Abstract
Freshwater habitats in Arctic landscapes provide essential food resources to predaceous terrestrial fauna. However, climate change threatens the continuation of aquatic resources as increased temperatures prompt widespread drying. We investigated how declines in surface waters in the Arctic may have implications for the diets of the wolf spider Pardosa glacialis (Araneae: Lycosidae), an abundant top predator in western Greenland known to consume both aquatic and terrestrial prey. We estimated the abundance of ground-dwelling prey taxa near and far from shallow ponds to compare resource availability for P. glacialis. We also measured the stable isotopes ratios of carbon and nitrogen of potential prey taxa and used linear mixing models to estimate the relative proportions of aquatic and terrestrial prey in the diets of P. glacialis collected near ponds versus drier, upland locations. Overall, near ponds there was a 3.7 times greater abundance of ground-dwelling organisms compared to locations far from ponds. Near ponds, depleted δ13C values of P. glacialis compared to the upland locations were explained by the consumption of aquatic insects, which were relatively depleted in 13C compared to terrestrial ground-dwelling and canopy taxa. Our linear mixing model indicated that the mean relative contribution of aquatic taxa to the diets of P. glacialis at pond locations was ~ 23% versus ~ 0% at upland locations, though these results were accompanied by significant uncertainty. Our results emphasize the importance of Arctic ponds in providing habitat to potential prey of terrestrial predators. We highlight how climate change, and the continued disappearance of aquatic habitats, could disrupt critical linkages between aquatic insects and terrestrial predators.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The Arctic is warming nearly four times faster than the rest of the globe due to Arctic amplification (Rantanen et al. 2022), a trend that is correlated with large-scale drying of surface waters, including lakes and ponds (Finger Higgens et al. 2019; Webb et al. 2022). Research has documented a 21% decrease in the number of lakes in a region in Western Greenland over the past 50 years (Finger Higgens et al. 2019). These freshwater systems are a prominent feature of Arctic landscapes, covering 20–40% of Arctic lowlands (Webb et al. 2022), which is more freshwater surface area than any other region worldwide (Grosse et al. 2013). They serve as important habitats for aquatic and terrestrial wildlife (Culp et al. 2022) and provide water and food resources to communities throughout the Arctic (Knopp et al. 2022 and refs therein). The input of terrestrial organic matter into these freshwater systems is also increasing in some parts of the Arctic (Hobbie et al. 1999; Crump et al. 2003; Prowse et al. 2006), which, coupled with changes in surface area, is affecting the role they play in carbon cycling. Earlier arrival of spring due to warming temperatures is also advancing the timing of ice melt on freshwater lakes and ponds (Visser and Holleman 2001; Cooper et al. 2014; Culler et al. 2015), which changes the phenology of aquatic species and their interactions in the broader Arctic ecosystem (Culler et al. 2015).
Abiotic changes, such as increasing temperatures and subsequent pond drying, are producing changes in freshwater food webs by modifying the distributions and abundances of plants, arthropods, and fish (Culp et al. 2022). This additionally impacts terrestrial predators that consume aquatic prey such as emerging insects (Murakami and Nakano 2002; Sabo and Power 2002; Sanzone et al. 2003; Paetzold et al. 2005). In the Arctic, aquatic insects can emerge in large numbers during short 2- to 4-week periods (Culler et al. 2015; Laske et al. 2021). After emergence, adult aquatic insects such as mosquitoes display an affinity for shallow ponds and lake margins where they oviposit (Danks and Corbet 1973; DeSiervo et al. 2022). Birds such as red-necked phalaropes build their nests on these shallow ponds where they have easy access to immature and adult aquatic insects as food (Culler, personal observation). Nutrient rich aquatic insects are consumed in many ecosystems by terrestrial arthropods, providing a linkage of nutrients between aquatic and terrestrial systems (Akamatsu et al. 2004; Kato et al. 2004; Krell et al. 2015). In addition, terrestrial predators also consume ground-dwelling soil invertebrates such as collembola (Eitzinger et al. 2019) that benefit from the high soil moisture levels found adjacent to freshwater habitats (Verhoef and Van Selm 1983; Kaneda and Kaneko 2011).
Notably, the extent to which terrestrial arthropod predators rely on food resources provisioned by freshwater habitats has not been thoroughly quantified. The spider Pardosa glacialis (Araneae: Lycosidae), a top predator in Western Greenland, is considered to have a diverse diet, consuming multiple prey items including emerging aquatic insects, herbivores, ground-dwelling detritivores, soil invertebrates, and conspecifics (Wirta et al. 2015; Eitzinger et al. 2019). A recent study found some evidence that freshwater habitats provide essential food resources for P. glacialis (Araneae: Lycosidae) in western Greenland (Culler et al. 2021). Wolf spiders near ponds had larger clutches (Culler et al. 2021), which could result from access to abundant resources in the form of emerging aquatic insects or higher abundances of soil invertebrates during critical periods of reproduction. If trophic connections between insectivorous terrestrial fauna like P. glacialis and freshwater and adjacent food webs are in fact critical, declines in surface waters in the Arctic may have profound implications for the broader terrestrial ecosystem.
Stable isotopes are a useful tool for elucidating aquatic and terrestrial food web linkages and the diet composition of terrestrial predators (Bastow et al. 2002; Akamatsu et al. 2004). Carbon has multiple isotopes, including 12C and 13C. The ratio of heavy to light carbon (13C:12C), also termed δ13C (per mil enrichment of 13C relative to a standard), is a signature that can be used to determine sources of carbon, as δ13C is incorporated by consumers at about the same ratio as their resource base (Post 2002; Boecklen et al. 2011). The ratio of heavy to light nitrogen, (15N:14N), also termed δ15N, is a signature that reflects the trophic position of organisms (Post 2002; Boecklen et al. 2011). Ratios of nitrogen isotopes in consumers become enriched from their resource base, which allows for the resolution of predators versus prey based upon their δ15N signature (Vander Zanden and Rasmussen 2001; Post 2002). Thus, the stable isotope signatures of carbon and nitrogen of an organism reflect what they eat. δ13C and δ15N values also vary between aquatic and terrestrial prey (e.g., Akamatsu et al. 2004). These values can be incorporated into linear mixing models, a quantitative method based on mass balance equations (Phillips and Gregg 2003), to quantify the relative proportion of different sources to an organism’s diet such as the proportional contribution of aquatic versus terrestrial insect prey (Bastow et al. 2002; Akamatsu et al. 2004).
The use of stable isotopes and linear mixing models to identify current trophic linkages presents an opportunity to help predict how the diets of terrestrial predators may shift with Arctic drying. We worked in western Greenland, where the surface area of freshwater ponds has declined by 15% in the past 50 years (Finger Higgens et al. 2019), to investigate the relative contributions of aquatic versus terrestrial prey in the diets of a dominant wolf spider species, Pardosa glacialis. In a preliminary study from three sites in Kangerlussuaq, Greenland in 2018, we found that P. glacialis collected near freshwater tundra ponds were significantly depleted in 13C compared to P. glacialis collected at paired upland locations with no ponds in proximity (Fig. 1; Culler et al. 2021). This suggested consumption of different food resources, e.g., more aquatic insects by spiders that forage near tundra ponds. Alternatively, the depletion in 13C of P. glacialis collected near ponds may be found if all prey taxa in that location, not just the aquatic insects, are also depleted in 13C. This would suggest that the diets of P. glacialis may be relatively robust to changes in freshwater habitats. Other factors, such as the relative abundances of soil invertebrates near versus far from ponds, may also play a role in observed differences in P. glacialis reproductive metrics (Culler et al. 2021).
Pardosa glacialis δ13C values (x axis) and δ15N values (y axis) in samples collected in 2018 (left, reproduced from Culler et al. 2021) and 2019 (right). P. glacialis from the pond locations were significantly depleted in 13C compared to upland locations (F1,47 = 23.9, p < 0.0001), and δ13C values did not vary by year (F1,47 = 0.02, p = 0.9). Location (F1,47 = 0.89, p = 0.35) and year (F1,47 = 0.18, p = 0.68) did not have significant effects on δ15N values. Small squares are individuals from the pond (brown) and upland (orange) locations. Circles are the mean values for the pond (brown) and upland (orange) locations with error bars representing ± 1 standard error
We tested these hypotheses by (1) quantifying the relative abundances of ground-dwelling soil invertebrates near ponds and at adjacent upland habitats, and (2) measuring the stable isotopes ratios of carbon and nitrogen in P. glacialis and in the main groups of arthropods that could serve as prey for P. glacialis in western Greenland. We considered aquatic, ground-dwelling, and canopy arthropods and tested if their δ13C and δ15N values varied across three sites and between pond and upland habitats to elucidate the diet of P. glacialis in wetter versus drier Arctic conditions. By deciphering the diet of this top predator across habitats, we aimed for better understanding how a warming, drying Arctic may alter its consumption patterns, impacting the flow of nutrients in a critical food web.
Methods
Study site
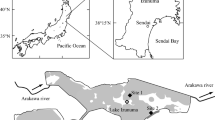
Field work was conducted in the tundra surrounding Kangerlussuaq, Greenland (67.01° N, 50.72° W) (Fig. 2) from 17 June to 20 July 2019, a period of time that coincides with peak arthropod activity and mosquito emergence (Culler et al. 2015, 2021). This area features low-lying shrubs (dwarf birch (Betula nana) and grey willow (Salix glauca)) and grasses adjacent to numerous smaller ponds and large freshwater lakes (Fig. 1, Heindel et al. 2015) that have overall been decreasing in extent since the 1960s (Finger Higgens et al. 2019). A 40-km road from the town of Kangerlussuaq to the margin of the Greenland Ice Sheet permitted access to field sites (Figs. 2, S1, Online Resource 1).
We selected three sites (Pond 1, Pond 1b, and Pond 3b; Fig. 2) that each had a shallow pond and were close to sites used previously by our research group (Fig. S1, Online Resource 1; Culler et al. 2015; DeSiervo et al. 2020; Culler et al. 2021). Each site had two locations: the margins of the pond and an upland location approximately 70–100 m away from the pond that was also separated by the road (Fig. S1, Online Resource 1).
Arthropod sampling overview
We used pitfall traps, sweep net sampling, soil extractions, and emergence traps from 24 June 2019 to 12 July 2019 to collect arthropods that could serve as prey for P. glacialis (Fig. 3). Potential prey were determined based on our field collections and previously known information about the feeding ecology and diets of P. glacialis (Wirta et al. 2015; Eitzinger et al. 2019). Previous studies have established that P. glacialis is known to consume multiple prey items including emerging aquatic insects, herbivores, detritivores, and conspecifics (Wirta et al. 2015; Eitzinger et al. 2019). We also hand-collected P. glacialis individuals between 24 June and 12 July from each of the locations (pond and upland) at each of the three sites.
The four techniques used to collect arthropods that could serve as prey for P. glacialis. Ground-dwelling prey were collected and quantified in pitfall traps, aquatic prey using sweep nets and emergence traps, and canopy prey using sweep nets. Ground-dwelling prey were extracted using Burlese (Tullgren) funnels to expose soil samples to an overhead heat source. All captures were sorted to the lowest practical taxonomic unit and counted. Created with BioRender.com
Composition and relative abundances of ground-dwelling prey
To collect, identify, and quantify potential ground-dwelling arthropod prey, we installed three pairs of pitfall traps at each pond and upland location at each site (Fig. S1, Online Resource 1). Three pairs were placed approximately equidistant along the margins of the pond (Fig. S1, Online Resource 1). At the upland sites, pairs of traps were placed randomly within similar vegetation of mixed grasses and dwarf shrubs. The two traps in a pair were spaced approximately 1 m apart. Each trap was a yellow plastic cup (5-cm radius, 10-cm depth) filled with water and a mild soap mixture. Due to their color, yellow cups may attract greater abundances of pollinating insects, such as bees and flies, potentially providing a bias for these taxa in our abundance data (Buchholz et al. 2010). However, we were primarily focused on ground-dwelling arthropods for which the color yellow is not likely to bias catches. Traps were installed on 24 June 2019 and sampled every 7 days until 12 July 2019 for a total of three collections (n = 108 traps). At each collection, we emptied the contents of each pair of pitfall traps into a container with 70% ethanol for storage. Pitfall samples were sorted in the lab, and all arthropods were counted and identified to the lowest practical taxonomic unit.
To assess if the composition of potential ground-dwelling arthropod prey varied among sites and between pond and upland locations, we conducted a MANOVA to test the effects of site (1, 1b, or 3b), location (pond or upland), and site * location on the abundances of the most common ground-dwelling taxa from the pitfall traps (proc glm, SAS v 9.4). We summed the total number of individuals of each taxon collected through the season in each replicate pair of traps and used a log(x + 1) transformation to normalize data prior to analysis. We also measured soil moisture at our sites to investigate relationships between soil moisture and ground-dwelling prey. At the second (7 July 2019) and third (12 July 2019) pitfall collection, we measured the soil moisture at each cup at each site’s near and upland locations (Delta-T HH2 moisture meter, Dynamax, Online Resource 2). From the soil moisture readings, we calculated the average soil moisture for each site’s near and upland location.
Additional prey collection
In addition to the quantitative pitfall sampling and analysis (described above), we aimed to identify additional prey taxa within aquatic and canopy habitats that would not easily be captured in a pitfall trap but may serve as a food resource for P. glacialis. We collected sweep net samples to identify potential prey taxa at each pond and upland habitat on 26 June, 1 July, and 12 July 2019. A sweep net sample typically consisted of approximately 3 min of sweeping the dominant vegetation type. Aquatic prey were further collected opportunistically by checking emergence traps that were installed on each of the three ponds. Additional potential prey from the soil community were identified by collecting 30 × 30 cm cubes of soil from each near and upland location at each site repeatedly over the season (n = 18 cubes). Soil samples were placed in Berlese (Tullgren) funnels with an overhead heat lamp for 12–24 h, which drove the arthropods out of the soil and downwards into a plastic collecting cup. Extractions were run a total of seven times. Due to time constraints, we were unable to quantify the density of prey collected using sweet nets, emergence traps, and Berlese (Tullgren) funnels. All arthropods from sweep, emergence, and extraction samples were frozen and later identified in the lab.
Stable isotope sample preparation and analysis
We prepared replicate samples of P. glacialis (n = 15 and n = 7 from pond and upland locations, respectively) and three replicate samples of each of 12 potential prey taxa from each location (pond or upland) from each site (1, 1b, or 3b) for stable isotope analysis. Springtails (Entognatha: Collembola) and beetle larvae (Insecta: Coleoptera) required pooling samples across sites and locations to reach the desired total biomass for stable isotope analysis. For smaller organisms, individuals were combined to reach ~ 1.0 mg and packaged in foil tins. Individual specimens over 1.0 mg were ground and reduced to 1.0 mg before packaging. 181 samples of P. glacialis and potential prey were sent to the Stable Isotope Facility at the University of California, Davis, for analysis of δ13C and δ15N.
We used linear mixed models to test if δ13C and δ15N of P. glacialis varied among the years of collection (2018 and 2019), pond and upland locations, and the interaction of year and location, including site as a random effect (proc mixed, SAS v 9.4). All other analyses were conducted with data collected in 2019 only. To test if the stable isotope signatures of prey taxa varied across sites and locations (pond or upland), we conducted a multiway analysis of variance (MANOVA) to test the effects of site (1, 1b, or 3b), location (pond or upland), and taxa on the δ13C and δ15N values of potential prey (proc glm, SAS v 9.4). The model included an interactive effect of taxa and location. We conducted an additional MANOVA to test if prey from the three habitats varied significantly in their δ13C and δ15N values (proc glm, SAS v 9.4). For both MANOVAs, we conducted post-hoc multiple means comparison tests with a Tukey-Cramer adjustment when appropriate. Some taxa were excluded from the MANOVAs due to a lack of replication.
Biplots of δ13C versus δ15N values of all taxa were created for each site to examine the alignment of signatures of P. glacialis with their potential prey. In addition, we used a three-source carbon and nitrogen isotope mixing model, IsoError, version 1.04 (Phillips and Gregg 2001), to determine the contribution of aquatic, ground-dwelling, and canopy prey sources to the diet of P. glacialis at the pond versus upland locations (all sites combined). The equations were:
where f is the mean proportion of each source in P. glacialis (the “mixture”), and α is the isotope fractionation coefficient during the feeding process, which we assumed to be + 1.0‰ and + 3.4‰ between spiders and their food sources for carbon and nitrogen, respectively (DeNiro and Epstein 1978, 1981; Minagawa and Wada 1984; McNabb et al. 2001; Vander Zanden and Rasmussen 2001; Oelbermann and Scheu 2002; Akamatsu et al. 2004). When f was estimated to be less than 0 for any given source by Eq. (1), we removed that source from the model (f = 0). The inputs to the model included the mean δ13C and δ15N isotopic signatures, standard deviations, and sample sizes for P. glacialis at either the pond or upland locations (2019 data only, the “mixtures”) and the mean δ13C and δ15N isotopic signatures, standard deviations, and sample sizes of prey individuals from the aquatic, ground-dwelling, and canopy habitats (2019 data, the three sources). The δ13C and δ15N isotopic signatures for prey individuals from each habitat were calculated by taking the average across all prey samples within each habitat, without weighting for relative abundance as this was not possible given our sampling effort, nor was it necessary input into the mixing model. We did not include P. glacialis as a potential source in this mixing model even though some studies report evidence of cannibalism (e.g., Koltz and Wright 2020).
Results
Spider and prey composition
From the pitfall trap, sweep net, emergence trap, and soil extraction sampling, we identified 12 taxa as potential prey, representing ground-dwelling, aquatic, and canopy habitats. We also confirmed that P. glacialis was the most common arthropod predator. Of the 5811 individuals collected in the pitfall traps, 326 were wolf spiders (Lycosidae, Arachnida: Araneae). P. glacialis was the most collected (87.3%) followed by Arctosa insignita (11.8%) and P. groenlandica (0.9%) (Fig. S2). 105 individuals were from six other families of Araneae (Theridiidae, Thomisidae, Linyphiidae, Philodromidae, Dictynidae, and Gnaphosidae) (Fig. S2). Of the remaining 5,485 individuals, 96% were in one of five ground-dwelling arthropod groups (Fig. 4), all of which were considered potential prey taxa for P. glacialis based on their previous studies (DeSiervo et al. 2020; Culler et al. 2021; Wirta et al. 2015): mites (Arachnida: Acari, n = 2678, 49% of all individuals), springtails (Entognatha: Collembola, n = 1356, 25% of all individuals), shore flies (Ephydridae, Insecta: Diptera, n = 502, 9% of all individuals), smaller/immature spider individuals (Arachnida: Araneae, n = 377, 7% of all individuals), and beetle larvae (Insecta: Coleoptera, n = 373, 7% of all individuals). Mites, springtails, and beetle larvae were also collected in the soil extraction samples. Three potential aquatic insect prey that were commonly collected in sweep nets and emergence traps in 2019 included adult mosquitoes (Culicidae), adult midges (Chironomidae), and adult caddisflies (Trichoptera). Caterpillars (Lepidoptera), leafhoppers (Cicadellidae), jumping plant lice (Psyllidae), and seed bugs (Lygaeidae) were the commonly collected potential canopy prey in the sweep nets and pitfall traps.
The abundance (per pair of traps) of the five most abundant ground-dwelling prey—mites, collembola, shore fly adults, small or immature spiders, and beetle larvae—collected at each site at the pond (brown) and upland (orange) locations. Total abundance (all taxa combined) is also shown on the right. Abundance is shown using a log(n + 1) transformation. Error bars are the back-transformed standard errors. The y-axes are scaled the same for comparative purposes
Composition and relative abundances of ground-dwelling prey
The abundances of the five ground-dwelling prey taxa varied significantly among sites and locations (pond vs. upland), with an overarching pattern that the pond locations had 3.7 times as many ground-dwelling individuals as the upland locations (Fig. 4). The overall MANOVA indicated a significant interaction of site and location on abundance (Wilks’ Lambda = 0.06, F10,16 = 5.12, p = 0.002), and this was driven entirely by patterns found in the mites, which were significantly more abundant at the pond location at Pond 1b and 3b but not at Pond 1 (F2,12 = 9.93, p = 0.003). Location was a significant predictor of ground-dwelling arthropod abundance in the overall MANOVA (Wilks’ Lambda = 0.15, F5,8 = 9.03, p = 0.004), attributable to higher relative abundances of springtails (F1,12 = 12.42, p = 0.004), shore flies (F1,12 = 21.17, p = 0.0006), beetle larvae (F1,12 = 9.37, p = 0.01), and mites (F1,12 = 18.57, p = 0.001) at the pond locations, where soil moisture tended to be higher (23.4–82.0%) compared to the upland sites (2.5–38.5%). There was no difference in abundances among locations of the smaller/immature spiders (F1,12 = 0.20, p = 0.66). Site was also a significant predictor of ground-dwelling arthropod abundance in the overall MANOVA (Wilks’ Lambda = 0.14, F10,16 = 2.65, p = 0.04). This was driven by patterns in springtails (F2,12 = 9.82, p = 0.003), which were significantly lower in abundance at Pond 3b, and mites (F2,12 = 4.98, p = 0.03), whose abundances varied significantly among the three sites (Fig. 4).
Variation in stable isotopes of Pardosa glacialis
Across 2018 and 2019, the mean (± SD) values of δ13C for P. glacialis were significantly lower at the pond locations (− 26.71 ± 1.09) compared to the upland locations (− 25.61 ± 0.94; F1,47 = 23.90, p < 0.0001; Fig. 1) with no variation explained by year (F1,47 = 0.02, p = 0.90) and no interactive effect of year and location (F1,47 = 0.31, p = 0.58). Location (F1,47 = 0.89, p = 0.35), year (F1,47 = 0.18, p = 0.68), and the interaction (F1,47 = 0.08, p = 0.78) did not have significant effects on δ15N values (4.9 ± 1.0 across years and locations; Fig. 1).
Variation in stable isotopes of prey
Stable isotope signatures of potential prey did not vary between pond and upland locations, but taxon was a significant predictor of δ13C and δ15N values (Wilks’ Lambda = 0.21, F18,208 = 13.48, p < 0.0001), as was site (Wilks’ Lambda = 0.46, F4,246 = 28.83, p < 0.0001). This was attributable to significant differences in δ13C (F9,105 = 2.72, p = 0.007) and δ15N (F9,105 = 32.63, p < 0.0001) among the potential prey taxa (Tables 1, 2) and significant differences in δ13C (F2,124 = 7.21, p = 0.001) and δ15N (F2,124 = 57.17, p < 0.0001) among taxa from the aquatic, ground-dwelling, and canopy habitats (Table 3). Aquatic taxa were significantly less enriched in 13C compared to ground-dwelling (p = 0.01) and canopy taxa (p = 0.001; Tables 1, 3; Fig. 5). Canopy taxa were significant less enriched in15N compared to aquatic (p < 0.0001) and ground-dwelling taxa (p < 0.0001; Tables 2, 3; Fig. 5). Due to the necessary pooling of springtail and beetle larvae samples to produce sufficient samples for stable isotope analysis, we lacked replicates for these taxa in our overall analysis. However, we note that their δ13C signatures were approximately equal to those of other ground-dwelling taxa including smaller spiders and mites (Table 1).
The δ13C (x axis) and δ15N (y axis) signatures of potential prey from the aquatic (blue), soil (brown), and canopy (green) habitats. Also shown are the δ13C and δ15N signatures of P. glacialis collected in 2019 at the pond and upland locations. Error bars are 1SEM. δ13C alignment and a 2 per mil difference of δ15N between P. glacialis and potential prey suggests prey could be a potential resource base for the predator
Compared to other ground-dwelling taxa, beetle larvae were relatively enriched, and springtails were relatively depleted in 15N (Table 2), indicating different trophic levels within the ground-dwelling community. Site was also a significant predictor of δ13C and δ15N values in the overall MANOVA (Wilks’ Lambda = 0.88, F4,208 = 3.39, p = 0.01) but this was driven entirely by patterns in δ13C (F2,105 = 4.55, p = 0.01). Prey were significantly enriched in 13C at Pond 3b (-26.6 ± 0.38) compared to Pond 1 (− 27.8 ± 0.38) and Pond 1b (− 28.0 ± 0.44), likely due to aquatic taxa, and especially adult midges, collected at Pond 3b that were significantly enriched in 13C compared to those from other locations (Fig. 5). The overall MANOVA indicated no effect of location (Wilks’ Lambda = 0.97, F2,104 = 1.45, p = 0.24) and no interaction between taxa and location (Wilks’ Lambda = 0.92 F18,208 = 0.46, p = 0.97) on δ13C and δ15N values.
Mixing model results and biplot analysis
Visual examination of the biplots and the mixing models provided two lines of evidence that feeding on aquatic insects at the pond locations may explain the depleted δ13C values of P. glacialis compared to upland location (δ13CPond = − 26.6 ± 0.3 (SE), δ13CUpland = − 26.0 ± 0.3 (SE); Table 3, Fig. 1). Aquatic insects indeed had lower δ13C values compared to the ground-dwelling and canopy taxa (Fig. 5, Table 1). The mean relative contribution of aquatic taxa to the diets of P. glacialis at pond locations was estimated to be 23.1%, although we note that the 95% confidence interval for this estimate overlapped 0 (Table 3). In contrast, at the upland location, the mean relative contribution of aquatic taxa was estimated as 0% from the three-source mixing model (Table 3). Interestingly, at Pond 3b, midges were significantly enriched in 13C (Fig. 5, see Discussion) and although not significant, the δ13C values of P. glacialis at this site seemed to track this enrichment, further suggesting that aquatic insects are part of their diet.
The δ13C values of P. glacialis at both the pond and upland locations also aligned with the δ13C values of potential prey from the soil and canopy habitats (in Fig. 5, see alignment of δ13C values of P. glacialis with those of the brown and green polygons), except at Pond 3b. The mixing model results suggest that P. glacialis was consuming a mixture of prey from different habitats (Table 3). Our results suggest that ground-dwelling taxa may contribute substantially to the diet of P. glacialis as they had the highest contributions to the diets of P. glacialis at both the pond (46.9%, 95%CI 0–100%) and upland locations (63.4%, 95%CI 43.7–83.1%) relative to aquatic and canopy taxa (Table 3). We note significant error associated with these estimates. Canopy taxa were estimated to make up 30.0% (95%CI 15.4–44.7%) and 35.9% (95%CI 15.8–55.9%) of the diets of P. glacialis at pond and upland locations, respectively (Table 3).
Biplot analysis further deciphered relationships between potential prey and P. glacialis on a site-by-site basis. At the Pond 1b site, the depleted δ13C values of P. glacialis near the pond (δ13CPond, Site 1b = − 27.2 ± 0.3 (SE)) could also be explained by the consumption of mites, which were very abundant near the pond (Fig. 4), relatively depleted in 13C near the pond (δ13C = − 29.4 ± 1.4) compared to other ground-dwelling taxa (Fig. 5), and approximately one trophic position lower than P. glacialis near the pond (δ15Nmites = 2.1 ± 1.4; δ15NP. glacialis = 5.3 ± 0.4; Fig. 5). At Pond 1b, the δ13C and δ15N values of P. glacialis at the upland location overlapped mostly with seed bugs from the canopy community (Fig. 4), which happened to be very abundant at that location (Bliska and Culler, personal observations). At Pond 3b, mites and shore flies were abundant ground-dwelling prey at the pond location (Fig. 4) and may be likely prey for P. glacialis based on the alignment of their δ13C and δ15N values with those of P. glacialis (Fig. 5).
Discussion
Our two key findings are that ground-dwelling prey were nearly four times as abundant near Arctic ponds than in drier upland locations and that aquatic insects are likely an important food resource for P. glacialis that forage near ponds. These findings, coupled with earlier research suggesting that P. glacialis near ponds have larger clutches (Culler et al. 2021), suggests that freshwater habitats and the organisms they support play an important role in the population dynamics of P. glacialis, a prolific Arctic predator.
Our initial motivation for this study was driven by field and lab observations of P. glacialis consuming mosquitoes and preliminary stable isotope data suggesting diet variation attributable to this aquatic-terrestrial linkage (Culler et al. 2021). We wished to quantify the proportion of P. glacialis diets coming from aquatic versus terrestrial sources. However, our results have led to a much more compelling and comprehensive understanding of the role that freshwater habitats play in the broader tundra food web. Freshwater habitats indeed support a diversity of aquatic and semi-aquatic organisms that are consumed by P. glacialis and other aquatic and terrestrial predators. However, our pitfall trap sampling additionally showed increased abundances of ground-dwelling and soil invertebrate taxa, especially for mites and collembola, adjacent to freshwaters where soil moisture was higher. Both mites and collembola are cited as important food sources for wolf spiders (Eitzinger et al. 2019). When considering declines in Arctic freshwater habitats, it is necessary to consider impacts on aquatic subsidies but also how the loss of these habitats affects nearby soil and ground-dwelling invertebrate communities that also support higher trophic levels. Soil organisms such as collembola may be particularly sensitive to climate change because of the effect that temperature has on soil moisture (Hodkinson et al. 1998; Koltz et al. 2018b).
The stable isotope signatures of carbon and nitrogen were significantly different among prey from the aquatic, soil, and canopy habitats and provided important inputs into the mixing model. As predicted, the three aquatic taxa considered in this study (mosquitoes, caddisflies, and midges) were generally depleted in 13C compared to our five terrestrial ground-dwelling taxa (mites, shore flies, small/immature spiders, beetle larvae, and collembola) and our four canopy taxa (caterpillars, leafhoppers, jumping plant lice, and seed bugs). δ15N values were less useful at distinguishing aquatic insects because the values were not statistically different from those of the soil invertebrates. Aquatic and terrestrial organisms vary in their δ13C signatures due to differences in carbon isotope fractionation in water compared to plants and soil (Bastow et al. 2002; Akamatsu et al. 2004). Within the signal for aquatic insects, we found significant site-level variation in δ13C for midges (Chironomidae, Fig. 5). Because we did not identify midges beyond the family level, we assume we captured and analyzed multiple species that have different feeding strategies (herbivorous and predaceous species), contributing to variable stable isotope signatures. Species-level variation in stable isotopes has been documented in this family (Reuss et al. 2013) and should be considered in future studies if the objective is to determine food web linkages at a finer resolution. Mosquitoes and caddisflies demonstrated less site-level variation in δ13C and had consistently depleted δ13C signatures relative to terrestrial taxa (Fig. 5). In sum, the δ13C and δ15N values of the prey taxa and their degree of alignment with the δ13C and δ15N values of P. glacialis provided an important visualization that could be compared to our mixing model results, which had significant uncertainty.
When we input our stable isotope data into the mixing models, we found that aquatic insects constituted about 20% of P. glacialis diet at pond margins compared to 0% at upland locations. We note significant uncertainty with these estimates (Table 3), and therefore do not base our interpretation on the mixing model alone. Other studies have documented wolf spiders consuming adult midges and mosquitoes, along with other emerging aquatic insects (Wirta et al. 2015; Eitzinger et al. 2019). A previous study in Kangerlussuaq noted that P. glacialis can consume up to eight mosquitoes per day (Culler et al. 2021) and we found a stronger alignment of P. glacialis near ponds with the stable isotope values of aquatic insects. While we are not able to quantify with confidence the relative proportion of P. glacialis diet coming from aquatic insects, we do assert aquatic insects are a food resource for those individuals foraging near ponds. These aquatic insects are rich in carbon and nitrogen and known to serve as resource subsidies to terrestrial predators in many systems (Akamatsu et al. 2004; Kato et al. 2004; Krell et al. 2015).
Our mixing model and stable isotope analyses revealed that terrestrial taxa also contribute to the diets of P. glacialis. The δ13C and δ15N signatures of canopy and ground-dwelling taxa were very distinct from one another (Tables 1, 2), which can be attributed to variation in δ13C and δ15N in their basal food sources (plants versus dead organic matter) (Marakov 2008; Craine et al. 2009; Tahmasebi et al. 2017). Although the δ15N values of canopy taxa were far below the δ15N signatures of P. glacialis (Fig. 5), the mixing model indicated that canopy taxa made up approximately a third of their diets at all locations, with high certainty (Table 3) compared to the soil and aquatic taxa. Our biplots revealed that all ground-dwelling taxa were likely prey for P. glacialis (Fig. 3), and the mixing models estimated the mean relative contribution of ground-dwelling taxa to be about 63% and 47% at the upland and pond locations, respectively (Table 3), though significant uncertainty prevents confidence in the exact magnitude of these estimates. Based on their abundance in pitfall traps (~ 75% of all captures), we hypothesize that springtails and mites are particularly important in the diets of P. glacialis. This is consistent with findings from Alaska, where researchers estimated that surface-dwelling predators like Lycosidae derived approximately 46% of their energy resources from the soil food web, noting that springtails and mites were particularly important for food web stability (Koltz et al. 2018a).
Our study offers a preliminary and non-exhaustive list of 12 taxa that may be prey for P. glacialis in the Kangerlussuaq area. Several taxa in our study were confirmed as prey for P. glacialis in East Greenland using DNA barcoding (Eitzinger et al. 2019), including Diptera (Culicidae and Chironomidae), Acari, Lepidoptera, Collembola, Hemiptera (Lygaeidae), and Araneae. Other prey that we included in this study, including two families of Hemiptera (Psyllidae, Cicadellidae), Trichoptera, Coleoptera, and Diptera (Ephydridae) were not confirmed with DNA barcoding. These taxa may not be present in East Greenland and/or they may not be a food resource for P. glacialis, which is a hypothesis that would require extensive field sampling, feeding trials, or the use of DNA barcoding at our site. A comparison using DNA barcoding of P. glacialis diets among Arctic locations and habitat types would reveal additional information that would support further exploration of the primary research objective in this study. DNA barcoding would also help us identify potential prey taxa that were missed with our field sampling.
Overall, our results suggest that aquatic insects are likely consumed by the most common terrestrial arthropod predator, P. glacialis. If aquatic insects are indeed serving as aquatic subsidies in this system, this linkage between aquatic and terrestrial systems is likely threatened by the continued disappearance of aquatic habitats. Prior research has shown that P. glacialis near tundra ponds had larger clutches (Culler et al. 2021), which could result from access along the margins of ponds to nutritious aquatic insects that subsidized reproduction. Upland from tundra ponds (i.e., not within access to emerging aquatic insects), P. glacialis produced 20% fewer eggs per egg sac (Culler et al. 2021), suggesting that P. glacialis may experience declines in reproduction and abundance because of decreases in aquatic subsidies in a warmer and drier Arctic. Closely related Lycosidae species, such as P. groenlandica, which are found in warmer and drier habitats (Culler et al. 2021), may become more dominant as the climate continues to change. Shifting population dynamics of wolf spiders may affect higher trophic levels in the Arctic food web, as birds and other terrestrial predators are known to consume spiders (Wirta et al. 2015), as well as insects that spiders consume.
As freshwater habitats decline in the Arctic (Webb et al. 2022), P. glacialis may increasingly on ground-dwelling and other terrestrial prey. Our study indicated that ground-dwelling taxa, including soil arthropods, may already play an important role in transferring carbon and nitrogen to upper trophic levels through predation by P. glacialis in upland areas. Prior research has similarly shown that the soil food web contributes significantly to carbon and nitrogen cycling in the Arctic (Koltz et al. 2018a). Our results suggest that the role of the soil food web in providing energy sources to terrestrial predators may even expand in a drier Arctic. Importantly, ground-dwelling prey will too experience consequences from a drier and warmer Arctic. Recent research has demonstrated that changes in freeze–thaw cycling caused by warming in the High Arctic have negatively impacted springtail abundance and species diversity (Koltz et al. 2018b; Gillespie et al. 2020). Surface detritivores are expected to decrease in abundance, and the impacts of warming have been projected to affect arthropod community composition about five times greater in drier habitats than in wetter habitats (Koltz et al. 2018b). Additionally, nitrogen availability is expected to further decline with declines in soil moisture and increases temperature in Greenland (Finger Higgens et al. 2021), which could have further indirect effects on soil-dwelling invertebrates. This necessitates further investigations as to the expanding role of ground-dwelling organisms in the diets of terrestrial predators and how this role may be tempered by climatic controls on their own populations.
Our study is one of the first to directly examine how a warming and drying Arctic may impact a top-order terrestrial predator via changes in prey availability. The decline in freshwater habitats, widespread surface water drying due to rapid permafrost thaw and subsequent drainage (Webb et al. 2022), and other related biotic changes (e.g., shrubification, Asmus et al. 2018) will cause changes in the relative abundances of aquatic, soil-dwelling, and canopy arthropods and the species within those habitats. Generalist predator species such as P. glacialis will probably respond by shifting their diets but may nonetheless experience declines in abundance (Culler et al. 2021). Although individual wolf spiders are small, in total they have about 80-fold higher biomass per hectare than mammalian predators in the Arctic (Koltz et al. 2018c). It follows that changes in their abundance can have cascading effects on ecosystem function (Koltz et al. 2018c), as well as on higher tropic levels that rely on wolf spiders for food (Wirta et al. 2015).
Data availability
Data are available in Online Resource 2.
References
Akamatsu F, Toda H, Okino T (2004) Food source of riparian spiders analyzed by using stable isotope ratios. Ecol Res 19:655–662. https://doi.org/10.1111/j.1440-1703.2004.00680.x
Asmus AL, Chmura HE, Høye TT, Krause JS, Sweet SK, Perez JH, Gough L (2018) Shrub shading moderates the effects of weather on arthropod activity in arctic tundra. Ecol Entomol 43:647–655. https://doi.org/10.1111/een.12644
Bastow JL, Sabo JL, Finlay JC, Power ME (2002) A basal aquatic-terrestrial trophic link in rivers: algal subsidies via shore-dwelling grasshoppers. Oecologia 131:261–268. https://doi.org/10.1007/s00442-002-0879-7
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 42:411–440. https://doi.org/10.1146/annurev-ecolsys-102209-144726
Buchholz S, Jess AM, Hertenstein F, Schirmel J (201) Effect of the colour of pitfall traps on their capture efficiency of carabid beetles (Coleoptera: Carabidae), spiders (Araneae) and other arthropods. Eur J Entomol 107: 277–280. https://doi.org/10.14411/eje.2010.036
Cooper MJ, Lamberti GA, Uzarski DG (2014) Spatial and temporal trends in invertebrate communities of Great Lakes coastal wetlands, with emphasis on Saginaw Bay of Lake Huron. J Great Lakes Res 40:68–182. https://doi.org/10.1016/j.jglr.2013.12.003
Craine JM, Elmore AJ, Aidar MP et al (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–992. https://doi.org/10.1111/j.1469-8137.2009.02917.x
Crump BC, Kling GW, Bahr M, Hobbie JE (2003) Bacterioplankton community shifts in an arctic lake correlate with seasonal changes in organic matter source. Appl Environ Microbiol 69:2253–2268. https://doi.org/10.1128/AEM.69.4.2253-2268.2003
Culler LE, Ayres MP, Virginia RA (2015) In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proc R Soc B 282:20151549. https://doi.org/10.1098/rspb.2015.1549
Culler LE, Stendahl AM, DeSiervo MH, Bliska HM, Virginia RA, Ayers MP (2021) Emerging mosquitoes (Aedes nigripes) as a resource subsidy for wolf spiders (Pardosa glacialis) in western Greenland. Polar Biol. https://doi.org/10.1007/s00300-021-02875-8
Culp JM, Goedkoop W, Christensen T, Christoffersen KS, Fefilova E, Liljaniemi P, Lento J (2022) Arctic freshwater biodiversity: establishing baselines, trends, and drivers of ecological change. Freshw Biol 67:1–13. https://doi.org/10.1111/fwb.13831
Danks HV, Corbet PS (1973) Sex ratios at emergence of two species of high-arctic Aedes (Diptera: Culicidae). Can Entomol 105:647–651. https://doi.org/10.4039/Ent105647-4
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506. https://doi.org/10.1016/0016-7037(78)90199-0
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351. https://doi.org/10.1016/0016-7037(81)90244-1
DeSiervo MH, Ayres MP, Virginia RA, Culler LE (2020) Consumer–resource dynamics in Arctic ponds. Ecology 101:e03135. https://doi.org/10.1002/ecy.3135
DeSiervo MH, Finger-Higgens RA, Ayres MP, Virginia RA, Culler LE (2022) Spatial and temporal patterns in Arctic mosquito abundance. Ecol Entomol 48:19–30. https://doi.org/10.1111/een.13198
Eitzinger B, Abrego N, Gravel D, Huotari T, Vesterinen EJ, Roslin T (2019) Assessing changes in arthropod predator–prey interactions through DNA-based gut content analysis—variable environment, stable diet. Mol Ecol 28:266–280. https://doi.org/10.1111/mec.14872
Finger Higgens RA, Chipman JW, Lutz DA, Culler LE, Virginia RA, Ogden LA (2019) Changing lake dynamics indicate a drier Arctic in Western Greenland. J Geophys Res Biogeosci 124:870–883. https://doi.org/10.1029/2018JG004879
Gillespie MAK, Alfredsson M, Barrio IC et al (2020) Status and trends of terrestrial arthropod abundance and diversity in the North Atlantic region of the Arctic. Ambio 49:718–731. https://doi.org/10.1007/s13280-019-01162-5
Grosse G, Jones BM, Arp CD (2013) Thermokarst lakes, drainage, and drained basins. In: Shroder JF (ed) Treatise on geomorphology, vol 8. Elsevier, Amsterdam, pp 325–353
Heindel RC, Chipman JW, Virginia RA (2015) The spatial distribution and ecological impacts of aeolian soil erosion in Kangerlussuaq, West Greenland. Ann Assoc Am Geogr 105:875–890. https://doi.org/10.1080/00045608.2015.1059176
Higgens RF, Pries CH, Virginia RA (2021) Trade-offs between wood and leaf production in arctic shrubs along a temperature and moisture gradient in West Greenland. Ecosystems 24:652–666. https://doi.org/10.1007/s10021-020-00541-4
Hobbie JE, Peterson BJ, Bettez N et al (1999) Impact of global change on the biogeochemistry and ecology of an Arctic freshwater system. Polar Res 18:207–214. https://doi.org/10.3402/polar.v18i2.6576
Hodkinson ID, Webb NR, Bale JS, Block W, Coulson SJ, Strathdee AT (1998) Global change and Arctic ecosystems: conclusions and predictions from experiments with terrestrial invertebrates on Spitsbergen. Arctic Alpine Res 30:306–13
Kaneda S, Kaneko N (2011) Influence of Collembola on nitrogen mineralization varies with soil moisture content. Soil Sci Plant Nutr 57:40–49
Kato C, Iwata T, Wada E (2004) Prey use by web-building spiders: stable isotope analyses of trophic flow at a forest-stream ecotone. Ecol Res 19:633–643. https://doi.org/10.1111/j.1440-1703.2004.00678.x
Knopp JA, Levenstein B, Watson A, Ivanova I, Lento J (2022) Systematic review of documented Indigenous Knowledge of freshwater biodiversity in the circumpolar Arctic. Freshw Biol 67:194–209
Koltz AM, Wright JP (2020) Impacts of female body size on cannibalism and juvenile abundance in a dominant arctic spider. J Anim Ecol 89:1788–1798
Koltz AM, Asmus A, Gough L, Pressler Y, Moore JC (2018a) The detritus-based microbial-invertebrate food web contributes disproportionately to carbon and nitrogen cycling in the Arctic. Polar Biol 41:1531–1545. https://doi.org/10.1007/s00300-017-2201-5
Koltz AM, Schmidt NM, Høye TT (2018b) Differential arthropod responses to warming are altering the structure of Arctic communities. R Soc Open Sci 5:171503. https://doi.org/10.1098/rsos.171503
Koltz AM, Classen AT, Wright JP (2018c) Warming reverses top-down effects of predators on belowground ecosystem function in Arctic tundra. PNAS 115:E7541–E7549. https://doi.org/10.1073/pnas.1808754115
Krell B, Röder N, Link M, Gergs R, Entling MH, Schäfer RB (2015) Aquatic prey subsidies to riparian spiders in a stream with different land use types. Limnologica 51:1–7. https://doi.org/10.1016/j.limno.2014.10.001
Laske SM, Gurney KEB, Koch JC, Schmutz JA, Wipfli MS (2021) Arctic insect emergence timing and composition differs across thaw ponds of varying morphology. Arct Antarct Alp Res 53:110–126. https://doi.org/10.1080/15230430.2021.1902249
McNabb DM, Halaj J, Wise DH (2001) Inferring trophic positions of generalist predators and their linkage to the detrital food web in agroecosystems: a stable isotope analysis. Pedobiologia 45:289–297. https://doi.org/10.1078/0031-4056-00087
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140. https://doi.org/10.1016/0016-7037(84)90204-7
Murakami M, Nakano S (2002) Indirect effect of aquatic insect emergence on a terrestrial insect population through by birds predation. Ecol Lett 5:333–337. https://doi.org/10.1046/j.1461-0248.2002.00321.x
Oelbermann K, Scheu S (2002) Stable isotope enrichment (δ 15N and δ 13C) in a generalist predator (Pardosa lugubris, Araneae: Lycosidae): effects of prey quality. Oecologia 130:337–344. https://doi.org/10.1007/s004420100813
Paetzold A, Schubert CJ, Tockner K (2005) Aquatic terrestrial linkages along a braided-river: riparian arthropods feeding on aquatic insects. Ecosystems 8:748–759. https://doi.org/10.1007/s10021-005-0004-y
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127:171–179. https://doi.org/10.1007/s004420000578
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269. https://doi.org/10.1007/s00442-003-1218-3
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Prowse TD, Wrona FJ, Reist JD, Gibson JJ, Hobbie JE, Lévesque LM, Vincent WF (2006) Climate change effects on hydroecology of Arctic freshwater ecosystems. Ambio 35:347–358. https://doi.org/10.1579/0044-7447(2006)35[347:CCEOHO]2.0.CO;2
Rantanen M, Karpechko AY, Lipponen A, Nordling K, Hyvärinen O, Ruosteenoja K, Vihma T, Laaksonen A (2022) The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ 3:168. https://doi.org/10.1038/s43247-022-00498-3
Reuss NS, Hamerlík L, Velle G, Michelsen A, Pedersen O, Brodersen KP (2013) Stable isotopes reveal that chironomids occupy several trophic levels within West Greenland lakes: implications for food web studies. Limnol Oceanogr 58:1023–1034. https://doi.org/10.4319/lo.2013.58.3.1023
Sabo JL, Power ME (2002) River–watershed exchange: effects of riverine subsidies on riparian lizards and their terrestrial prey. Ecology 83:1860–1869. https://doi.org/10.1890/0012-9658(2002)083[1860:RWEEOR]2.0.CO;2
Sanzone DM, Meyer JL, Martí E, Gardiner EP, Tank JL, Grimm NB (2003) Carbon and nitrogen transfer from a desert stream to riparian predators. Oecologia 134:238–250. https://doi.org/10.1007/s00442-002-1113-3
Tahmasebi F, Longstaffe FJ, Zazula G, Bennett B (2017) Nitrogen and carbon isotopic dynamics of subarctic soils and plants in southern Yukon Territory and its implications for paleoecological and paleodietary studies. PLoS ONE 12:e0183016. https://doi.org/10.1371/journal.pone.0183016
Verhoef HA, Van Selm AJ (1983) Distribution and population dynamics of Collembola in relation to soil moisture. Ecography 6:387–388
Visser ME, Holleman LJ (2001) Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc R Soc B 268:289–294. https://doi.org/10.1098/rspb.2000.1363
Webb EE, Liljedahl AK, Cordeiro JA, Loranty MM, Witharana C, Lichstein JW (2022) Permafrost thaw drives surface water decline across lake-rich regions of the Arctic. Nat Clim Change 12:841–846. https://doi.org/10.1038/s41558-022-01455-w
Wirta HK, Weingartner E, Hambäck PA, Roslin T (2015) Extensive niche overlap among the dominant arthropod predators of the High Arctic. Basic Appl Ecol 16:86–92. https://doi.org/10.1016/j.baae.2014.11.003
Zanden MJV, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066. https://doi.org/10.4319/lo.2001.46.8.2061
Acknowledgements
We thank Angie Spickard and Alex Stendahl for help with field work, the CH2MHill Polar Services team for excellent logistical support and Naalakkersuisut (the Government of Greenland) for permission to conduct research in Greenland. We also thank AA, BB, and CC for providing valuable feedback on this manuscript during the review process.
Funding
This study was supported by a U.S. National Science Foundation award to LEC, MPA, and RAV (#1748137). Additional funding was provided to HMB through a Raynolds International Expedition Grant from the John Sloan Dickey Center for International Understanding at Dartmouth College and the Jerry Manne Fund in the Department of Environmental Studies at Dartmouth College. This material is based upon work supported by (while LEC was serving at) the National Science Foundation.
Author information
Authors and Affiliations
Contributions
H.B., L.C., M.A., and R.V. designed the research project. H.B., L.C., M.A., and M.D. conducted field experiments and collected all samples. H.B. and L.C. processed stable isotope samples and analyzed the data. L.C. and M.D. created data visualizations. H.B. and L.C. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the special issue on the “Pathways and impacts of biotically-mediated marine and other stored nutrient transfer between polar ecosystems”, coordinated by Peter Convey, Katarzyna Zmudczyńska-Skarbek, and Stef Bokhorst.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bliska, H.M., DeSiervo, M.H., Virginia, R.A. et al. Declining freshwater habitats in Greenland may shift diets of the ubiquitous wolf spider Pardosa glacialis. Polar Biol (2024). https://doi.org/10.1007/s00300-024-03259-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00300-024-03259-4