Abstract
Low bioavailability of the vital element iron (Fe) limits primary production in large regions of the Southern Ocean, thus impacting phytoplankton community structures. Primary productivity seems to be particularly sensitive to the reduced form of iron (Fe(II)), which is thought to be the most readily bioavailable redox form of Fe in the ocean. Here, we investigated the impact of temperature (3 °C, 5 °C and 7 °C) and Fe(II) additions (+ 5 nM) on growth of two Southern Ocean phytoplankton species Fragilariopsis cylindrus and Phaeocystis antarctica in coastal and open ocean water. At all tested temperatures, growth rates of P. antarctica were significantly higher with added iron, compared to the treatments without added iron in both waters. Temperature only had a significant effect on the growth rate of this species when it was raised to 7 °C in all treatments. For F. cylindrus, growth rates only significantly increased with iron addition at 7 °C in both water types. Temperature did not affect the growth rate of F. cylindrus except for a significant reduction without iron addition at 7 °C in coastal water. These results highlight the complex interactions between Fe bioavailability and temperature on Southern Ocean phytoplankton growth. Thus, certain Southern Ocean phytoplankton species may have higher growth rates in regions of the ocean that will warm the most and possibly experience greater Fe supply under future climate conditions, such as coastal regions. This may result in changes in phytoplankton community structures with implications for carbon sequestration efficiency under future climate conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Southern Ocean (SO) contributes ~ 33% to the transfer of global carbon (C) from the atmosphere into a zonal belt along the Antarctic Circumpolar Current (Arrigo et al. 1999; Schlitzer 2002). It is further characterised by its richness in macronutrients such as nitrate and phosphate and its growth limiting iron (Fe) concentrations, leading to the definition of High Nutrient Low Chlorophyll (HNLC) ocean regions. Concentrations of Fe in the open SO are usually < 1 nM (Sedwick et al. 1997; Bowie et al. 2002) and can range from 0.7 nM in deeper waters (Boye et al. 2001) to < 0.2 nM in surface waters (Schallenberg et al. 2018). In contrast, coastal areas, which extend from land masses to the continental shelf, are richer in Fe due to higher flux from a greater variety of sources (e.g., atmospheric supply, sediment leaching; (Tagliabue et al. 2017). Iron is crucial for phytoplankton growth in the oceans due to its essential role in cellular photosynthesis and respiration reactions. Most of the Fe in seawater is bound to ligands (L), which aid in keeping Fe from precipitating and sinking to depth, therefore allowing a more consistent supply of Fe to phytoplankton (Maldonado and Price 1999; Hunter and Boyd 2007; Shaked and Lis 2012).
The uptake of Fe by phytoplankton is described as a two-step process of iron reduction from Fe(III) to Fe(II) followed by transport across the cell membrane (Maldonado and Price 2002). This process can be further distinguished into: (1) the use of transporter compounds like siderophores, (2) the use of Fe(II) transporters which carry Fe(II) across the membrane through oxidation, and (3) the initial reduction of Fe(III) to Fe(II) at the cell surface before 4) transportation into the cell via oxidation mechanisms (Shaked et al. 2005; Salmon et al. 2006; Morel et al. 2008).
The bioavailability of Fe for phytoplankton differs between its chemical forms. Dissolved iron (dFe; operationally defined as < 0.2 µm) is often considered a proxy for the ‘bioavailable’ form, while the reduced redox species of iron in the ocean (dFe(II)) is considered even more bioavailable (Rich and Morel 1990; Kuma and Matsunaga 1995; Hassler and Schoemann 2009; Shi et al. 2010; Shaked and Lis 2012; Lis et al. 2015; Trimborn et al. 2017a).
Of the two main Fe redox species (dFe(II) and dFe(III)), dFe(III) is generally more thermodynamically stable in seawater at its current pH of ~ 8.1, while dFe(II) is transitory due to its rapid oxidation to dFe(III), making its measurement challenging (King et al. 1995; Bowie et al. 2002; Croot and Laan 2002; Hansard and Landing 2009). Dissolved Fe(II) occurs in picomolar concentrations, which is typically equal to only 4–13% of the total dFe in open ocean surface waters (Bowie et al. 2002), but can be much higher in coastal areas (> 1 nM) due to leaching from sediments (Kuma et al. 1992).
The oxidation rate of dFe(II), and therefore the period it is available to phytoplankton, is controlled by oxygen (O2) and hydrogen peroxide (H2O2) concentrations, temperature and pH (Eqs. 1–4; Haber and Weiss 1932; Millero et al. 1987; Moffett and Zika 1987; Millero and Izaguirre 1989; Millero and Sotolongo 1989).
Therefore, seawater temperature and pH have direct impacts on Fe chemistry and Fe bioavailability and will thus be impacted by climate change (Hoffmann et al. 2012).
Seawater temperatures have already increased by 0.85 °C since the industrial revolution, due to the increased emission of greenhouse gases into the atmosphere and are predicted to rise a further 1.4–3.7 °C by the end of this century (Bindoff et al. 2019). This significant increase will impact many marine organisms, including phytoplankton. Each species of these single celled organisms has its own thermal tolerance window (Pörtner 2002; Boyd 2019). Ocean warming will therefore have direct effects on species composition (Noiri et al. 2005; Lacour et al. 2017), which can have impacts for carbon export and carbon flow through trophic levels in different ocean environments.
This study tested the effect of increasing seawater temperature (3 °C, 5 °C and 7 °C) and iron addition on the growth of P. antarctica and F. cylindrus in open ocean and coastal seawater (Fig. 1). The initial temperature was chosen as it is a representative temperature for both species, while the other temperature treatments were chosen based on predicted future climate scenarios (Bindoff et al. 2019).
Experimental hypotheses: coastal waters are often high in iron (Fe) and therefore also high in primary production, whereas Fe is lacking in open ocean water, leading to low primary production. Once 5 nM of dissolved Fe are added and the temperatures are elevated from 3 to 5 °C or 7 °C, it is assumed that this will have little to no impact on the primary producers in coastal areas but result in decreased growth rates in open ocean water, with temperature being the dominant factor
Based on the two assumptions that: (1) growth itself is directly affected by temperature, and (2) increasing temperatures decrease Fe bioavailability due to increased dFe(II) oxidation rates to Fe(III), the following hypotheses were formulated (Fig. 1):
-
(1)
In open ocean water without Fe addition, an increase in temperature will lead to a decrease in growth for both species. Here, any potential positive direct effect of increasing temperature on growth is outweighed by a further reduced dFe(II) availability in the already low Fe water.
-
(2)
dFe(II) additions in open ocean water will increase the growth rates of both species compared to the low Fe treatments. However, elevated temperatures will increase the growth rate of F. cylindrus and decrease the growth rate of P. antarctica.
-
(3)
In coastal water, growth rates of both species will be higher compared to open ocean water because of the higher background Fe concentrations. Increasing temperatures will affect the growth rate of both species similarly as in the open ocean water under Fe addition (see Hypothesis 2).
-
(4)
dFe(II) addition will cause no or very little additional increase in growth rate in coastal water. Here, increasing temperatures will affect growth of both species similarly as in the open ocean water under Fe addition (see Hypothesis 2).
Methods
Experimental overview
Fragilariopsis cylindrus and P. antarctica were grown in coastal and in open ocean seawater under different temperature and Fe conditions in 28 mL polycarbonate (PC) screw capped vials (Thermo Fisher). A closed small-vessel system was chosen because it allowed us to measure growth rates without opening the sample vials during the experiment and limit Fe contamination. After inoculating 600 µL of either P. antarctica or F. cylindrus cultures into the vials, they were placed into a rack in a cold room at 3.2 ± 0.6 °C for 16 days and in two temperature blocks at 5 °C ± 0.5 °C and 7 °C ± 0.5 °C (n = 6) respectively (see Fig. 2). To three vials from each treatment, 5 nM dFe(II) (ammonium iron (II) sulphate hexahydrate (NH4)2Fe(SO4)2·6H2O) were added while the other three were incubated in the unaltered coastal or open ocean water.
Cleaning
All processing and sampling was carried out under a class 100 laminar airflow hood in a 3 °C cold room at the Institute of Marine and Antarctic Studies (IMAS) in Hobart, Tasmania. Trace metal clean protocols (Cutter et al. 2017) were used to prevent trace metal contamination. This included initial 2% Decon baths, followed by thorough rinses with ultra-high pure water (UHP, Barnstead, 18.2 MΩ). After washing all equipment in a 6 M hydrochloric acid bath [HCl; in-house distilled acid using a Savillex perfluoroalkoxy-polymer (PFA) still, DST-1000] for 1 month, everything was rinsed seven times with UHP water. Three initial preconditioning rinsing steps were done with the respective seawater used for the experiments for each vial. The pipette tips were sterile microwaved for five minutes in UHP water to prevent bacterial contamination for further culture work. This was followed by three HCl acid (distilled) and seven UHP water rinses.
Seawater
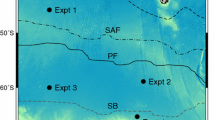
The coastal seawater was collected from Kingston beach (Kingston seawater—KISW, 42 ° 98′ S, 147 ° 32′ E), Tasmania in January 2018. An acid cleaned 400 µm mesh was used to prefilter any large grazers and particles, prior to 0.2 µm filtration (PALL, Acropak 200) under a class 100 laminar flow hood into a trace metal clean carboy (Nalgene, 20 L, LDPE). The open ocean water was collected on the SR3-GEOTRACES GS01 voyage in the SO on board of the RV Investigator in January/February 2018, using a trace metal rosette as described in Holmes et al. (2019) at an open ocean station (55° 93′ S, 140° 41′ E, from depths between 100 and 700 m). The open ocean water was 0.2 µm filtered (PALL, Acropak 200) directly on board.
While open ocean water was collected in the SO, coastal water was collected at the coastline of Tasmania during summer, with a considerably different composition. Therefore, an extensive analysis of these two water types was done. Both water types were stored for aging in large containers (Nalgene, 20 L, LDPE) in the dark at 4 °C for at least a month prior to the experiments to ensure the complete oxidation of dFe(II), which usually happens within minutes to hours (Millero et al. 1987).
Study organisms
The haptophyte P. antarctica and the diatom F. cylindrus were collected and isolated from Antarctic pack ice (Davis station, East Antarctica) in 2015. All cultures were grown under cool white, fluorescent light (50 µmol photon m−2 s−1, 12:12 light:dark cycle, Osram) at 2 °C ± 1 °C prior to the experiment. P. antarctica was cultured in L1 medium, while the diatom F. cylindrus was kept in Aquil medium before they were inoculated into the two distinct seawaters (coastal and open ocean). Both species were washed in either coastal or open ocean seawater three times to reduce the amount of residual ethylenediaminetetraacetic acid (EDTA) left from either L1 or Aquil. The final concentrations of EDTA for the experiments using P. antarctica and F. cylindrus were calculated to be 0.8 nM and 0.7 nM, respectively.
Temperature, pH and salinity
The seawater temperatures in the rack and the temperature block were measured daily using a built-in pH meter probe (Hach HQ40D, Probe No. PHC10101). Salinity and pH of the seawater were measured initially at 20 °C using a conductivity probe (Orion 013005MD, Thermo scientific) and the same pH meter. Seawater pH and salinity values are given in Table 1.
Macronutrients
Prior to the experiment, samples from coastal and open ocean water were filtered through a 0.2 µm syringe filter (PES, Millex GP) into PC vials (15 mL) and frozen at – 80 °C until analysis. Phosphate (PO43−), nitrate (NO3−) and silicic acid (Si(OH)4) concentrations were measured within 12 months of sampling, using a 4 channel LACHAT Quick-Chem 8500 auto analyser, following the Quick-Chem-methods by Diamond (2008a, b) and Liao (2008). Macronutrient concentrations at the beginning of the experiment are given in Table 1.
Trace elements
Samples for dissolved trace metals (Cd, Co, Cu, Fe, Ga, Mn, Ni, Pb, Ti, V, Zn) were collected in 125 mL LDPE bottles (Nalgene; 0.2 µm filtered, Millex, GP), acidified with distilled HCl to pH 1.8 and stored for at least a month. The dissolved trace metal concentrations were determined using an offline combination of a seaFAST S2 pico (ESI, Elemental Scientific, USA) multi-element extraction system with a Nobias Chelate-PA1 column, followed by analysis on a sector field inductively coupled plasma mass spectrometer (SF-ICPMS, Element 2 Thermo Fisher Scientific, Inc. (Wuttig et al. 2019). A preconcentration factor of 53.33 was achieved by preconcentrating 40 mL of inline buffered sample onto a Nobias PA1 column. Afterwards, the column was eluted with 750 µL of 1.7M distilled nitric acid (HNO3). To obtain blank values, acidified UHP water samples were analysed without any additions. The blank (acidified UHP water with no addition) was subtracted from the sample values. The detection limit for Fe was determined as three times the standard deviation of the acidified UHP blank and was 0.002 nmol kg−1 with a recovery for Fe of 101%, which is within the error of the measurement. Trace metal concentrations at the beginning of the experiments are given in Table 1.
Voltammetry
Concentrations of iron binding organic ligands in the two oceanic water types, were determined by Competitive Ligand Exchange-Adsorptive Cathodic Stripping voltammetry (CLE-AdCSV). The system (757 VA Computrace, Metrohm, Switzerland) uses a hanging mercury drop electrode, a glassy carbon counter electrode, and a silver/silver chloride reference electrode (provided with an inner electrode submerged in a 3M KCl solution, Metrohm) which acts as bridge electrolyte. A 2-(2 tiazolylazo)-p-cresol (TAC) was used as the competing ligand (Croot and Johansson 2000). In order to maintain pH, 100 µL of a 1 M stock EPPS buffer solution was added to 20 mL seawater and mixed. Additions of Fe were made from a 10 mM Fe(III) stock in 1% Q-HCl ranging from 0 to 20.4 nM. After an equilibration time of 3 h, 100 µL of a 0.01 M TAC solution was added into each Teflon vial. After overnight equilibration, the content of all Teflon vials was analysed following procedures outlined in Croot and Johansson (2000).
Total dFe concentrations and relative peak height (intensity, nA) were measured using the software ProMCC (Omanović et al. 2015). The values of \(\alpha_{{{\text{Fe}}^{\prime } {\text{TAC2}}}}^{\prime }\) and \(K_{{{\text{Fe}}^{\prime } {\text{TAC2}}}}^{\prime }\) were obtained from seawater salinity (Croot and Johansson 2000). The Langmuir/Gerringa (Gerringa et al. 1995) and Ruzic/van den Berg (Ružić 1982; Van den Berg 1982) methods were used for the simultaneous calculations of total concentration and conditional stability constants to determine the natural Fe-binding organic ligand fraction. Total Fe values derived from earlier SF-ICP-MS analysis were used for the calculations. Ancillary parameters were further calculated as follows: the excess ligand concentration (L′) is calculated as the difference between L and dFe concentrations, whereas the inorganic Fe concentration (Fe′) was calculated according to Eq. 5:
The concentration of organically-bound Fe, expressed as percentage, was calculated as %FeL = 100 ([dFe] − [Fe′])/[dFe]. The side reaction coefficient for Fe complexation with the natural ligand (log α′Fe′L) was obtained as the logarithmic sum between \({\text{K}}_{{{\text{Fe}}^{\prime } {\text{L}}}}^{\prime }\) and L. Results are shown in Table 2, the raw data used can be found under the public repository. https://doi.org/10.25959/S2DK-CV95.
Growth rates
Growth rates were calculated using in vivo chlorophyll a fluorescence (Turner Designs Model 10-AU). The 28 mL vials were dark adapted for 10 min and cooled on ice during measurement. The specific growth rates (µ day−1) were calculated from linear regressions of each replica of the Ln in vivo fluorescence or cell counts versus time (t) for exponentially growing cultures, where N0 and N1 are the densities at the beginning and end of an exponential growth phase (Eq. 6).
Irradiance was 50 ± 5 µmol photons m−2 s−1, measured with a 4π quantum sensor (model QSL2100, Biospherical Instruments) in a 12:12 light:dark cycle. The growth rates are reported at the public repository.
Statistical analysis
All statistical analyses were conducted using IBM SPSS (version 27 and 29). Multiple comparison tests (univariate/Tukey Posthoc tests) were used to assess the difference through significance by grouping into species, coastal and open ocean water and with or without added dFe(II). The dependent variable was the growth rate, the fixed factor was temperature.
ANOVAs were used to assess the impacts of natural iron concentrations vs. additions of 5 nM dFe(II) and the impact of temperature between the different treatments. For specific information on each temperature and dFe(II) additions, a pairwise comparison of variables was undertaken using a 2-way ANOVA. All testing was done at the 95% confidence level. The data used can be found in the public repository mentioned above.
Results
Seawater characterization
The nutrient concentrations in the open ocean seawater were 19.64 µM for nitrate and 1.29 µM for phosphate (Table 1). This corresponds to an N:P ratio of 15.2:1, which is close to the Redfield ratio of 16:1 (Redfield 1934). The coastal water concentrations for nitrate were 0.37 µM and 0.32 µM for phosphate (ratio: 1.15:1). The silica (Si) concentration was 22.47 µM in the open ocean water. No Si measurements were taken for coastal waters. High values of ammonia were measured in the open ocean water (3.06 µM) compared to coastal water (0.25 µM).
Except for cadmium (Cd), vanadium (V) and nickel (Ni), all trace metals analysed were higher in the coastal water when compared to the open ocean water. This is especially pronounced for Fe, manganese (Mn), lead (Pb) and zinc (Zn). Smaller differences were found for cobalt (Co), copper (Cu), gallium (Ga) and titanium (Ti) (Table 1).
Growth response to temperatures and dFe(II) in coastal and open ocean water
Tukey’s HSD Post-Hoc Tests revealed, that without Fe addition, P. antarctica had its highest growth rate in coastal water at 7 °C (0.31 ± 0.01 µ day−1, n = 3). This rate was significantly higher compared to growth at 3 °C and 5 °C (0.26 ± 0.01 µ day−1, n = 3, p = 0.006 and 0.25 ± 0.02 µ day−1, n = 3, p = 0.003 respectively). In open ocean water (no Fe addition), P. antarctica grew best at 7 °C (0.29 ± 0.04 µ day−1, n = 3), which was not significantly different to the growth rate at 3 °C (0.24 ± 0.01 µ day−1, n = 3, p = 0.164). The growth rate at 5 °C (0.18 ± 0.01 µ day−1, Fig. 3), however, was significantly lower compared to growth rates at 3 °C (n = 3, p = 0.048 and 7 °C, n = 3, p = 0.005).
The highest growth rate of F. cylindrus in coastal water without iron added was found at 3 °C (0.20 ± 0.03 µ day−1, n = 3), which did not differ significantly from that at 5 °C (0.20 ± 0.03 µ day−1, n = 3, p = 0.99) but decreased significantly to 0.05 ± 0.06 µ day−1 when incubated at 7 °C (n = 3, p = 0.010). In open ocean water without Fe addition, F. cylindrus also had the highest growth rate at 3 °C (0.25 ± 0.02 µ day−1, n = 3) which did not change significantly when grown at 5 °C and 7 °C (0.22 ± 0.02 µ day−1, n = 3, p = 0.379 and 0.22 ± 0.04 µ day−1, n = 3, p = 0.269 respectively).
For P. antarctica, the growth rates in coastal water at 3 °C and 5 °C with dFe(II) addition were the same at 0.31 ± 0.02 µ day−1 (n = 3). However, there was a significant increase at 7 °C to a growth rate of 0.43 ± 0.04 µ day−1 (n = 3, p = 0.040 for both). In open ocean water, the growth rate increased even more with the addition of dFe(II) at 7 °C (0.48 ± 0.04 µ day−1), which was significantly higher compared to the growth rate at 3 °C and 5 °C (0.33 ± 0.03 µ day−1, n = 3, p = 0.005 and 0.35 ± 0.02 µ day−1, n = 3, p = 0.001 respectively).
Fragilariopsis cylindrus also had a higher growth rate when 5 nM dFe(II) was added. Its growth in coastal water displayed a steady but non-significant increase from 3 °C (0.21 ± 0.08 µ day−1, n = 3) to 5 °C (0.27 ± 0.04 µ day−1, n = 3, p = 0.665) and was also non-significant from 5 to 7 °C (0.29 ± 0.07 µ day−1, n = 3, p = 0.910). In open ocean water, there was a slight non-significant decline in growth from 3 °C (0.27 ± 0.02 µ day−1, n = 3) to 5 °C (0.25 ± 0.1 µ day−1, n = 3, p = 0.654) with a significant increase again when incubated at 7 °C (0.31 ± 0.02 µ day−1, n = 3, p = 0.019) compared to growth at 5 °C (n = 3, p = 0.057).
Combined impacts of temperature and Fe additions on growth
One-way ANOVAs showed that in coastal water, P. antarctica grew significantly better when Fe was added at 3 °C (F1,4 = 20.45, p = 0.010), 5 °C (F1,2 = 15.43, p = 0.021) and at 7 °C (F1,4 = 30.63, p = 0.045, Fig. 3). In open ocean water, the difference in growth when Fe was added was significant at 3 °C (F1,4 = 30.38, p = 0.008), 5 °C (F1,4 = 122.10, p = 0.001) and 7 °C (F1,4 = 28.00, p = 0.045), respectively. In contrast to this, F. cylindrus only showed significant differences in growth rate in coastal water upon the Fe addition at 7 °C (F1,4 = 25.41, p = 0.012). In open ocean water, F. cylindrus showed similar trends as in coastal water and the addition of Fe significantly increased the growth rate at 7 °C only (F1,4 = 11.76, p = 0.027).
A two-way ANOVA for the combined treatments revealed that for P. antarctica there was no significant interaction (level 0.5) between Fe additions and temperature on growth in coastal water (F2,12 = 4.85, p = 0.269), whereas the interaction of Fe addition and temperature changes had a significant impact on growth in open ocean water (F2,12 = 4.70, p = 0.029). For F. cylindrus, the combined treatments of Fe additions and temperature increases were significant for growth in both water types (F2,12 = 7.90, p = 0.013 and F2,12 = 3.65, p = 0.025, respectively).
Iron-binding organic ligands
The ligand concentrations were 19.30 ± 1.1 nM for open ocean water (n = 3), and 15.0 ± 0.5 nM for coastal water (n = 3, Table 2). The ligand to Fe (L:dFe) ratio was very high for the open ocean water (128.67), but low for coastal water (1.29). The binding strength value \({\text{logK}}_{{{\text{Fe}}^{\prime } {\text{L}}}}^{\prime }\) was 11.21 ± 0.05 (n = 3) for open water, and 11.86 ± 0.08 for (n = 3) coastal water. The freely available Fe′ was 0.05 pM in open ocean water and 4.72 pM in coastal water. For both samples > 99% of dFe was complexed by organic ligands. The \({\text{log}}\alpha_{{{\text{Fe}}^{\prime } {\text{L}}}}^{\prime }\) revealed a lower reactivity (3.49) for open ocean water, and a higher reactivity (4.06) for coastal water.
Discussion
Growth rates of P. antarctica and F. cylindrus
Phaeocystis antarctica and F. cylindrus are ecologically important SO species, making them useful as model organisms for ocean warming scenarios. P. antarctica can form large blooms which makes it an important contributor to the ocean carbon cycle (Smith Jr. et al. 1991), but it is also a key player for the sulfur cycle (DiTullio et al. 2000). F. cylindrus also plays an important role in ocean carbon fixation as it is one of the most widespread cold-water diatoms in the world’s ocean (Mock and Hoch 2005). Without Fe addition, the growth rate of P. antarctica increased in both seawaters when temperature was increased from 3 to 7 °C. This contradicts our assumption that temperature increase would decrease iron bioavailability and reduce growth in low Fe open ocean water. However, dFe(II) additions did result in higher growth rates in both waters and further amplified the increase in growth rates with increasing temperature (Fig. 3). Growth of P. antarctica was likely limited by low iron bioavailability in both waters and the increasing temperature did not affect Fe(II) oxidation enough to significantly affect biological uptake. High ligand concentrations might have been also responsible for the results.
Other studies report a two-fold increase in the growth rate of P. antarctica when up to 1 µM Fe (replete) was added at 2–3 °C (Alderkamp et al. 2012; Strzepek et al. 2019). This increase was much larger compared to our study where the growth rate of P. antarctica increased from 0.24 ± 0.01 to 0.33 ± 0.03 with dFe(II) addition at 3 °C in open ocean water (Fig. 3). The use of natural water vs. artificial seawater medium (Aquil) may have accounted for some of the variation seen between studies (Luxem et al. 2017; Andrew et al. 2019; Strzepek et al. 2019). One reason could be freshly added vitamins in artificial seawater media. A study by Trimborn et al. (2017b) used natural seawater and added fresh vitamins. Their results displayed similar growth rates (0.4 µ day−1) as found by Strzepek et al. (2019) and Alderkamp et al. (2012).
For F. cylindrus, we had expected an increase in growth with increasing temperatures under Fe replete conditions as shown by Pančić et al. (2015) which was not supported by our findings. In both seawaters, this diatom had the lowest growth rates at 7 °C in the treatments without iron, though this was counteracted with dFe(II) addition (Fig. 3). Alderkamp et al. (2012) report lower growth rates in Fe-limited conditions (0.05 µ day−1) and in Fe-replete cultures (0.16 µ day−1) at 2 °C. These latter results do not compare to our findings for open or coastal water as the growth rates were always higher (e.g. open ocean water 3 °C: −Fe: 0.25 µ day−1, +Fe: 0.27 µ day−1; coastal water 3 °C: − Fe: 0.20 µ day−1, + Fe: 0.21 µ day−1). The study by Pančić et al. (2015) shows that there is a large difference in the physiological response of different strains of F. cylindrus to temperature. It is possible that similar strain specific reactions to iron limitation could explain the difference between our findings and those of Alderkamp et al. (2012).
For P. antarctica, a thermal window ranging from – 1 to + 8 °C was reported, with its optimum growth at 6 °C and a sharp decrease in growth rate at 7 °C (Boyd 2019). Based on these findings we assumed that P. antarctica would grow best in our 5 °C treatment and that growth would decrease at 7 °C. However, in both water types we observed the highest growth rates at 7 °C, regardless of Fe treatment. In open ocean water however, P. antarctica did not grow as well, regardless of the dFe(II) addition which could be due to a limitation in other nutrients such as N and P, or lack of micronutrients which are usually added to artificial seawaters. In contrast, F. cylindrus did not grow well (0.05 ± 0.06 µ day−1) in coastal water at 7 °C when no additional Fe was added. Since we did not analyse the Si concentrations in the coastal water used, we can’t say if low Si limitation could have affected the growth rate of this diatom. Since both species can generally grow at 7 °C, further strain specific physiological investigation using higher temperatures would be required to examine the thermal tolerance window to try to explain their growth in future, presumably warmer oceans. This however may not be realistic as a temperature above 7 °C already exceeds the expected temperature changes within the foreseeable future, based on the predictions from latest IPCC report (Bindoff et al. 2019).
Iron binding organic ligands
Both water types used in this study had high ligand concentration, which bound most of the Fe (> 99%) that was present before the addition. We had assumed that an addition of 5 nM dFe(II) would create an Fe(II) ‘saturated’ condition for a short period, with unknown rates for uptake or organic ligand binding. This boost of dFe(II) could have led to a retarded oxidation of dFe(II) due to binding to excess ligands (Roy et al. 2008), which we assumed might result in increased growth in both species in both water types, regardless of the temperature treatment. We indeed observed higher growth rates in all treatments when dFe(II) was added (Fig. 3).
The ratio of ligands to the overall Fe concentration may also have been an important factor in the availability of dFe(II) and therefore the resulting growth. The open ocean water had a very high ligand to Fe ratio (128:1) whereas the ratio in coastal water was lower at 1.29:1. A speculative answer for why both species grew well upon the addition of 5 nM might be related to the specific reactivity (\({\text{log}}\alpha_{{{\text{Fe}}^{\prime } {\text{L}}}}^{\prime }\)) of the ligands in each water type, which was generally low in open ocean water (3.49) and in coastal water (4.06). While this difference may not be significant, open ocean water was low in total Fe concentrations and high but less reactive ligand concentrations compared to coastal water. In coastal water on the other hand, ligands were more reactive with higher Fe concentrations and Fe-ligand concentrations.
Additionally, the open ocean water had a slightly lower ligand binding strength (11.21 ± 0.05) compared to the coastal water (11.86 ± 0.08). Although the difference is small, it may have facilitated Fe availability in open ocean water compared to coastal water.
Conclusions
Additions of dFe(II) resulted in a direct biological response in both water types and for both species. It is likely that the added dFe(II) was bound by excess ligands, sustaining it in the dissolved form, leaving it ‘ready to use’ and freely available for phytoplankton.
Temperature increases combined with dFe(II) addition led to a higher growth for P. antarctica in open ocean (0.33 µ day−1 at 3 °C vs. 0.48 µ day−1 at 7 °C) and coastal (0.26 µ day−1 at 3 °C vs. 0.43 µ day−1 at 7 °C) water. Overall, we did not observe any indication that warming in low iron waters would decrease Fe bioavailability and therefore reduce growth of the tested species as hypothesized. For F. cylindrus, no increased growth rate was observed at higher temperatures. This contradicts the findings of Pančić et al. (2015). Generally, the different changes in growth rates with increasing temperature might have implications for shifts in phytoplankton community composition under future climate scenarios, which may result in changes for carbon export and food web structures as both species make a large contribution to primary production in the SO.
Many SO phytoplankton studies use cold water species with optimum growths at temperatures between 0 and 5 °C. P. antarctica and F. cylindrus were chosen based on their greater temperature range. We suggest future studies look at natural plankton communities including different strains of the same species, to provide better insight into community composition changes from warming effects.
Change history
08 October 2023
The ORCiD https://orcid.org/0000-0003-4010-5918 for author Kathrin Wuttig was inadvertently omitted.
References
Alderkamp AC, Kulk G, Buma AG, Visser RJ, Van Dijken GL, Mills MM, Arrigo KR (2012) The effect of iron limitation on the photophysiology of Phaeocystis antarctica (Prymnesiophyceae) and Fragilariopsis cylindrus (Bacillariophyceae) under dynamic irradiance. J Phycol 48:45–59. https://doi.org/10.1111/j.1529-8817.2011.01098.x
Andrew SM, Morell HT, Strzepek RF, Boyd PW, Ellwood MJ (2019) Iron availability influences the tolerance of Southern Ocean phytoplankton to warming and elevated irradiance. Front Mar Sci. https://doi.org/10.3389/fmars.2019.00681
Arrigo KR, Robinson DH, Worthen DL, Dunbar RB, DiTullio GR, VanWoert M, Lizotte MP (1999) Phytoplankton community structure and the drawdown of nutrients and CO2 in the Southern Ocean. Science 283:365–367. https://doi.org/10.1126/science.283.5400.365
Bindoff N, Cheung W, Kairo J, Arístegui J, Guinder V, Hallberg R, Hilmi N, Jiao N, Karim M, Levin L, O’Donoghue S, Purca Cuicapusa S, Rinkevich B, Suga T, Tagliabue A, Williamson P (2019) Changing ocean, marine ecosystems, and dependent communities. In: Pörtner H-O, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A, Petzold J, Rama B, Weyer NM (eds) IPCC special report on the ocean and cryosphere in a changing climate. Cambridge University Press, Cambridge, pp 447–587. https://doi.org/10.1017/9781009157964.007
Bowie AR, Achterberg EP, Sedwick PN, Ussher S, Worsfold PJ (2002) Real-time monitoring of picomolar concentrations of iron(II) in marine waters using automated flow injection-chemiluminescence instrumentation. Environ Sci Technol 36:4600–4607. https://doi.org/10.1021/es020045v
Boyd PW (2019) Physiology and iron modulate diverse responses of diatoms to a warming Southern Ocean. Nat Clim Change 9:148–152. https://doi.org/10.1038/s41558-018-0389-1
Boye M, van den Berg CMG, de Jong JTM, Leach H, Croot P, de Baar HJW (2001) Organic complexation of iron in the Southern Ocean. Deep Sea Res Part I Oceanogr Res Pap 48:1477–1497. https://doi.org/10.1016/S0967-0637(00)00099-6
Croot PL, Johansson M (2000) Determination of iron speciation by cathodic stripping voltammetry in seawater using the competing ligand 2-(2-thiazolylazo)-p-cresol (TAC). Electroanalysis 12:565–576. https://doi.org/10.1002/(SICI)1521-4109(200005)12:8%3c565::AID-ELAN565%3e3.0.CO;2-L
Croot PL, Laan P (2002) Continuous shipboard determination of Fe(II) in polar waters using flow injection analysis with chemiluminescence detection. Anal Chim Acta 466:261–273. https://doi.org/10.1016/S0003-2670(02)00596-2
Cutter G, Casciotti K, Croot P, Geibert W, Heimbuerger LE, Lohan MC, Planquette H, Flierdt T (2017) Sampling and sample-handling protocols for GEOTRACES cruises. http://www.geotraces.org/images/stories/documents/intercalibration/Cookbook.pdf
Diamond D (2008) Determination of nitrate/nitrite in brackish or seawater by flow injection analysis. QuickChem method, LaChat Instruments, Loveland
Diamond D (2008) Determination of orthophosphate in brackish or seawater by flow injection analysis. QuickChem method, LaChat Instruments, Loveland
DiTullio GR, Grebmeier JM, Arrigo KR, Lizotte MP, Robinson DH, Leventer A, Barry JP, VanWoert ML, Dunbar RB (2000) Rapid and early export of Phaeocystis antarctica blooms in the Ross Sea, Antarctica. Nature 404:595–598. https://doi.org/10.1038/35007061
Gerringa LJA, Herman PMJ, Poortvliet TCW (1995) Comparison of the linear Van den Berg/Ružić transformation and a non-linear fit of the Langmuir isotherm applied to Cu speciation data in the estuarine environment. Mar Chem 48:131–142. https://doi.org/10.1016/0304-4203(94)00041-B
Haber F, Weiss J (1932) Über die katalyse des hydroperoxydes. Sci Nat 20:948–950. https://doi.org/10.1007/BF01504715
Hansard SP, Landing WM (2009) Determination of iron(II) in acidified seawater samples by luminol chemiluminescence. Limnol Oceanogr Methods 7:222–234. https://doi.org/10.5194/bg-6-2281-2009
Hassler CS, Schoemann V (2009) Bioavailability of organically bound Fe to model phytoplankton of the Southern Ocean. Biogeosciences 6:2281–2296. https://doi.org/10.5194/bg-6-2281-2009
Hoffmann LJ, Breitbarth E, Boyd PW, Hunter KA (2012) Influence of ocean warming and acidification on trace metal biogeochemistry. Mar Ecol Prog Ser 470:191–205. https://doi.org/10.3354/meps10082
Holmes TM, Wuttig K, Chase Z, van der Merwe P, Townsend AT, Schallenberg C, Tonnard M, Bowie AR (2019) Iron availability influences nutrient drawdown in the Heard and McDonald Islands region, Southern Ocean. Mar Chem 211:1–14. https://doi.org/10.1016/j.marchem.2019.03.002
Hunter KA, Boyd PW (2007) Iron-binding ligands and their role in the ocean biogeochemistry of iron. Environ Chem 4:221–232. https://doi.org/10.1071/EN07012
King DW, Lounsbury HA, Millero FJ (1995) Rates and mechanism of Fe(II) oxidation at nanomolar total iron concentrations. Environ Sci Technol 29:818–824. https://doi.org/10.1021/es00003a033
Kuma K, Matsunaga K (1995) Availability of colloidal ferric oxides to coastal marine phytoplankton. Mar Biol 122:1–11. https://doi.org/10.1007/BF00349272
Kuma K, Nakabayashi S, Suzuki Y, Kudo I, Matsunaga K (1992) Photo-reduction of Fe(III) by dissolved organic substances and existence of Fe(II) in seawater during spring blooms. Mar Chem 37:15–27. https://doi.org/10.1016/0304-4203(92)90054-E
Lacour T, Larivière J, Babin M (2017) Growth, chl a content, photosynthesis, and elemental composition in polar and temperate microalgae. Limnol Oceanogr 62:43–58. https://doi.org/10.1002/lno.10369
Liao N (2008) Determination of ammonia in brackish or seawater by flow injection analysis. LaChat Instruments, Loveland
Lis H, Shaked Y, Kranzler C, Keren N, Morel FM (2015) Iron bioavailability to phytoplankton: an empirical approach. ISME J 9:1003–1013. https://doi.org/10.1038/ismej.2014.199
Luxem KE, Ellwood MJ, Strzepek RF (2017) Intraspecific variability in Phaeocystis antarctica’s response to iron and light stress. PLoS ONE 12:e0179751. https://doi.org/10.1371/journal.pone.0179751
Maldonado MT, Price NM (1999) Utilization of iron bound to strong organic ligands by plankton communities in the subarctic Pacific Ocean. Deep Sea Res Part II Top Stud Oceanogr 46:2447–2473. https://doi.org/10.1016/S0967-0645(99)00071-5
Maldonado MT, Price NM (2002) Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae). J Phycol 37:298–310. https://doi.org/10.1046/j.1529-8817.2001.037002298.x
Millero FJ, Izaguirre M (1989) Effect of ionic strength and ionic interactions on the oxidation of Fe(II). J Solut Chem 18:585–599. https://doi.org/10.1007/BF00664239
Millero FJ, Sotolongo S (1989) The oxidation of Fe(II) with H2O2 in seawater. Geochim Cosmochim Acta 53:1867–1873. https://doi.org/10.1016/0016-7037(89)90307-4
Millero FJ, Sotolongo S, Izaguirre M (1987) The oxidation kinetics of Fe(II) in seawater. Geochim Cosmochim Acta 51:793–801. https://doi.org/10.1016/0016-7037(87)90093-7
Mock T, Hoch N (2005) Long-term temperature acclimation of photosynthesis in steady-state cultures of the polar diatom Fragilariopsis cylindrus. Photosynth Res 85:307–317. https://doi.org/10.1007/s11120-005-5668-9
Moffett JW, Zika RG (1987) Reaction kinetics of hydrogen peroxide with copper and iron in seawater. Environ Sci Technol 21:804–810. https://doi.org/10.1021/es00162a012
Morel FMM, Kustka AB, Shaked Y (2008) The role of unchelated Fe in the iron nutrition of phytoplankton. Limnol Oceanogr 53:400–404. https://doi.org/10.4319/lo.2008.53.1.0400
Noiri Y, Kudo I, Kiyosawa H, Nishioka J, Tsuda A (2005) Influence of iron and temperature on growth, nutrient utilization ratios and phytoplankton species composition in the western subarctic Pacific Ocean during the SEEDS experiment. Prog Oceanogr 64:149–166. https://doi.org/10.1016/j.pocean.2005.02.006
Omanović D, Garnier C, Pižeta I (2015) ProMCC: an all-in-one tool for trace metal complexation studies. Mar Chem 173:25–39. https://doi.org/10.1016/j.marchem.2014.10.011
Pančić M, Hansen PJ, Tammilehto A, Lundholm N (2015) Resilience to temperature and pH changes in a future climate change scenario in six strains of the polar diatom Fragilariopsis cylindrus. Biogeosciences 12:4235–4244. https://doi.org/10.5194/bg-12-4235-2015
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol 132:739–761. https://doi.org/10.1016/S1095-6433(02)00045-4
Redfield AC (1934) On the proportions of organic derivatives in sea water and their relation to the composition of plankton. University Press of Liverpool, Liverpool
Rich HW, Morel FMM (1990) Availability of well-defined iron colloids to the marine diatom Thalassiosira weissflogii. Limnol Oceanogr 35:652–662. https://doi.org/10.4319/lo.1990.35.3.0652
Roy EG, Wells ML, King DW (2008) Persistence of iron(II) in surface waters of the western subarctic Pacific. Limnol Oceanogr 53:89–98. https://doi.org/10.4319/lo.2008.53.1.0089
Ružić I (1982) Theoretical aspects of the direct titration of natural waters and its information yield for trace metal speciation. Anal Chim Acta 140:99–113. https://doi.org/10.1016/S0003-2670(01)95456-X
Salmon TP, Rose AL, Neilan BA, Waite TD (2006) The FeL model of iron acquisition: nondissociative reduction of ferric complexes in the marine environment. Limnol Oceanogr 51:1744–1754. https://doi.org/10.4319/lo.2006.51.4.1744
Schallenberg C, Bestley S, Klocker A, Trull TW, Davies DM, Gault-Ringold M, Eriksen R, Roden NP, Sander SG, Sumner M, Townsend AT, Merwe P, Westwood K, Wuttig K, Bowie A (2018) Sustained upwelling of subsurface iron supplies seasonally persistent phytoplankton blooms around the southern Kerguelen plateau, Southern Ocean. Geophys Res Oceans 123:5986–6003. https://doi.org/10.1029/2018JC013932
Schlitzer R (2002) Carbon export fluxes in the Southern Ocean: results from inverse modeling and comparison with satellite-based estimates. Deep Sea Res Part II Top Stud Oceanogr 49:1623–1644. https://doi.org/10.1016/S0967-0645(02)00004-8
Sedwick PN, Edwards PR, Mackey DJ, Griffiths FB, Parslow JS (1997) Iron and manganese in surface waters of the Australian subantarctic region. Deep Sea Res Part I Oceanogr Res Pap 44:1239–1253. https://doi.org/10.1016/S0967-0637(02)00021-3
Shaked Y, Lis H (2012) Disassembling iron availability to phytoplankton. Front Microbiol 3:123. https://doi.org/10.3389/fmicb.2012.00123
Shaked Y, Kustka AB, Morel FMM (2005) A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol Oceanogr 50:872–882. https://doi.org/10.4319/lo.2005.50.3.0872
Shi D, Xu Y, Hopkinson BM, Morel FM (2010) Effect of ocean acidification on iron availability to marine phytoplankton. Science 327:676–679. https://doi.org/10.1126/science.1183517
Smith WO Jr, Codispoti LA, Nelson DM, Manley T, Buskey EJ, Niebauer HJ, Cota GF (1991) Importance of Phaeocystis blooms in the high-latitude ocean carbon cycle. Nature 352:514–516. https://doi.org/10.1038/352514a0
Strzepek RF, Boyd PW, Sunda WG (2019) Photosynthetic adaptation to low iron, light, and temperature in Southern Ocean phytoplankton. Proc Natl Acad Sci USA 116:4388–4393. https://doi.org/10.1073/pnas.1810886116
Tagliabue A, Bowie AR, Boyd PW, Buck KN, Johnson KS, Saito MA (2017) The integral role of iron in ocean biogeochemistry. Nature 543:51–59. https://doi.org/10.1038/nature21058
Trimborn S, Brenneis T, Hoppe CJM, Laglera LM, Norman L, Santos-Echeandía J, Völkner C, Wolf-Gladrow D, Hassler CS (2017a) Iron sources alter the response of Southern Ocean phytoplankton to ocean acidification. Mar Ecol Prog Ser 578:35–50. https://doi.org/10.3354/meps12250
Trimborn S, Thoms S, Brenneis T, Heiden JP, Beszteri S, Bischof K (2017b) Two Southern Ocean diatoms are more sensitive to ocean acidification and changes in irradiance than the prymnesiophyte Phaeocystis antarctica. Physiol Plant 160:155–170. https://doi.org/10.1111/ppl.12539
Van den Berg C (1982) Determination of copper complexation with natural organic ligands in seawater by equilibration with MnO2 I. Theory Mar Chem 11:307–322. https://doi.org/10.1016/0304-4203(82)90028-7
Wuttig K, Townsend AT, van der Merwe P, Gault-Ringold M, Holmes T, Schallenberg C, Latour P, Tonnard M, Rijkenberg MJA, Lannuzel D, Bowie AR (2019) Critical evaluation of a seaFAST system for the analysis of trace metals in marine samples. Talanta 197:653–668. https://doi.org/10.1016/j.talanta.2019.01.047
Acknowledgements
We are grateful to the captain, crew, and scientists onboard the SR3 expedition (IN2018_V01) of the R.V Investigator. For water collection and filtration, we would like to thank Pauline Latour, Pier van der Merwe, Melanie Gault-Ringold, Christine Weldrick and Matt Corkill. We further would like to thank Damon Britton and Catriona Hurd for analysis of the seawater nutrient concentrations, Pier van der Merwe, Melanie Gault-Ringold and Pauline Latour for their assistance with preparing the samples for SF-ICP-MS analyses and Ashley Townsend for analysing them. Further we would like to thank Andrew McMinn for providing the phytoplankton cultures, Fraser Kennedy for giving insights into culturing techniques and Robert Strzepek for providing comments on the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was supported under the Australian Research Council’s Special Research Initiative for Antarctic Gateway Partnership (Project ID SR140300001). We are grateful for funding provided by the Australian Government’s Cooperative Research Centres Program through the Antarctic Climate and Ecosystems Cooperative Research Centre (ACE CRC), for the nutrient analysis provided by the ACE CRC, and the analysis of the trace metal samples using SF-ICP-MS funded by the Australian Research Council LIEF scheme (LEO989539).
Author information
Authors and Affiliations
Contributions
HA, KW and ARB designed the research. HA conducted the research. HA, LH, KW, ARB, CG and TH interpreted the data. HA wrote the manuscript with help from LH, TH, KW and ARB. All authors read, reviewed, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares neither conflict of interest nor competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aflenzer, H., Hoffmann, L., Holmes, T. et al. Effect of dissolved iron (II) and temperature on growth of the Southern Ocean phytoplankton species Fragilariopsis cylindrus and Phaeocystis antarctica. Polar Biol 46, 1163–1173 (2023). https://doi.org/10.1007/s00300-023-03191-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03191-z