Abstract
Terrestrial animals are key elements in the cycling of elements in the Arctic where nutrient availability is low. Waste production by herbivores, in particular urine deposition, has a crucial role for nitrogen (N) recycling, still, it remains largely unexplored. Also, experimental evidence is biased toward short-term studies and Arctic regions under high herbivore pressure. In this study, we aimed to examine the fate of N derived from urine in a nutrient poor tundra heath in West Greenland, with historical low level of herbivory. We performed a pulse labelling with 15N-urea over the plant canopy and explored ecosystem N partition and retention in the short-term (2 weeks and 1 year) and longer-term (5 years). We found that all vascular plants, irrespective of their traits, could rapidly take up N-urea, but mosses and lichens were even more efficient. Total 15N enrichment was severely reduced for all plants 5 years after tracer addition, with the exception of cryptogams, indicating that non-vascular plants constituted a long-term sink of 15N-urea. The 15N recovery was also high in the litter suggesting high N immobilization in this layer, potentially delaying the nutrients from urine entering the soil compartment. Long-term 15N recovery in soil microbial biomass was minimal, but as much as 30% of added 15N remained in the non-microbial fraction after 5 years. Our results demonstrate that tundra plants that have evolved under low herbivory pressure are well adapted to quickly take advantage of labile urea, with urine having only a transient effect on soil nutrient availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Permafrost-affected soils are large reservoirs of organic matter and nitrogen (N) (Harden et al. 2012; Hugelius et al. 2014; Voigt et al. 2020). Although recent evidence suggests hotspots of N availability in some Arctic ecosystems such as in nearshore coastal zones (Sanders et al. 2022) and permafrost peatlands (Ramm et al. 2022), most Arctic soils are strongly N limited with small fractions of labile inorganic N (Templer et al. 2012; Maslov and Makarov 2016). Long cold winter season with below-zero temperatures, cool summer and high soil acidity constrain microbial activity and limit N mineralization in tundra soils (Robinson 2001; Weintraub and Schimel 2003; Morsdorf et al. 2019). Despite reports of direct uptake of low molecular weight amino acids or proteins by tundra plants (Chapin et al. 1993; Schimel and Chapin 1996; Hill et al. 2011; Ravn et al. 2017), most soil N in Arctic soils are bound into complex organic compounds and are not directly available for plant uptake (Kielland 1994; Neff et al. 2003; Read and Perez-Moreno 2003). In addition, a large proportion of N is also immobilized in soil microbial biomass and plant–microbe competition for soil available N is fierce (Jonasson et al. 1996; Michelsen et al. 1999; Liu et al. 2018). In ecosystems with such constraints on nutrient availability, any mechanism acting on N sources and N recycling would profoundly affect plant productivity and soil biogeochemical cycles.
Herbivores play a key role in a large range of ecosystems by strongly influencing plant community composition and nutrient cycling via three main mechanisms occurring simultaneously; selective foraging, vegetation and soil trampling and excreta deposition (Olff and Ritchie 1998; Bardgett and Wardle 2003; Pastor and Cohen 2006). While senescent vegetation enters the soil as slowly-decomposing litter, herbivores convert vegetation into labile and nutrient rich dung and urine from which nitrogen is released rapidly into the soil thus stimulating microbial activity, soil nutrient availability, and plant productivity (Bazely and Jefferies 1985; Day and Detling 1990; Hobbs 1996; Frank et al. 2004). Plants can take up urea CO(NH2)2, the main N compound in urine, directly through foliage absorption (Uscola et al. 2014) or in the soil by root absorption of either intact molecule of urea (Fujii and Hayakawa 2022) with the presence of high and low affinity transporters in plant cells (Wang et al. 2008) or in mineralized forms (Mugasha and Pluth 1994). Both in plants and in the soil, urea is rapidly hydrolyzed into ammonium by urea degrading enzymes and in plant cells N compounds derived from urea are later assimilated by glutamine synthetase (Wang et al. 2008). Experimental evidence of the role of urine for N cycling in natural ecosystems is scarce, particularly at high latitude environments, with most evidence emerging from highly managed agricultural systems where urea is applied as a fertilizer at high doses.
Barthelemy et al. (2018) explored the short-term fate of urea, in a tundra heath and a graminoid dominated vegetation in northern Norway, with both sites under a high herbivore pressure by a large population of reindeer (albeit a lower density in the tundra health) and many species of rodents with cyclic population outbreaks. Urea was applied as trace amounts of 15N-urea over the plant canopy to simulate urine deposition, a method to follow the fate of 15N-urea without altering its natural behavior in the ecosystem (Robinson 2001). A quick and high 15N recovery in plant canopy was observed shortly after tracer addition, with low 15N-recovery in soil and microbial communities (Barthelemy et al. 2018). This study demonstrated that tundra plants under a high herbivore pressure seem to be able to rapidly benefit from N compounds derived from urine and that urea is an important element in the N budget of naturally grazed tundra ecosystems. The fate of N-urea is unclear for ecosystems with low herbivore density where plants could be less adapted to take advantage of N derived from urine excretion (i.e. lower urea transport activity). However, it is also possible that plant uptake of N-urea could be greater for tundra plants experiencing lower levels of herbivore disturbance (i.e. less defoliation and trampling) which can reduce foliar and root nutrient absorption. Tundra ecosystems are highly sensible to the ongoing rapid and dramatic climate change (IPCC 2013) and changes in Arctic herbivore activity, density and distribution have been reported (Koltz et al. 2022). As the Arctic warms, shifts in geographical range for small and large mammal populations are expected (Baltensperger and Huettmann 2015; Mallory and Boyce 2018; Cuyler et al. 2020) and can give herbivores access to new territories with previously minor herbivore pressure or no mammal herbivory history with consequences for biogeochemical cycles, particularly N recycling.
Tundra ecosystems host a large variety of plant functional groups with strikingly different traits and life strategies (Bjorkman et al. 2018). Plant coexistence in nutrient-poor environments is achieved by a strong resource partitioning of available nutrients in space (i.e. root depth), in time (i.e. differential uptake during the growing season) and chemical forms (i.e. organic and inorganic forms) (McKane et al. 2002). Mosses and lichens, ubiquitous in tundra, take up most of their nutrients from the atmosphere and from wet or dry deposition (Jonsdottir et al. 1995; Eckstein and Karlsson 1999; Dahlman et al. 2004; Ayres et al. 2006; Krab et al. 2008), decompose slowly and show high internal nutrient recycling (Eckstein 2000; Lang et al. 2009). Hence, they likely capture most of the incoming N compounds from urine and retain it for a long time thus reducing N uptake by microbes and vascular plants. In tundra, a large proportion of vascular plants are associated with mycorrhizal fungi (Michelsen et al. 1996, 1998; Barthelemy et al. 2017) that provide an advantage for N uptake from the dominating organic N sources compared to non-mycorrhizal vascular plants, which rely mainly on inorganic N sources (Read and Perez-Moreno 2003; Schimel and Bennett 2004). In the short term, different tundra plant species seem to be equally efficient in the uptake and retention of 15N-urea (Barthelemy et al. 2018). However, in the long term, 15N compounds derived from urine may eventually enter the soil compartment through decomposition of 15N-enriched litter and become available for uptake by vascular plants and soil microorganisms. Then, differences in partitioning of 15N from urine should be observed between mycorrhizal plants with shallow root systems close to the surface layer and non-mycorrhizal plants with deeper root systems. Soil microbial communities might also efficiently compete for incoming N derived from urine.
Ecosystem N retention, the ability of plant-soil-microbe pools to retain N, is an important function of terrestrial ecosystems (de Vries and Bardgett 2016; Wang et al. 2018). Understanding at which proportion incoming N can be lost to the surroundings by leaching or volatilization is vital in tundra ecosystems where microbial decomposition and mineralization activities are strongly limited. In the Arctic, belowground biomass is proportionally high and a large fraction the ecosystem N pool is found in microbial biomass and decomposing litter (Jonasson et al. 1999; Poorter et al. 2012; Iversen 2014; Zhu et al. 2016). Hence, there is a crucial need for studies exploring N retention in aboveground and belowground part of the system. Interactions between plants and soil microbes are likely to be vital in controlling retention and loss of incoming nutrients from urine. Further, urea volatilization rates following urine excretion and nutrient leaching toward deeper soil layers are also important factors in determining N-urea retention in the system. Retention of urine-derived N may be large in ecosystems dominated by slow growing and resource conservative plant species and their associated fungal microbial communities and provide a long-term source of nutrients supporting plant productivity. Long-term ecosystem N retention studies in the Arctic are rare, and to our knowledge, the fate of N from urine in the plant–microbe-soil compartments of tundra ecosystem in longer terms has never been documented.
In this study, we explored the N availability and ecosystem stocks by analyzing 15N natural abundance (δ15N) and N pool sizes in a mesic, nutrient-poor tundra heath in Greenland. We furthermore analyzed the partitioning and retention of 15N labelled urea in aboveground and belowground N pools in both the short-term (2 weeks and 1 year) and the long-term (5 years). Trace amounts of 15N labelled urea was applied above the plant canopy to mimic the natural return of nutrients in the form of urine. The study was performed at Disko Island, West Greenland with historically low herbivore pressure, where no large herbivores are present and only a low density of small herbivores. We hypothesized that: (1) Most of the 15N-urea applied above the vegetation is rapidly assimilated into the plant canopy. We expect 15N plant enrichment in our system to differ from 15N enrichment reported for tundra under high herbivore pressure, with higher uptake under low herbivore disturbance due to less defoliation and trampling; (2) Mosses and lichens are the most efficient plant groups to capture 15N-urea; (3) In the long term (5 years), 15N recovery in soil and microbial biomass will increase and plant species with different traits and mycorrhizal status will differ in 15N recoveries. Plants with acquisitive traits are expected to show larger decline in 15N over time compared to plants with more conservative traits; (4) 15N retention is high due to low soil nutrient availability and a dominance of resource-conservative plant species.
Materials and methods
Study site
The study was conducted in a mesic tundra heath in Blæsedalen Valley at Disko Island, West-Greenland (69° 15′ N 53° 34′ W) (Fig. 1). The climate is characteristic for low Arctic tundra, with mean annual air temperature − 3.0 °C (1991–2011), July the warmest month with a mean temperature at 7.9 °C and March the coldest month with a mean temperature at − 14.0 °C. The mean annual precipitation is 400 mm with approximately 60% falling as rain (1994–2006), with data from the closest climatic station, 3 km from the study site (Hollesen et al. 2015). The soil type can be classified as a Haplic Cryosol (FAO)/Orthic Gelisol (USDA) and soils consist of a thin (5–10 cm) organic layer overlaying glacially rebedded basaltic sediments of a volcanic origin. The vegetation, a mesic tundra heath (Fig. 1b), is dominated by deciduous and evergreen dwarf shrubs with the most common vascular plant species being Betula nana, Salix glauca, Vaccinium uliginosum, Empetrum hermaphroditium and Cassiope tetragona, while grasses and forbs are rare (Blok et al. 2016). Bryophytes and lichens are also an important part of the total plant biomass at the research site. The vegetation is interspaced with patches of exposed bare ground. Disko Island lacks large vertebrate herbivores, and small-sized vertebrate herbivores, such as the Arctic hare (Lepus arcticus) and the rock ptarmigan (Lagopus muta), have very low densities. Soil pH measured as Milli-Q H2O to dry soil in a 1: 10 ratio is 5.08 ± 0.09 at 0–5 cm and 5.81 ± 0.03 at 5–10 cm depth, and carbon to nitrogen ratio (C:N) is 22.50 (± 1.15) at 0–5 cm and 15.15 (± 0.40) at 5–10 cm depth (means ± SE, n = 5) (D'Imperio et al. 2017). Atmospheric N deposition at the site is low, < 0.1 g m−2 (Kanakidou et al. 2016).
Location of Disko Island in West Greenland (coordinate reference system: WGS 84 / NSIDC Sea Ice Polar Stereographic North) (a) and satellite scene of the southern part of Disko Island with Blæsedalen valley where the 15N-urea study was conducted (left panel) and corresponding terrestrial cover classes (right panel) (b). The ten experimental plots are located along the red line. Modified from D'Imperio et al. (2017) with permission
Experimental design
At the peak of the growing season, in July 2012, 10 experimental sites at least 10 m apart were established at Blæsedalen. Sites were selected to have similar topography, plant community composition, and percentage of bare ground. Within each site, two plots (1 × 1 m) were established and randomly allocated to control or to the 15N enriched urea addition manipulation. The 15N-labelled urea (Urea-15N, 98% 15N, Cambridge Isotope Laboratories, Inc., USA) was diluted in 2 L of water from a nearby lake and sprayed uniformly over the vegetation at a dose of 0.1 g N m−2, on the 14 of July 2012. Control plots received the equivalent amount of non-15N enriched lake water. In this study, we aimed to mimic natural urine deposition and the 15N enriched urea plots did not receive any additional non-enriched water to wash off the added tracer from the vegetation. The lake was located in a remote location and nutrient poor, with a concentration of 0.312 mg L−1 total N and 0.024 mg L−1 total P (on 22 July 2017). Each plot received a negligible 0.000624 g N m−2 with water, but 0.1 g N m−2 with the 15N addition. Applying this trace amount of urea over the vegetation, which is 15N-enriched relative to any occurring natural level, allowed us to trace the N-urea in the different ecosystem N pools but precluded fertilization effect (Barthelemy et al. 2018).
Sampling
Aboveground biomass (standing plant biomass including foliage and twigs) was collected 10 days (24 July 2012, short-term recovery), and 5 years (20 July 2017, long-term recovery) after the 15N-urea addition from 25 cm × 25 cm subplots in each experimental plot. To estimate mid-term 15N-urea recovery in the vegetation, we harvested aboveground part of plants in 19 cm × 19 cm subplots in 6 randomly selected sites 1 year after the tracer addition (20 July 2013). All fresh plant material was stored at 4 °C until further processing. The harvested plants were sorted by species, except for graminoids, mosses and lichens that were treated collectively. The litter layer (dead leaves, dead moss pieces and twigs and fragments thereof, inevitably mixed with some soil) was collected in the same sub-plots and included both fresh litter and older plant remains that were not separated. The litter layer was differentiated from the organic horizon below, the latter here defined as finely fragmented dead plant material and decaying organic matter. The above-ground plant biomass was divided into the following nine compartments; Graminoids, Forbs, B. nana, Other deciduous shrub species, E. hermaphroditum, Other evergreen shrub species, Lichens, Mosses and Litter. The complete species composition and abundance for the Forbs, Other deciduous shrubs and Other evergreen shrubs, are presented in the Online Resource 1. After sampling, all the aboveground plant parts were washed with water to remove any remaining 15N-urea deposition on the surface of the plants, oven-dried (60 °C, 48 h) and weighed.
Belowground plant biomass was collected in all experimental plots 10 days and 5 years after tracer addition. After 10 days, root biomass was sampled at 0–5 cm depth, which corresponds to the most active root zone at the site (one soil core, area 44 cm2) while after 5 years, root biomass was sampled at 0–5 cm depth (two soil cores, area 14 cm2) and also at 5–10 cm and 10–20 cm (three soil cores, area 21 cm2). All samples were kept at 4 °C until further processing in the laboratory. Roots were separated from soil, washed to remove remaining organic and mineral matter, oven-dried (60 °C, 48 h) and weighed. After 5 years, the collected roots were sorted into fine (< 1 mm) and coarse (> 1 mm) roots.
To estimate 15N-urea recovery in the soil and microbial N pools and soil nutrient availability at the site, soil samples were collected in each experimental plot 10 days and 5 years after tracer addition. Ten days after tracer addition, two or three soil cores (area 14 cm2) were taken at 0–5 cm and 5 years after tracer addition, soil was retrieved from the same cores from which also root biomass was sampled at 0–5 cm, 5–10 cm and 10–20 cm depth. Undecomposed dead plant material were removed and the fresh samples separated in roots and soil by sieving (mesh 2 mm). The soil was kept at 4 °C until further processing.
Chemical analysis
Above- and belowground plant samples were ground to fine powder and subsamples encapsulated into preweighed tin capsules. The δ15N and elemental N concentration was measured on an Isoprime isotope ratio mass spectrometer (Isoprime Ltd., Cheadle Hulme, UK) coupled to a CN elemental analyser (Eurovector, Milan, Italy), at the Stable Isotope Facility at Department of Biology, University of Copenhagen.
For each soil sample, soil water content was measured gravimetrically (105 °C, 12 h) and organic matter content was determined by loss-on-ignition (475 °C, 12 h). A 10 g soil subsample was shaken with 70 ml of demineralized water for 1h and filtered through glass fibre filters (Whatman No 1). Another 10 g soil subsample was fumigated with chloroform (CHCl3) vapor (50 ml chloroform in glass vial in vacuum desiccator) for 24 h in order to release N contained in the microbial biomass before shaking with water (Brookes et al. 1985; Clemmensen et al. 2008). The soil and fumigated extracts were kept frozen until further analyses. Total extractable N (Ne) was determined by persulfate digestion by oxidizing all N to NO3− (Williams et al. 1995) and then analyzed by flow injection analysis (FIASTAR 5000 analyzer, FOSS Analytical; application note AN 5201from the manufacturer). The microbial N content (Nmic) was calculated by subtracting the total extractable N in the non-fumigated extracts (Ne) from the N in the fumigated extracts (Nf) with a microbial correction factor KEN of 0.4 (Jonasson et al. 1996; Clemmensen et al. 2008). In 2017, NH4+, NO3− and PO43− were determined for the non-fumigated soil extracts using FIASTAR 5000 analyzer application notes AN 5220, AN 5201 and AN 5241 from the manufacturer.
To determine extractable δ15N (δ15Ne) and microbial δ15N (δ15Nmic), a subsample of 10 ml of digested extracts collected 10 days after tracer addition (in 2012) were prepared for isotope analysis following a modification of the acid trap diffusion technique (Barthelemy et al. 2018). Five years after tracer addition (in 2017), fumigated extracts (30–50 ml) and soil extracts (70 ml) were freeze-dried for isotope analysis as in Ravn et al. (2017); and Pedersen et al. (2020). The acidified diffusion discs were analysed using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK) at the Stable Isotope Facility, University of California, Davis. The freeze-dried extracts were analysed at the Stable Isotope Facility at Department of Biology, University of Copenhagen.
Isotopic calculations
Enrichment and natural abundance of 15N was measured as 15N/14N ratio in plant and soil fractions (Barthelemy et al. 2018). The natural abundance of 15N was expressed as
where Rsample is the isotope ratio of the sample (15N/14N) relative to a standard calibrated against atmospheric N2.
The urea-15N enrichment in each ecosystem N pools was calculated as the 15N atomic frequency in excess as
where Atom % tracer is the 15N atomic frequency of a sample collected in the 15N urea addition plot and the Atom % background is the 15N atomic frequency of the corresponding sample collected in the non-labelled plot.
The percentage tracer recovery in each ecosystem N pool was estimated as
where Atom 15N excess is the urea-15N enrichment (%), N is the N content (%), Mass is the total mass of each pool of biomass or soil (g m−2) and the total amount of the tracer added corresponds to the 0.1 g m−2 of the isotopic labelled urea. Tracer recovery was corrected for the fractional abundance of 15N in the tracer (98 Atom % 15N). We assumed negligible N isotope fractionation during tracer movement in the different ecosystem pools (Robinson 2001) and no difference in 15N natural abundance within the N pools from 2 weeks, 1 year and 5 years after tracer addition.
Mycorrhizal colonization analysis
Mycorrhizal colonization analysis was conducted for the 10 most common plant species at the site; the graminoids Carex bigelowii, the forbs Tofieldia pusilla, Pyrola grandiflora, Pedicularis flammea, Bistorta vivipara, the deciduous shrubs S. glauca, B. nana, V. uliginosum and the evergreen shrubs E. hermaphroditum and C. tetragona. Root samples were harvested 5 years after tracer addition (in 2017) from 10 individuals close to the experimental plots by tracing the fine roots from the stem and main roots down into the soil. Root samples were thoroughly washed with water and stored in 70% ethanol until further processing. Root sample preparation for mycorrhizal analysis and colonization rates of fungi can be found in the Online Resource 2.
Statistics
All variables were log-transformed prior analysis to fulfill the assumptions of normality and homoscedasticity, with the exception of soil nutrient availability, which fulfilled the model’s assumption. We performed one-way analysis of variance (ANOVA) followed by post hoc Tukey’s HSD (Honestly Significance Difference) to investigate differences between soil depth for soil nutrient (NH4+, NO3− and PO43−) concentration, root biomass, and root, bulk soil N, soil extractable N, microbial N pool size, 15N natural abundance, 15N enrichment and 15N recovery. To test differences in 15N natural abundance between aboveground plant groups and plant litter we conducted one-way ANOVA followed by post hoc Tukey’s HSD test. Differences in soil nutrient availability, biomass and N pool size between control and 15N enriched plots were tested with one-way ANOVA. Differences in plant biomass, plant, soil and microbial N pool sizes, 15N enrichment and 15N recovery between years were tested using linear mixed effect model with time following tracer addition as fixed factor and plot identity as a random effect. This analysis avoids temporal pseudoreplication by taking into account the sampling of the same plots at 2 weeks, 1 year and 5 years after tracer addition. Linear contrasts were used to examine the differences between years. For each linear model, Akaike’s information criteria and residual plots were used to assess the fit of the models. All statistical analyses were performed using the statistical package R (R Core Team 2022).
Results
Biomass and N pool size
Plant litter, deciduous shrubs and mosses were the largest N pools and contained collectively 80 to 85% of the total aboveground N pool (Fig. 2a), which did not differ between sampling years. The N pool of the different plant groups remained unchanged across the 5 years period except that the N pool was significantly lower after 2 weeks compared to 1 year in deciduous shrubs (Tukey’s HSD, p = 0.034). Larger amount of N was stored in plant aboveground than in plant belowground biomass (Fig. 2a, b). Both roots and microbial N had larger N pools in 0–5 cm compared to deeper horizons (Tukey’s HSD, p < 0.001) while the opposite pattern was found for soil extractable N and total N (Tukey’s HSD, p < 0.001) (Fig. 2b and Online Resource 3). In 0–5 cm, total extractable N differed between the two sampling years (Tukey’s HSD, p < 0.001) while roots and microbial N pools remained unchanged (Fig. 2b). Detailed analysis of soil nutrient availability is presented in the Online Resource 3 and aboveground and belowground plant biomass is presented in the Online Resource 1.
Nitrogen pool (g m−2) in each 15N-enriched plot for aboveground ecosystem pools 2 weeks, 1 year and 5 years after tracer addition (a) and for belowground ecosystem pools 2 weeks and 5 years after tracer addition (b). TEN: Total extractable N. Three soil horizons were considered in 2017 (5 years after tracer addition). Bars represent means for each ecosystem N pool and the error bars account for the standard error of the total N pool for each sampling year. n = 10 in each ecosystem compartment except for aboveground N pools at 1 year (n = 6)
15N natural abundance
Graminoids had significantly higher 15N natural abundance (δ15N) than all other plant functional groups (Fig. 3 and Online Resource 4), and the δ15N of evergreen shrubs and E. hermaphroditum were significantly lower than the δ15N of lichens, mosses, and forbs (Fig. 3 and Online Resource 4). The δ15N of mixed litter was significantly lower than that of live graminoids (Tukey’s HSD, p < 0.001). Fine roots had a higher δ15N than coarse roots (Fig. 3, ANOVA, F1,43, p = 0.008), and δ15N in fine roots was higher at 5–10 cm depth compared to 0–5 cm (Tukey’s HSD, p = 0.004) and 10–20 cm (Tukey’s HSD, p < 0.001) whereas δ15N of coarse roots was similar in all depths. Soil and microbial N pools had higher δ15N values compared to plants (Fig. 3). Soil extractable N had lower δ15N values at 0–5 cm depth in comparison to 5–10 cm (Tukey’s HSD, p = 0.004) and 10–20 cm depth (Tukey’s HSD, p = 0.013) (Fig. 3). Microbial N pool δ15N did not differ with soil depth.
δ15N natural abundance (‰) for the nine plant groups (B. nana, Other DS: Other deciduous shrubs, E. hermaphroditum, Other ES: Other evergreen shrubs, Forbs, Graminoids, Lichens, Mosses and Litter), and for Fine and Coarse roots, TN: Total soil N, TEN: Total extractable N and Microbial N. δ15N natural abundance was measured in 2017 (5 years after tracer addition) in each control plot. Three soil horizons were considered for the belowground ecosystem compartment. Mean values ± standard errors are presented. n = 10 in each ecosystem compartment except for E. hermaphroditum, graminoids, coarse roots at 5–10 cm (n = 7 for all), Other evergreen shrubs (n = 4) and TEN (n = 8)
Initial 15N recovery and enrichment
The total recovery of 15N in plant N pools was 26.2% two weeks after the addition of the 15N-labeled urea (Fig. 4). Aboveground plant biomass (Fig. 4a) was the largest sink of the added 15N compared to belowground plant parts (total recovery of 0.4%, Fig. 4b). Mosses and B. nana had the highest 15N recovery of the plant fractions (recovery of 5.6% and 4.2% respectively), followed by E. hermaphroditum with a recovery of 2.2% (Fig. 4a and Online Resource 5). Deciduous shrubs and E. hermaphroditum had higher 15N recovery than graminoids and forbs while the other plant groups did not differ (Online Resource 5). Plant litter, with a recovery of 11.0%, had a higher recovery than of any of live plant species (Tukey’s HSD, p < 0.001 for all). Lichens had the highest 15N enrichment (0.6 15N excess in atom %) compared to the other plant groups (Tukey’s HSD, p < 0.001 for all, Fig. 6a). Mosses were also 15N enriched compared to graminoids (Tukey’s HSD, p < 0.001) and forbs (Tukey’s HSD, p < 0.001), while the other plant functional groups did not differ (Fig. 6a). Initial 15N enrichment in roots, soil extractable N and microbial N was low (Fig. 6b).
15N recovery (%) for aboveground ecosystem pools 2 weeks, 1 year and 5 years after tracer addition (a). Bars represent means for each ecosystem pool and the error bars account for the standard error of the total recovery for each sampling year. 15N recovery (%) for Roots 2 weeks and 5 years after tracer addition (b). Mean values ± standard errors are presented. For aboveground recovery, n = 10 in each compartment at 2 weeks and 5 years and n = 6 at 1 year
Longer-term 15N recovery and enrichment
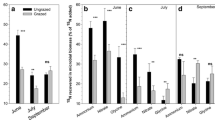
One year after tracer addition total aboveground 15N enrichment (p < 0.001) and 15N recovery (p < 0.001, 50% increase) were higher than initial 15N enrichment and recovery (Linear mixed effects model, Fig. 4a). This increase was driven by an increase in 15N enrichment for deciduous shrubs (p = 0.012), graminoids (p = 0.021), lichen (p = 0.026) and in the litter layer (p = 0.001) (Linear mixed effects model). Five years after tracer addition total plant and soil extractable and microbial 15N recovery was 24% lower than after 1 year (Linear mixed effects model, p < 0.05), with aboveground plants (incl. litter) still being the largest sink of the added 15N-urea with a recovery of 20.7% (Figs. 4 and 5). Total aboveground 15N recovery and 15N recovery of the three largest 15N sinks, B. nana, mosses and litter after 5 years did not differ from the initial recovery after two weeks (Online Resource 5) while 15N recovery in deciduous shrubs, E. hermaphroditum, and forbs decreased after 5 years (Online Resource 5). Total 15N enrichment in aboveground plants was 42% lower after 5 years compared to the initial 15N enrichment after 2 weeks (Linear mixed effects model, p < 0.001, Fig. 5a). A large decrease in 15N enrichment was observed for all vascular plant groups (Linear mixed effects model, p < 0.001, 78% decrease) but not for mosses and lichens (29% decrease, Fig. 6a) which still had the highest 15N enrichment (Linear mixed effects model, p < 0.001), with no difference between the other groups.
Aboveground 15N enrichment (Atom % 15N excess) 2 weeks, 1 year and 5 years after tracer addition (a). The nine plant groups are B. nana, Other DS: Other deciduous shrubs, E. hermaphroditum, Other ES: Other evergreen shrubs, Forbs, Graminoids, Lichens, Mosses and Litter. Belowground 15N enrichment (Atom % 15N excess) 2 weeks and 5 years after tracer addition (b). The belowground N pools are Roots, TEN: Total extractable N and Microbial N. Three soil horizons were considered in 2017 (5 years after tracer addition). Mean values ± standard errors are presented. In cases where the standard errors are not visible, they are smaller than the data point. Aboveground 15N enrichment, n = 10 in each compartment for at 2 weeks and 5 years (variation between 4–10 depending on the compartment) and n = 6 at 1 year (variation between 2–6 depending on the compartment). Belowground 15N enrichment, n = 10 in each compartment except for TEN (n = 9) and microbial N (n = 8)
Plant roots had a higher 15N enrichment after 5 years compared to the initial enrichment (+0.09% enrichment, Linear mixed effects model, p = 0.001, Fig. 6b), but were still a weak sink of the 15N-urea with a recovery of 0.38% (Fig. 4b). Root 15N enrichment was higher at 0–5 cm compared to 10–20 cm depth (Tukey’s HSD, p < 0.001). Both tracer 15N recovery and 15N enrichment were still low in extractable N and microbial N pools after 5 years compared to aboveground N pools (Figs. 4, 5, 6). 15N enrichment in extractable N was higher after 5 years compared to the initial enrichment (Linear mixed effects model, p = 0.0023) but still low for all soil depths, while 15N enrichment in microbial N did not differ (Fig. 6b). Microbial N 15N enrichment was on average 72% higher at 5–10 cm soil layer than at 0–5 cm (Tukey’s HSD, p < 0.001) and 10–20 cm (Tukey’s HSD, p = 0.001) layers.
Recovery in the extractable N pool increased with depth (Tukey’s HSD, p = 0.009) while the microbial 15N recovery did not. Soil total 15N recovery (0–20 cm depth) was 30% of added 15N after 5 years, with higher recovery at 5–10 cm compared to 0–5 cm depth (Tukey’s HSD, p < 0.0112, Fig. 5c).
Discussion
In this study, we applied the main N component of urine as trace amount of 15N labelled urea over the plant canopy. We traced the 15N-urea pathway from plant canopy to belowground ecosystem compartments over a 5-year period in a mesic tundra heath with low level of herbivory. In agreement with our first hypothesis, we found that most of the 15N-urea was rapidly assimilated into the plant canopy, with high 15N enrichment in all aboveground plant parts 2 weeks after tracer addition. This is in line with previous studies reporting rapid foliar absorption rates of 15N urea by deciduous shrubs at the same site (Blok et al. 2016), by tundra plants in northern Norway (Barthelemy et al. 2018) and by plants from other biomes (Dong et al. 2002; Uscola et al. 2014; Ruan and Gerendas 2015). All vascular and non-vascular plants, independently of differences in traits, were efficient in absorbing applied 15N-urea and they did so in proportion to their abundance in the vegetation. Our results support that urine can be an easy and rapid source of N for Arctic plant species in nutrient-poor tundra ecosystems, in a much faster way than nutrients released during the slow process of plant litter decomposition. Interestingly, short-term 15N enrichment in plants was of the same magnitude as 15N enrichment in tundra plants adapted to high level of herbivory when normalized per biomass (Barthelemy et al. 2018), indicating that plants with historically low level of herbivory might be as efficient as highly grazed plants in the uptake of N compounds derived from urine. While this study does not investigate the exact molecular and physiological mechanisms behind tundra plant urea uptake, it suggests that, in partial contrast to our first hypothesis, foliar and root absorption of N-urea is not affected by the level of herbivory pressure on the ecosystem.
In accordance with our second hypothesis, mosses and lichens were the largest sink of the 15N from the applied 15N urea compared to the other plant functional groups. Cryptogams are known to efficiently incorporate large amount of mineral and organic nutrients with their highly absorptive surfaces (Weber and Vancleve 1981; Crittenden 1989; Jonsdottir et al. 1995; Eckstein and Karlsson 1999; Krab et al. 2008). Interestingly, while vascular plant 15N enrichment was strongly reduced 5 years after tracer addition, 15N enrichment for mosses and lichens was almost identical to the initial 15N enrichment measured at 2 weeks. Mosses are long-living, decompose slowly and have a high internal nutrient turnover (Eckstein and Karlsson 1999; Eckstein 2000; Lang et al. 2009).
Mosses and lichens can constitute a large part of the diet for some herbivores, for example in early spring for pink-footed geese (Anser brachyrhynchus) (Fox et al. 2006) and in winter for Svalbard reindeer (Rangifer tarandus platyrhynchus) (Zhao et al. 2019) and Norwegian lemming (Lemmus lemmus) (Saetnan et al. 2009). Other herbivores, including reindeer and muskox (Ovibos moschatus) which are widely present in the Arctic, forage selectively on forbs, graminoids and deciduous shrubs during the growing season (Nieminen and Heiskari 1989; Kristensen et al. 2011). This indicates that most N compounds derived from urine can be incorporated and sequestered into non-forage plant species for some important herbivore populations in the Arctic. In tundra ecosystems under moderate or high herbivore pressure, the N-urea captured by cryptogams is likely to be released to other ecosystem compartments after a few years, via herbivores’ foraging behavior or trampling. For instance, geese grubbing activities (Speed et al. 2010), reindeer and muskox trampling (Gornall et al. 2009; Mosbacher et al. 2019) or rodents peaks (Rydgren et al. 2007) are all well known to strongly reduce the cryptogam layer, potentially enabling more nutrients to enter the soil system. However, in Arctic systems with historically low levels of herbivory, mosses and lichens can constitute a long-term sink of N by immobilizing N compounds for years and making them unavailable to vascular plants and soil microbes.
The litter layer was also an important sink of the 15N from the applied 15N urea. Initially, plant litter from recently senesced 15N enriched plant materials would account only for a small fraction of the 15N enrichment in the litter since the first sampling occurred at the peak of the growing season, 2 weeks after tracer addition. This initial high 15N enrichment and recovery in the litter fraction is more likely the result of high microbial immobilization associated with the decomposition of plant litter. Free-living saprotrophic fungi are important colonizers of the litter layer and ascomycetes and basidiomycetes dominated the surface litter fungal communities at the site (Christiansen et al. 2017). Fungal communities control the early stage of plant litter decomposition by the release of hydrolytic exoenzymes in the litter layer (Read and Perez-Moreno 2003; Schimel and Bennett 2004) and their activities in arctic ecosystems are often limited by N availability rather than carbon availability (Liu et al. 2018). A large increase in 15N enrichment and in 15N recovery (twice as high as the initial recovery at 2 weeks) was observed in the litter layer 1 year after tracer addition. This is probably due to the deposition of 15N enriched foliar material and leaching from plants during plant senescence at the end of the first growing season. After 5 years, 15N enrichment and recovery in litter decreased compared to the levels observed at 1 year but were still as high as the initial levels measured at 2 weeks. Fungal enzymatic activities and litter decomposition rates are strongly controlled by ground surface litter microclimate, in particular temperature and moisture regimes. Litter mass and carbon loss from decomposing plant litter is much lower in our mesic heath tundra sites compared to an adjacent sedge vegetation with higher moisture availability (Christiansen et al. 2017). Our findings suggest that in dry tundra ecosystems with slow litter decomposition, a large fraction of N compounds from urine incorporated in plant biomass can be sequestered in the litter layer for many years before entering the soil compartment.
Initial 15N enrichment was relatively low in soil extractable N and microbial biomass, despite that the microbial N pool is of similar magnitude as that of plants (Fig. 2b). When applied from above the vegetation, 15N is rapidly sequestered by plant canopy and the litter layer at the soil surface. The very small 15N enrichment measured in roots 2 weeks after tracer addition also supports this idea. Our results contrast with the much higher 15N recovery (40–80%) in soil and large microbial immobilization observed for studies where 15N compounds have been injected directly in tundra soil (Jonasson et al. 1996; Grogan et al. 2004; Nordin et al. 2004; Clemmensen et al. 2008). Our findings also indicate that urine would have a fast effect on plants rather than a slow indirect effect via stimulation of microbial activity and organic matter decomposition, leading to increased soil nutrient availability from which plants will benefit (van der Wal et al. 2004). Both the 15N enrichment and 15N recovery in the plant community increased 1 year after tracer addition. Most likely, some 15N compounds derived from the added urea have moved from aboveground plants to the litter and belowground compartments over the first year following tracer addition (i.e. due to plant senescence and root exudation). Plants subsequently could have acquired some of this 15N in the soil, causing the observed increase in 15N recovery in deciduous shrubs, graminoids and litter after 1 year.
We expected in our third hypothesis that in the short term (1st year) aboveground plant biomass should capture and retain most of the incoming N from the urea (either as intact urea molecule or in mineralized forms), while in the long-term N compounds derived from urea should eventually reach the soil matrix via plant turnover. We found mixed support for this hypothesis. Five years after tracer addition, belowground 15N enrichment in extractable and microbial fractions was very low and 15N recovery in microbial pool N minimal, with c. 1% 15N recovered in 0–5, 5–10 and 10–20 cm soil depth. On the other hand, other fractions of the 15N gradually released from microbes over the five years were absorbed to non-labile particles in the soil matrix, as demonstrated by the higher (5–15%) total soil 15N recovery after 5 years in the same soil depths. We did not find any support for our hypothesis that 15N enrichment should differ between plants with different traits and morphology as 15N enrichment decreased equally for all vascular plant species. Evergreen shrubs with a slower turnover than fast growing graminoids and forbs did not have a higher capacity to retain the 15N compounds from the applied 15N urea. This indicates a high reallocation of nutrients to woody stems or belowground biomass before plant senescence: a physiological mechanism which can be typical for nutrient limited Arctic ecosystems (Aerts and Chapin 2000).
The large difference in 15N natural abundance between co-occurring plant species reflects varying uptake of N between mycorrhizal and non-mycorrhizal plants and a strong niche partitioning in plant nutrient uptake based on chemical N form and mycorrhizal fungal associates (Michelsen et al. 1996, 1998; Barthelemy et al. 2017). All shrubs, evergreen or deciduous, were associated with either ericoid or ectomycorrhizal fungi, whereas graminoids were mainly non-mycorrhizal. Still, only small differences in 15N enrichment by urea addition were observed between vascular plant species, indicating that mycorrhizal symbioses did not provide any competitive advantage to acquire 15N compounds compared to non-mycorrhizal plants. It is likely that, even if plants absorbed intact urea through their foliage, 15N-urea would, when reaching the soil, be rapidly hydrolyzed into 15N-ammonium by urease, an extracellular enzyme (Fujii et al. 2018). Then, the 15N form in the soil would have been 15N-ammonium, a mineral form of N easily taken up by both mycorrhizal and non-mycorrhizal plants.
Our fourth hypothesis suggested that 15N loss would be small in this tundra heath system with a closed N cycling. Our results largely confirmed that our study system was indeed N-limited. Soil nutrient availability (both NH4+ and NO3−) and plant N pools were significantly lower in our site compared to other tundra heaths in Greenland (Michelsen et al. 1998) and in Scandinavia (Barthelemy et al. 2017). The wide range in plant 15N natural abundance and low 15N natural abundance in the soil matrix is often found in nutrient limited Arctic ecosystems (Michelsen et al. 1996, 1998; Hobbie and Hobbie 2006; Barthelemy et al. 2018). Yet, a considerable fraction of N from urea was lost from the ecosystem with a 5-year retention of approx. 20% in plants and approx. 30% in soil to 20 cm depth. We suggest that a rapid volatilization shortly after the application of the 15N-urea may contribute to initial loss of 15N from our tundra ecosystem. While volatilization generally is considered low in Arctic ecosystems, it could occur for the 15N-urea deposited over the plant canopy or the fraction in the soil compartment. When urea is applied to vegetation, like via natural herbivore urination, a large proportion is immobilized in the litter layer, where a higher temperature and pH may promote urea hydrolysis and the conversion of ammonium into volatile ammonia (Ernst and Massey 1960). Soil and plant surfaces can reach 28 °C at the site and in 2014 surfaces were on average 7.7 ± 0.9 °C warmer than air temperature at 1.5 m height during summer (Lindwall et al. 2016). Hence, it is likely that a proportion of the urea added over the vegetation has been lost into volatile ammonia. Precipitation events following the application of urea are also critical for ammonia volatilization rates (Engel et al. 2011; Holcomb et al. 2011). Yet, meteorological data retrieved from the Greenland Ecosystem Monitoring Database reported no rainfall between the 15N urea application and the first sampling 2 weeks later, which likely prevented most of the urea from moving deeper into the soil matrix, below our sampling profile. In the longer term, 15N ecosystem losses may result from the downward movement of 15N compounds along the soil profile and the following 15N leaching. This is supported by the highest 15N recovery for soil extractable N found at the deepest soil horizon at 10–20 cm depth after 5 years. Microbial denitrification has been often reported to explain N loss through the conversion of nitrate into atmospheric N (Groffman and Tiedje 1989; Aulakh et al. 1992), but denitrification is moderate in the dry heath tundra (Xu et al. 2023) with low soil moisture, cold temperatures, low nitrate concentration, and aerobic conditions prevailing along the soil profile and in the litter layer.
Our 15N tracer study in a tundra vegetation with low herbivore density demonstrates that urea can be rapidly captured by the plant canopy, in particular by mosses and lichens, and that plants under low herbivore pressure are as efficient as highly grazed plants in taking advantage of this incoming N source from urine. Whereas vascular plant 15N enrichment strongly declined over the long-term, mosses and lichens retained the same amount of labelled N as initially even after five years, demonstrating that in the absence of herbivores, cryptogams may constitute a very long-term sink of N. By contrast, although short-term microbial N uptake contributed to ecosystem retention of added urea-N, long-term 15N recovery in this pool was small, indicating that herbivores can increase the quality of their forage plants more rapidly via urine deposition than via changes in microbial activities and coupled increase in soil nutrient availability. However, N-urea losses from the system right after urine excretion by N volatilization and leaching may be large despite a strong nutrient-limitation of the ecosystem. Our findings suggest that herbivores colonizing new territories (i.e. herbivore reintroduction or range expansion due to climate change (Olofsson and Post 2018) could rapidly increase plant litter quality by the deposition of nutrient rich and labile urine. Herbivores will benefit immediately from this improved forage quality, in contrast to other mechanisms also enhancing N cycling but operating at longer time scale such as slow vegetation shift to more palatable plants due to selective grazing.
Data availability
All data that support the findings of this study is openly available. Sample data are available in Dryad with the name: Short- and long-term plant and microbial uptake of 15N-labelled urea in a mesic tundra heath, West Greenland, with DOI https://doi.org/10.5061/dryad.m0cfxpp92.
References
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. In: Fitter AH, Raffaelli DG (eds) Advances in ecological research, vol 30. vol 30. Elsevier Academic Press Inc., San Diego, pp 1–67
Aulakh MS, Doran JW, Mosier AR (1992) Soil denitrification—significance, measurement, and effects of management. Springer, New York
Ayres E, van der Wal R, Sommerkorn M, Bardgett RD (2006) Direct uptake of soil nitrogen by mosses. Biol Lett 2:286–288. https://doi.org/10.1098/rsbl.2006.0455
Baltensperger AP, Huettmann F (2015) Predicted shifts in small mammal distributions and biodiversity in the altered future environment of Alaska: an open access data and machine learning perspective. PLoS ONE 10:21. https://doi.org/10.1371/journal.pone.0132054
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268. https://doi.org/10.1890/02-0274
Barthelemy H, Stark S, Kytoviita MM, Olofsson J (2017) Grazing decreases N partitioning among coexisting plant species. Funct Ecol 31:2051–2060. https://doi.org/10.1111/1365-2435.12917
Barthelemy H, Stark S, Michelsen A, Olofsson J (2018) Urine is an important nitrogen source for plants irrespective of vegetation composition in an Arctic tundra: Insights from a N-15-enriched urea tracer experiment. J Ecol 106:367–378. https://doi.org/10.1111/1365-2745.12820
Bazely DR, Jefferies RL (1985) Goose feces - a source of nitrogen for plant-growth in a grazed salt-marsh. J Appl Ecol 22:693–703. https://doi.org/10.2307/2403222
Bjorkman AD et al (2018) Plant functional trait change across a warming tundra biome. Nature. https://doi.org/10.1038/s41586-018-0563-7
Blok D, Elberling B, Michelsen A (2016) Initial stages of tundra shrub litter decomposition may be accelerated by deeper winter snow but slowed down by spring warming. Ecosystems 19:155–169. https://doi.org/10.1007/s10021-015-9924-3
Brookes PC, Kragt JF, Powlson DS, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: the effects offumigation time and temperature. Soil Biol Biochem 17:831–835
Chapin FS, Moilanen L, Kielland K (1993) Preferential use of organic nitrogen for growth by a nonmycorrhizal arctic sedge. Nature 361:150–153. https://doi.org/10.1038/361150a0
Christiansen CT et al (2017) Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Global Change Biol 23:406–420. https://doi.org/10.1111/gcb.13362
Clemmensen KE, Sorensen PL, Michelsen A, Jonasson S, Strom L (2008) Site-dependent N uptake from N-form mixtures by arctic plants, soil microbes and ectomycorrhizal fungi. Oecologia 155:771–783. https://doi.org/10.1007/s00442-008-0962-9
Crittenden PD (1989) Nitrogen relations of mat-forming lichens. Cambridge University Press, Cambridge
Cuyler C et al (2020) Muskox status, recent variation, and uncertain future. Ambio 49:805–819. https://doi.org/10.1007/s13280-019-01205-x
Dahlman L, Persson J, Palmqvist K, Nasholm T (2004) Organic and inorganic nitrogen uptake in lichens. Planta 219:459–467. https://doi.org/10.1007/s00425-004-1247-0
Day TA, Detling JK (1990) Grassland patch dynamics and herbivore grazing preference following urine deposition. Ecology 71:180–188. https://doi.org/10.2307/1940258
de Vries FT, Bardgett RD (2016) Plant community controls on short-term ecosystem nitrogen retention. New Phytol 210:861–874. https://doi.org/10.1111/nph.13832
D’Imperio L, Nielsen CS, Westergaard-Nielsen A, Michelsen A, Elberling B (2017) Methane oxidation in contrasting soil types: responses to experimental warming with implication for landscape-integrated CH4 budget. Glob Change Biol 23:966–976. https://doi.org/10.1111/gcb.13400
Dong SF, Cheng LL, Scagel CF, Fuchigami LH (2002) Nitrogen absorption, translocation and distribution from urea applied in autumn to leaves of young potted apple (Malus domestica) trees. Tree Physiol 22:1305–1310. https://doi.org/10.1093/treephys/22.18.1305
Eckstein RL (2000) Nitrogen retention by Hylocomium splendens in a subarctic birch woodland. J Ecol 88:506–515. https://doi.org/10.1046/j.1365-2745.2000.00480.x
Eckstein RL, Karlsson PS (1999) Recycling of nitrogen among segments of Hylocomium splendens as compared with Polytrichum commune: Implications for clonal integration in an ectohydric bryophyte. Oikos 86:87–96. https://doi.org/10.2307/3546572
Engel R, Jones C, Wallander R (2011) Ammonia volatilization from urea and mitigation by NBPT following surface application to cold soils. Soil Sci Soc Am J 75:2348–2357. https://doi.org/10.2136/sssaj2011.0229
Ernst JM, Massey MF (1960) The effects of several factors on volatilization of ammonia formed from urea in the soil. Soil Sci Soc Am J 24:87–90
Fox TA, Francis IS, Bergersen E (2006) Diet and habitat use of Svalbard pink-footed geese Anser brachyrhynchus during arrival and pre-breeding periods in Adventdalen. Ardea 94:691–699
Frank DA, Evans RD, Tracy BF (2004) The role of ammonia volatilization in controlling the natural N-15 abundance of a grazed grassland. Biogeochemistry 68:169–178. https://doi.org/10.1023/b:Biog.0000025736.19381.91
Fujii K, Hayakawa C (2022) Urea uptake by spruce tree roots in permafrost-affected soils. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2022.108647
Fujii K, Yamada T, Hayakawa C, Nakanishi A, Funakawa S (2018) Another bottleneck for nitrogen mineralization in temperate forest soils: Arginine metabolism in microorganisms. Soil Biol Biochem 126:22–30. https://doi.org/10.1016/j.soilbio.2018.08.005
Gornall JL, Woodin SJ, Jonsdottir IS, Van der Wal R (2009) Herbivore impacts to the moss layer determine tundra ecosystem response to grazing and warming. Oecologia 161:747–758. https://doi.org/10.1007/s00442-009-1427-5
Groffman PM, Tiedje JM (1989) Denitrification in north temperate forest soils - Spatial and temporal patterns at the landscape and seasonal scales. Soil Biol Biochem 21:613–620. https://doi.org/10.1016/0038-0717(89)90053-9
Grogan P, Michelsen A, Ambus P, Jonasson S (2004) Freeze-thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biol Biochem 36:641–654. https://doi.org/10.1016/j.soilbio.2003.12.007
Harden JW et al (2012) Field information links permafrost carbon to physical vulnerabilities of thawing. Geophys Res Lett 39:6. https://doi.org/10.1029/2012gl051958
Hill PW et al (2011) Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat Clim Chang 1:50–53. https://doi.org/10.1038/nclimate1060
Hobbie JE, Hobbie EA (2006) N-15 in symbiotic fungi and plants estimates nitrogen and carbon flux rates in Arctic tundra. Ecology 87:816–822. https://doi.org/10.1890/0012-9658(2006)87[816:Nisfap]2.0.Co;2
Hobbs NT (1996) Modification of ecosystems by ungulates. J Wildl Manage 60:695–713. https://doi.org/10.2307/3802368
Holcomb JC, Sullivan DM, Horneck DA, Clough GH (2011) Effect of irrigation rate on ammonia volatilization. Soil Sci Soc Am J 75:2341–2347. https://doi.org/10.2136/sssaj2010.0446
Hollesen J et al (2015) Winter warming as an important co-driver for Betula nana growth in western Greenland during the past century. Global Change Biol 21:2410–2423. https://doi.org/10.1111/gcb.12913
Hugelius G et al (2014) Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 11:6573–6593. https://doi.org/10.5194/bg-11-6573-2014
IPCC (2013) Climate Change 2013: The physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Iversen CM (2014) Using root form to improve our understanding of root function. New Phytol 203:707–709. https://doi.org/10.1111/nph.12902
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV, Callaghan TV (1996) Microbial biomass C, N and P in two arctic soils and responses to addition of NPK fertilizer and sugar: implications for plant nutrient uptake. Oecologia 106:507–515. https://doi.org/10.1007/bf00329709
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV (1999) Responses in microbes and plants to changed temperature, nutrient, and light regimes in the arctic. Ecology 80:1828–1843. https://doi.org/10.1890/0012-9658(1999)080[1828:Rimapt]2.0.Co;2
Jonsdottir IS, Callaghan TV, Lee JA (1995) Fate of added nitrogen in a moss sedge arctic community and effects of increased nitrogen deposition. Sci Total Environ 160–61:677–685. https://doi.org/10.1016/0048-9697(95)04402-m
Kanakidou M et al (2016) Past, present, and future atmospheric nitrogen deposition. J Atmos Sci 73:2039–2047. https://doi.org/10.1175/jas-d-15-0278.1
Kielland K (1994) Amino-acid-absorption by arctic plants - Implications for plant nutrition and nitrogen cycling. Ecology 75:2373–2383. https://doi.org/10.2307/1940891
Koltz AM, Gough L, McLaren JR (2022) Herbivores in Arctic ecosystems: effects of climate change and implications for carbon and nutrient cycling. Ann N Y Acad Sci 1516:28–47. https://doi.org/10.1111/nyas.14863
Krab EJ, Cornelissen JHC, Lang SI, van Logtestijn RSP (2008) Amino acid uptake among wide-ranging moss species may contribute to their strong position in higher-latitude ecosystems. Plant Soil 304:199–208. https://doi.org/10.1007/s11104-008-9540-5
Kristensen DK, Kristensen E, Forchhammer MC, Michelsen A, Schmidt NM (2011) Arctic herbivore diet can be inferred from stable carbon and nitrogen isotopes in C-3 plants, faeces, and wool. Can J Zool 89:892–899. https://doi.org/10.1139/z11-073
Lang SI et al (2009) An experimental comparison of chemical traits and litter decomposition rates in a diverse range of subarctic bryophyte, lichen and vascular plant species. J Ecol 97:886–900. https://doi.org/10.1111/j.1365-2745.2009.01538.x
Lindwall F, Svendsen SS, Nielsen CS, Michelsen A, Rinnan R (2016) Warming increases isoprene emissions from an arctic fen. Sci Total Environ 553:297–304. https://doi.org/10.1016/j.scitotenv.2016.02.111
Liu XY et al (2018) Nitrate is an important nitrogen source for Arctic tundra plants. Proc Natl Acad Sci USA 115:3398–3403. https://doi.org/10.1073/pnas.1715382115
Mallory CD, Boyce MS (2018) Observed and predicted effects of climate change on Arctic caribou and reindeer. Environ Rev 26:13–25. https://doi.org/10.1139/er-2017-0032
Maslov MN, Makarov MI (2016) Transformation of nitrogen compounds in the tundra soils of Northern Fennoscandia. Eurasian Soil Sci 49:757–764. https://doi.org/10.1134/s1064229316070073
McKane RB et al (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71. https://doi.org/10.1038/415068a
Michelsen A, Schmidt IK, Jonasson S, Quarmby C, Sleep D (1996) Leaf N-15 abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non- and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia 105:53–63. https://doi.org/10.1007/bf00328791
Michelsen A, Quarmby C, Sleep D, Jonasson S (1998) Vascular plant N-15 natural abundance in heath and forest tundra ecosystems is closely correlated with presence and type of mycorrhizal fungi in roots. Oecologia 115:406–418. https://doi.org/10.1007/s004420050535
Michelsen A, Graglia E, Schmidt IK, Jonasson S, Sleep D, Quarmby C (1999) Differential responses of grass and a dwarf shrub to long-term changes in soil microbial biomass C, N and P following factorial addition of NPK fertilizer, fungicide and labile carbon to a heath. New Phytol 143:523–538. https://doi.org/10.1046/j.1469-8137.1999.00479.x
Morsdorf MA et al (2019) Deepened winter snow significantly influences the availability and forms of nitrogen taken up by plants in High Arctic tundra. Soil Biol Biochem 135:222–234. https://doi.org/10.1016/j.soilbio.2019.05.009
Mosbacher JB, Michelsen A, Stelvig M, Hjermstad-Sollerud H, Schmidt NM (2019) Muskoxen modify plant abundance, phenology, and nitrogen dynamics in a High Arctic fen. Ecosystems 22:1095–1107. https://doi.org/10.1007/s10021-018-0323-4
Mugasha AG, Pluth DJ (1994) Distribution and recovery of 15N-urea in a tamarack/black spruce mixed stand on a drained minerotrophic peatland. For Ecol Manage 68:353–363. https://doi.org/10.1016/0378-1127(94)90056-6
Neff JC, Chapin FS, Vitousek PM (2003) Breaks in the cycle: dissolved organic nitrogen in terrestrial ecosystems. Front Ecol Environ 1:205–211. https://doi.org/10.1890/1540-9295(2003)001[0205:Bitcdo]2.0.Co;2
Nieminen M, Heiskari U (1989) Diets of freely grazing and captive reindeer during summer and winter. Rangifer 9:17–34
Nordin A, Schmidt IK, Shaver GR (2004) Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 85:955–962. https://doi.org/10.1890/03-0084
Olff H, Ritchie ME (1998) Effects of herbivores on grassland plant diversity. Trends Ecol Evol 13:261–265. https://doi.org/10.1016/s0169-5347(98)01364-0
Olofsson J, Post E (2018) Effects of large herbivores on tundra vegetation in a changing climate, and implications for rewilding. Philos Trans R Soc b: Biol Sci 373:8. https://doi.org/10.1098/rstb.2017.0437
Pastor J, Cohen YTH (2006) The role of large herbivores in ecosystem nutrient cycles. Cambridge University Press, Cambridge
Pedersen EP, Elberling B, Michelsen A (2020) Foraging deeply: depth-specific plant nitrogen uptake in response to climate-induced N-release and permafrost thaw in the High Arctic. Global Change Biol 26:6523–6536. https://doi.org/10.1111/gcb.15306
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna ULR https://www.R-project.org/.
Ramm E et al (2022) Alder-induced stimulation of soil gross nitrogen turnover in a permafrost-affected peatland of Northeast China. Soil Biol Biochem 172:13. https://doi.org/10.1016/j.soilbio.2022.108757
Ravn NMR, Elberling B, Michelsen A (2017) The fate of (CN)-C-13-N-15 labelled glycine in permafrost and surface soil at simulated thaw in mesocosms from high arctic and subarctic ecosystems. Plant Soil 419:201–218. https://doi.org/10.1007/s11104-017-3322-x
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems - a journey towards relevance? New Phytol 157:475–492. https://doi.org/10.1046/j.1469-8137.2003.00704.x
Robinson D (2001) delta N-15 as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162. https://doi.org/10.1016/s0169-5347(00)02098-x
Ruan JY, Gerendas J (2015) Absorption of foliar-applied urea-N-15 and the impact of low nitrogen, potassium, magnesium and sulfur nutritional status in tea (Camellia sinensis L.) plants. Soil Sci Plant Nutr 61:653–663. https://doi.org/10.1080/00380768.2015.1027134
Rydgren K, Okland RH, Pico FX, de Kroon H (2007) Moss species benefits from breakdown of cyclic rodent dynamics in boreal forests. Ecology 88:2320–2329. https://doi.org/10.1890/06-1634.1
Saetnan ER, Gjershaug JO, Batzli GO (2009) Habitat use and diet composition of norwegian lemmings and field voles in central Norway. J Mammal 90:183–188. https://doi.org/10.1644/07-mamm-a-259.1
Sanders T et al (2022) Seasonal nitrogen fluxes of the Lena river delta. Ambio 51:423–438. https://doi.org/10.1007/s13280-021-01665-0
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602. https://doi.org/10.1890/03-8002
Schimel JP, Chapin FS (1996) Tundra plant uptake of amino acid and NH4+ nitrogen in situ: Plants compete well for amino acid N. Ecology 77:2142–2147. https://doi.org/10.2307/2265708
Speed JDM, Cooper EJ, Jonsdottir IS, van der Wal R, Woodin SJ (2010) Plant community properties predict vegetation resilience to herbivore disturbance in the Arctic. J Ecol 98:1002–1013. https://doi.org/10.1111/j.1365-2745.2010.01685.x
Templer PH et al (2012) Sinks for nitrogen inputs in terrestrial ecosystems: a meta-analysis of N-15 tracer field studies. Ecology 93:1816–1829. https://doi.org/10.1890/11-1146.1
Uscola M, Villar-Salvador P, Oliet J, Warren CR (2014) Foliar absorption and root translocation of nitrogen from different chemical forms in seedlings of two Mediterranean trees. Environ Exp Bot 104:34–43. https://doi.org/10.1016/j.envexpbot.2014.03.004
van der Wal R, Bardgett RD, Harrison KA, Stien A (2004) Vertebrate herbivores and ecosystem control: cascading effects of faeces on tundra ecosystems. Ecography 27:242–252. https://doi.org/10.1111/j.0906-7590.2004.03688.x
Voigt C et al (2020) Nitrous oxide emissions from permafrost-affected soils. Nat Rev Earth Environ 1:420–434. https://doi.org/10.1038/s43017-020-0063-9
Wang WH, Kohler B, Cao FQ, Liu LH (2008) Molecular and physiological aspects of urea transport in higher plants. Plant Sci 175:467–477. https://doi.org/10.1016/j.plantsci.2008.05.018
Wang FP et al (2018) Ecosystem nitrogen retention is regulated by plant community trait interactions with nutrient status in an alpine meadow. J Ecol 106:1570–1581. https://doi.org/10.1111/1365-2745.12924
Weber MG, Vancleve K (1981) Nitrogen dynamics in the forest floor of interior alaska black spruce ecosystems. Can J for Res 11:743–751. https://doi.org/10.1139/x81-106
Weintraub MN, Schimel JP (2003) Interactions between carbon and nitrogen mineralization and soil organic matter chemistry in arctic tundra soils. Ecosystems 6:129–143. https://doi.org/10.1007/s10021-002-0124-6
Williams BL, Shand CA, Hill M, O’Hara C, Smith S, Young ME (1995) A procedure for the simultaneous oxidation of total soluble N and P in extracts of fresh and fumigated soils and litters. Commun Soil Sci Plant Anal 26:91–106
Xu WY, Frendrup LL, Michelsen A, Elberling B, Ambus PL (2023) Deepened snow in combination with summer warming increases growing season nitrous oxide emissions in dry tundra, but not in wet tundra. Soil Biol Biochem 180:12. https://doi.org/10.1016/j.soilbio.2023.109013
Zhao LZ, Colman AS, Irvine RJ, Karlsen SR, Olack G, Hobbie EA (2019) Isotope ecology detects fine-scale variation in Svalbard reindeer diet: implications for monitoring herbivory in the changing Arctic. Polar Biol 42:793–805. https://doi.org/10.1007/s00300-019-02474-8
Zhu Q, Iversen CM, Riley WJ, Slette IJ, Vander Stel HM (2016) Root traits explain observed tundra vegetation nitrogen uptake patterns: Implications for trait-based land models. J Geophys Res Biogeosci 121:3101–3112. https://doi.org/10.1002/2016jg003554
Acknowledgements
We thank Carin Olofsson, Regin Rønn, Gosha Sylvester, Esben Nielsen and Daan Blok for their crucial help during the sample processing. This study was supported by the grants from the Knut and Alice Wallenberg Foundation awarded to Hélène Barthelemy and by the Independent Research Fund Denmark, DFF Grants no. 0135-00140B and 2032-00064B to Anders Michelsen.
Funding
Open access funding provided by University of Bergen (incl Haukeland University Hospital).
Author information
Authors and Affiliations
Contributions
The project was conceived and designed by HB, AM and JO. HB, LAN and AM conducted the fieldwork campaigns. HB and LAN prepared all samples and HB and LAN conducted the laboratory analyses with the assistance of AM, SS and MV. LAN and AM performed preliminary data analyses and HB conducted the full data analyses and drafted the manuscript. All authors provided feedbacks on the manuscript through several rounds of revision.
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barthelemy, H., Nobel, L.A., Stark, S. et al. Short- and long-term plant and microbial uptake of 15N-labelled urea in a mesic tundra heath, West Greenland. Polar Biol 47, 1–15 (2024). https://doi.org/10.1007/s00300-023-03209-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03209-6