfluesjøen may move to deeper water in the littoral zone or move into the pelagic or profundal zone. Therefore, the number of fish in the profundal zone in Svalbard lakes such as Lake Vårfluesjøen may be much lower than in temperate lakes, due to the absence of competition from the youngest age classes, and also because the larger/ older anadromous charr are feeding in seawater. Thus, the predation pressure from Arctic charr on the profundal living M. segerstralei in Lake Vårfluesjøen is probably relatively low, preventing the M. segerstralei population to go extinct.
Most Mysis species seem to be important predators of zooplankton (Langeland 1988; Hessen and Kjellberg 1995; Spencer 1999; Stockwell et al. 2020), but the species are also predated by both pelagic and benthic-feeding fish (Bailey 1972; Isaac et al. 2012). Further, most Mysis species are suggested to conduct a vertical diurnal migration, staying in deep water during the day, and feeding in the upper water during the night (Beeton and Bowers 1982; Moen and Langeland 1989). In a recent review, however, Stockwell et al. (2020) claimed that surprisingly few studies had paid attention to the benthic components of Mysis ecology and emphasized that some mysids may remain at the bottom also at night, i.e., do not necessarily migrate vertically. This phenomenon of vertical migration is characteristic of plankton in deep-water situations and is probably linked to prey avoidance. While this behavior is evidently common in most freshwater species (i.e., M. relicta, M. salemaai, M. diluviana) and perhaps also in marine mysids, it may not be typical of M. segerstralei, a species most often found in shallow freshwater lagoons, with no possibility to escape to darkness (Risto Väinölä, pers. comm.).
On the other hand, M. segerstralei has also been found in the more than 30 m deep lake Pulmankijärvi, although their potential migratory behavior has not been reviewed (Audzijonyte and Väinölä 2005). In Lake Vårfluesjøen, we set the gillnets in the afternoon and pulled or checked them the day after. The low CPUE in the pelagic does not support the hypothesize that resident Arctic charr migrates between the pelagic and the profundal zone. Further, the stomach contents in resident Arctic charr caught in the pelagic showed no dominance of M. segerstralei but contained approximately the same amount of other food items as Arctic charr captured in the littoral zone such as caddisflies, copepods (C. abyssorum), and chironomid pupae, indicating a rather potential horizontal migration between the littoral and pelagic habitats. Further, the small portion of zooplankton eaten by resident charr captured in the pelagic zone and probably being the only potential food item for M. segerstralei in the surface area, also suggest that diurnal migration is hardly a successful strategy for M. segerstralei in Lake Vårfluesjøen. This is also in accordance with the conclusions from Stockwell et al. (2020) and Risto Väinölä (pers. comm).
In conclusion, the marine relict, M. segerstralei, may survive well in the deeper areas in Lake Vårfluesjøen, due to low predation pressure from a relatively small resident Arctic charr population, and also favored by a low water transparency caused by high glacial silt content of the water mass during summer.
Data availability
The data are available from the corresponding author upon reasonable request.
References
Aas M (2007) Bestandsdynamikk og habitatbruk hos anadrom og stasjonær røye (Salvelinus alpinus) i Straumsjøen; en grunn innsjø på Svalbard. MSc thesis, Norwegian University of Life Sciences
Aass P (1969) Crustacea, especially Lepidurus arcticus Pallas, as brown trout food in Norwegian mountain reservoirs. Rep Inst Freshw Res Drottningholm 49:183–201
Amundsen P-A, Gabler H-M, Staldvik FJ (1996) A new approach to graphical analysis of feeding strategy from stomach contents data—modification of the Costello (1990) method. J Fish Biol 48:607–614. https://doi.org/10.1111/j.1095-8649.1996.tb01455.x
Andrews JT (1973) Maps of the maximum postglacial marine limit and rebound for the former Laurentide Ice Sheet (the National Atlas of Canada). Arct Alp Res 5:41–48. https://doi.org/10.1080/00040851.1973.12003677
Audzijonyte A, Väinölä R (2005) Diversity and distributions of circumpolar fresh- and brackish-water Mysis (Crustacea: Mysida): descriptions of Mysis relicta Lovén, 1862, Mysis salemaai n. sp., Mysis segerstralei n. sp. and Mysis diluviana n. sp., based on molecular and morphological characters. Hydrobiologia 544:89–141. https://doi.org/10.1007/s10750-004-8337-7
Audzijonyte A, Väinölä R (2006) Phylogeographic analyses of a circumarctic coastal and a boreal lacustrine mysid crustacean, and evidence of fast postglacial mtDNA rates. Mol Ecol 15:3287–3301. https://doi.org/10.1111/j.1365-294X.2006.02998.x
Aura R-L, Benesh D, Palomaki R, Valtonen ET (2015) The natural history of Echinorhynchus bothniensis Zdzitowiecki and Valtonen, 1987 (Acanthocephala) in a high Arctic lake. Folia Parasitol. https://doi.org/10.14411/fp.2015.051
Bailey MM (1972) age, growth, reproduction, and food of the Burbot, Lota lota (Linnaeus), in Southwestern Lake Superior. Trans Am Fish Soc 101:667–674. https://doi.org/10.1577/1548-8659(1972)101%3c667:AGRAFO%3e2.0.CO;2
Beeton AM, Bowers JA (1982) Vertical migration of Mysis relicta Lovén. Hydrobiologia 93:53–61. https://doi.org/10.1007/BF00008098
Bergane V (2018) Sjørøya (Salvelinus alpinus) i Linnévassdraget, Svalbard: livshistorietrekk og populasjonsstruktur ti år etter innføring av kvotebasert fangst. MSc thesis, Norwegian University of Life Sciences
Bjøru B, Sandlund OT (1995) Differences in morphology and ecology within a stunted Arctic char population. Nordic J Freshw Res 71:163–172
Borgstrøm R, Isdahl T, Svenning M-A (2015) Population structure, biomass, and diet of landlocked Arctic charr (Salvelinus alpinus) in a small, shallow high Arctic lake. Polar Biol 38:309–317. https://doi.org/10.1007/s00300-014-1587-6
Butler M, Miller MC, Mozley S (1980) Macrobenthos. In: Hobbie JE (eds) Limnology of tundra ponds. Barrow, Alaska. Dowden, Hutchinson & Ross, Inc, Stroudsburg, Pennsylvania, pp 297–339
Dege W (1938) Spitzbergen-Expedition Deutscher Studenten 1936. Geomorphologische Forschungen im nördlichen Andréeland (Nord-Spitzbergen). Inaugural Dissertation, Münster, Lengericher Handesldruckerei, Lengerich, Germany
Eloranta AP, Kahilainen KK, Amundsen P-A et al (2015) Lake size and fish diversity determine resource use and trophic position of a top predator in high-latitude lakes. Ecol Evol 5:1664–1675. https://doi.org/10.1002/ece3.1464
Enckell PH (1980) Kräftdjur. Bokförlaget Signum, Lund
Gregersen F (2006) Long-term variation in diet of Arctic char, Salvelinus alpinus, and brown trout, Salmo trutta: effects of changes in fish density and food availability. Fish Manag Ecol 13:243–250. https://doi.org/10.1111/j.1365-2400.2006.00500.x
Grossnickle NE (1982) Feeding habits of Mysis relicta—an overview. Ecol Mysidacea. https://doi.org/10.1007/BF00008103
Gullestad N (1973) Ferskvannsbiologiske undersøkelser på Svalbard 1962–1971. Fauna 26:225–232
Hammar J (1982) Arctic char in Arctic regions. Fauna Flora 77:85–92
Hammar J (2000) Cannibals and parasites: conflicting regulators of bimodality in high latitude Arctic char, Salvelinus alpinus. Oikos 88:33–47. https://doi.org/10.1034/j.1600-0706.2000.880105.x
Hammar J (2014) Natural resilience in Arctic charr Salvelinus alpinus: life history, spatial and dietary alterations along gradients of interspecific interactions. J Fish Biol 85:81–118. https://doi.org/10.1111/jfb.12321
Hammar J (2023) Natural selection at the edge of life: allelic polymorphism and recruitment in high latitude Arctic char (Salvelinus alpinus) generated and maintained by environmental extremes. Diversity. https://doi.org/10.3390/d15010074
Hessen D, Kjellberg G (1995) Krepsdyret Mysis relicta i sitt naturlige miljø. In: Borgstrøm R, Jonsson B, L’Abée-Lund J (eds) Ferskvannsfisk. Økologi, kultivering og utnytting. Norges forskningsråd, Oslo, pp 167–173
Holmquist C (1959) Problems on Marine-glacial relicts on account of investigations on the genus Mysis. Berlingska Boktryckeriet, Lund, Sweden
Isaac EJ, Hrabik TR, Stockwell JD, Gamble AE (2012) Prey selection by the Lake Superior fish community. J Great Lakes Res 38:326–335. https://doi.org/10.1016/j.jglr.2012.02.017
Jeppesen E, Christoffersen K, Landkildehus F et al (2001) Fish and crustaceans in northeast Greenland lakes with special emphasis on interactions between Arctic charr (Salvelinus alpinus), Lepidurus arcticus and benthic chydorids. Hydrobiologia 442:329–337. https://doi.org/10.1023/A:1017508211819
Johnson L (1962) The Relict Fauna of Greiner Lake, Victoria Island, N.W.T Canada. J Fish Res Bd Can 19:1105–1120. https://doi.org/10.1139/f62-073
Johnson L (1964) Marine-Glacial relicts of the Canadian Arctic Islands. Syst Zool 13:76–91. https://doi.org/10.2307/2411827
Johannsson O, Leggett M, Rudstam L et al (2011) Diet of Mysis relicta in Lake Ontario as revealed by stable isotope and gut content analysis. Can J Fish Aquat Sci 58:1975–1986. https://doi.org/10.1139/cjfas-58-10-1975
Klemetsen A, Amundsen P-A, Grotnes P et al (2002) Takvatn through 20 years: long-term effects of an experimental mass removal of Arctic Charr, Salvelinus alpinus, from a subarctic lake. Environ Biol Fish 64:39–47. https://doi.org/10.1023/A:1016062421601
Knudsen R, Eloranta A, Siwertsson A et al (2019) Introduction of Mysis relicta (Mysida) reduces niche segregation between deep-water Arctic charr morphs. Hydrobiologia 840:245–260. https://doi.org/10.1007/s10750-019-3953-4
Koksvik J, Næsje T, Grossnickle N (1995) Mysis relicta kan endre innsjøens økosystem. In: Borgstrøm R, Jonsson B, L’Abée-Lund J (eds) Ferskvannsfisk. Økologi, kultivering og utnytting. Norges forskningsråd, Oslo, pp 174–183
Langeland A (1988) Decreased zooplankton density in a mountain lake resulting from predation by recently introduced Mysis relicta. SIL Proceed 1922–2010(23):419–429. https://doi.org/10.1080/03680770.1987.11897956
Moen V, Langeland A (1989) Diurnal vertical and seasonal horizontal distribution patterns of Mysis relicta in a large Norwegian lake. J Plankt Res 11:729–745. https://doi.org/10.1093/plankt/11.4.729
Nilsson NA (1967) The role of size-biased predation in competition and interactive segregation in fish. In: Gerking SD (ed) Ecology of freshwater fish production. Blackwell Scientific Publications, Oxford, pp 303–325
Olofsson O (2018) Studien Über die Süsswasserfauna Spitzbergens: Beitrag zur Systematik, Biologie und Tiergeographie der Crustaceen und Rotatorien. Zoologiska Bidrag från Uppsala 6. Forgotten Books, Uppsala
Rikardsen A, Amundsen P-A, Bodin P (2003) Growth and diet of Arctic charr post-smolt after their return to freshwater. Ecol Freshw Fish 12:74–80. https://doi.org/10.1034/j.1600-0633.2003.00001.x
Rudstam LG, Hetherington AL, Mohammadian AM (1999) Effect of temperature on feeding and survival of Mysis relicta. J Great Lakes Res 25:363–371. https://doi.org/10.1016/S0380-1330(99)70745-8
Scharf J, Krappe M, Koschel R, Waterstraat A (2008) Feeding of European cisco (Coregonus albula and C. lucinensis) on the glacial relict crustacean Mysis relicta in Lake Breiter Luzin (Germany). Limnologica 38:147–158. https://doi.org/10.1016/j.limno.2007.12.001
Skogstad OC, Skogstad Ø (2006) Dynamikk og ressursbruk hos anadrom og resident røye (Salvelinus alpinus) i Vårfluesjøen på Svalbard etter flere års fredning av bestanden. MSc thesis, Norwegian University of Life Sciences
Spencer C (1999) Impact of predation by Mysis relicta on zooplankton in Flathead Lake, Montana, USA. J Plankt Res 21:51–64. https://doi.org/10.1093/plankt/21.1.51
Spikkeland I, Kinsten B, Kjellberg G et al (2016) The aquatic glacial relict fauna of Norway—an update of distribution and conservation status. Fauna Norvegica 36:51–65. https://doi.org/10.5324/fn.v36i0.1994
Stockwell JD, O’Malley BP, Hansson S et al (2020) Benthic habitat is an integral part of freshwater Mysis ecology. Fresh Biol 65:1997–2009. https://doi.org/10.1111/fwb.13594
Svenning M-A (1992) Fiskeribiologiske undersøkelser i røyevassdrag på Svalbard i perioden 1987–1990. University of Tromsø, Tromsø
Svenning M-A (1993) Life history variations and polymorphism in Arctic charr, Salvelinus alpinus on Svalbard and in Northern Norway. Ph.D. Dissertation, University of Tromso
Svenning M-A (2015) Miljøvariable i innsjøer på Svalbard; vanntemperatur, lys- og isforhold. NINA Mini-report 575
Svenning M-A, Borgstrøm R (1995) Population structure in landlocked Spitsbergen Arctic charr. Sustained by cannibalism? Nordic J Freshw Res 71:424–431
Svenning M-A, Gullestad N (2002) Adaptations to stochastic environmental variations: the effects of seasonal temperatures on the migratory window of Svalbard Arctic charr. Environ Biol Fish 64:165–174. https://doi.org/10.1007/978-94-017-1352-8_13
Svenning M-A, Klemetsen A, Olsen T (2007) Habitat and food choice of Arctic charr in Linnévatn on Spitsbergen, Svalbard: the first year-round investigation in a High Arctic lake. Ecol Freshw Fish 16:70–77
Svenning M-A, Bergane V, Borgstrøm R (2020) Sjørøya i Linnévassdraget. Sluttrapport til Svalbards miljøvernfond. NINA Rapport 1825
Väinölä R (1993) Zoogeography of “glacial relict” crustaceans in northern Fennoscandia: a molecular systematic approach. Oulanka Reports ISSN 0358–3651(12):109–113
Vik JO (2002) Feedback and population regulation by piscivory in Arctic charr Salvelinus alpinus and brown trout Salmo trutta. Ph. D. Dissertation, Agricultural University of Norway
Walseng B, Jensen TC, Dimante-Deimantovica I et al (2018) Freshwater diversity in Svalbard: providing baseline data for ecosystems in change. Polar Biol 41:1995–2005. https://doi.org/10.1007/s00300-018-2340-3
Acknowledgements
We thank the Governor of Svalbard for helicopter transport, and the allowance to use their cabin at Lake Vårfluesjøen. Jennifer Stien is acknowledged for improving the English, Brian Dempson for comments and inputs on an earlier version of the manuscript, and Oddvar Hanssen for confirming the identification of M. segerstralei. We are grateful for the suggestions and corrections given by the editor and two unknown referees to improve the manuscript. This work was partly funded by the Norwegian Institute for Nature research.
Author information
Authors and Affiliations
Contributions
Conceptualization, MAS; Methodology, MAS and RB; Field sampling, OCS and ØS; Writing original draft, review and editing, MAS and RB; Project administration, MAS; Data curation, visualization and funding acquisition, MAS; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Svenning, MA., Skogstad, O.C., Skogstad, Ø. et al. Mysis segerstralei, an unexpected but important prey for resident Arctic charr (Salvelinus alpinus) in a Svalbard lake. Polar Biol (2024). https://doi.org/10.1007/s00300-024-03261-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00300-024-03261-w