Abstract
Polar deserts contain unique and sensitive communities responsive to climate-associated habitat changes. However, unlike temperate desert ecosystems, characterizing and predicting the responses of polar ecosystems to environmental change remains a significant challenge due to logistical constraints. Here we aim to demonstrate the use of a custom-designed Polar Desert Environmental Chamber (PDEC) to perform off-continent experimental ecological research. We did so by characterizing the structure and composition of arid edaphic bacterial communities collected from the McMurdo Dry Valleys during a simulated wetting event. The results were discussed in light of previous field observations. Rapid structural and compositional changes were observed during wetting and re-drying treatments. Those were driven by changes in the relative abundance of coexisting taxa, which fluctuated asynchronously over time in response to the treatments. While selection was the main ecological factor influencing communities during dry conditions or the initial wetting, with prolonged exposure to wetness, neutral processes began to drive community assembly. Ultimately, these observations reflect different adaptative responses from microbial taxa to water stress, which can be argued as beneficial to increasing resilience in polar deserts. Our findings demonstrate that experiments conducted in PDEC provide valuable contextual data on community response to environmental change and can accelerate our ability to assess biological thresholds to change within polar desert ecosystems. We advocate that, with careful consideration of key emulated environmental attributes, laboratory-based Antarctic research can complement fieldwork to achieve a nuanced and evidence-based understanding of the ecology of Antarctica’s ice-free regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terrestrial Antarctica encompasses the largest polar desert and one of the most extreme environments on Earth. The continent is mostly ice-covered, resulting in the majority of its terrestrial biodiversity being constrained to the 1% ice-free soil, patchily distributed across the continent (Lee et al. 2017). The largest ice-free region on the continent is the McMurdo Dry Valleys (MDV), a hyperarid and ultraoligotrophic polar desert (Doran et al. 2002; Bockheim et al. 2007; Cary et al. 2010). Due to the lack of precipitation, liquid water is only ephemerally available during the Austral summer when the combination of air temperature, relative humidity, wind speed, and solar radiation favour the melting of glaciers and the ablation of permafrost (Law and Van Dijk 1994; Fountain et al. 2014). The intensity and frequency of these physical processes can increase water availability by expanding lakes and transiently wet areas such as streams, moats, and water tracks, thereby affecting this region’s ecology.
Antarctic ice-free regions are considered natural laboratories, well-suited for studying the relationship between environmental variability, biodiversity, and ecosystem functioning due to the lack of trophic complexity (Convey et al. 2014). In these regions, microorganisms make up for most biomass and diversity, exhibiting a diverse repertoire of metabolic strategies that facilitate survival and acquisition of energy and carbon despite the severe moisture and nutrient limitations (Ortiz et al. 2021). Microbial distribution, diversity, and function are intimately linked to historical and contemporary abiotic conditions (Lee et al. 2019; Bottos et al. 2020). As a result, these are arguably the most responsive biological entities to environmental change, functioning as biological sentinels for climate change in the system (Monteiro et al. 2022). Field observations and in situ manipulative experiments have driven tremendous progress in predicting and demonstrating the sensitivity of microbial response to present-day changes in Antarctic environmental conditions, particularly to changes in water availability (McKnight et al. 2007; Tiao et al. 2012; Van Horn et al. 2014; Buelow et al. 2016; Niederberger et al. 2019; Monteiro et al. 2022). Initial observations by McKnight et al. (2007) detected an increase in primary productivity from a former dried cyanobacterial mat 1 day after the channel, which had not received substantial water flow for two decades, was re-hydrated. Later, an unprecedented 4-year experimental manipulation involving the transplantation of a mummified seal carcass in the MDV revealed the capacity of the Antarctic soil microbiome to respond to contemporary alterations in relative humidity in timeframes considerably shorter than previously thought (Tiao et al. 2012). More recently, Niederberger et al. (2019), through the simulation of a natural wetting event by re-diverting a meltwater stream into a historically dry soil area in the Miers Valley, showed significant structural and compositional shifts in the soil microbial communities over 7 weeks. These compositional changes co-occurred with the onset of significant declines in the relative abundance of Actinobacteriota, a recognized dominant phylum in dry soils, and an increase of Cyanobacteria and other taxa commonly found in MDV wetted areas, such as Proteobacteria and Bacteroidetes (Monteiro et al. 2022). However, there is still limited information regarding the thresholds and subsequent ability of the Antarctic soil microbiome to recover from changes in its local environment.
Many aspects of change in Antarctic ice-free regions manifest slowly and incrementally over time, and tracking ecological changes over decades is logistically challenging to execute (Convey and Peck 2019). Firstly, the restricted time frame allocated to field access significantly limits any continuous monitoring of biological response to environmental change. Without the constant collection of time series data, the risk of ecological bias within predictions increases by the mismatch between the range of scales at which climate change and biological response occur during temporal studies (Bütikofer et al. 2020). Currently, field manipulations require an extraordinary logistic and personal effort to be achieved, such as the presence of researchers on-site for the duration of the experiment (Niederberger et al. 2019) or, in the case of long-term manipulations, the decision to leave the experiment in place unattended in remote locations year-round without control for unpredictable events (McKnight et al. 2007; Tiao et al. 2012). The latter poses a risk to the quality of the research since it increases the chances of incorporating extraneous variables, making interpreting results difficult or inaccurate. Secondly, strict international regulations plus the requirement for permits and substantial financial and logistic support impose significant limitations on the performance of experimental field manipulations in parts of the continent. For instance, in the MDV, only a few experiments have been successfully performed in situ (McKnight et al. 2007; Tiao et al. 2012; Van Horn et al. 2014; Buelow et al. 2016; Niederberger et al. 2019). Lastly, research activities can leave long-lasting impacts on the soils and their communities (Hughes et al. 2015; O’Neill et al. 2015), which contradicts one of the Antarctic Treaty’s founding principles, the preservation of the continent.
Given the relatively low biological complexity present on the Antarctic continent compared to more temperate or tropical regions, experimental manipulations conducted off-continent, under settings representative of the natural environment, can create a powerful means of hypothesis testing without the permit, temporal and logistical limitations, or anthropogenic impacts associated with field studies. When conducted in parallel with and validated by in-situ observations, laboratory experiments enable the collection of time series from long and short-term controlled disturbances while reducing the complexity of confounding variables and ecological processes in the field. The latter gives a deeper and more controlled perspective on the niche-based and stochastic processes that govern community assembly during environmental change. Moreover, considering the expected increase in the timing and frequency of wetting events around ice-free areas (Fountain et al. 2014; Lee et al. 2017), off-continent laboratory experiments can manipulate brief or prolonged cycles of wetting events, helping to identify the ecological thresholds and tipping points of biological communities. The question remains: can we emulate ice-free Antarctic systems and biological responses in the laboratory?
Here we demonstrate the ability to study the impacts of environmental change on biological communities from the MDV using a Polar Desert Environmental Chamber (PDEC) custom-designed to emulate permafrost ablation, air temperature, relative humidity, and light conditions observed in Antarctic ice-free systems. We validated the PDEC by comparing the response of the MDV soil microbiome to wetting events with previous field observations, particularly to a 7-week wetting experiment performed in Miers Valleys (Niederberger et al. 2019). We subjected previously collected MDV soils to a continuous wetting treatment (8 weeks) and re-drying treatment (4 weeks wet/4 weeks dry). We demonstrate that with adequate and controlled infrastructure, manipulative laboratory experiments are a complementary approach to Antarctic field studies, providing a mechanism for addressing predictions for biological response to single or multiple disturbances. Work of this kind can be expanded to incorporate a variety of perturbations expected to occur in Antarctic ice-free regions, including warming, productivity changes, or species introduction, coupled to activity measurements, which can be performed without the temporal, logistic, and permit constraints nor the anthropogenic impacts associated with field-based work.
Methods
Soil sampling and processing
During the New Zealand Antarctic field season in 2018, surface soil samples (0–2 cm) geochemically characterized as dry were aseptically collected with a sterile spatula 10 m from the shore of Lake Hoare (Eastern side) in Taylor Valley, in the McMurdo Dry Valleys, Antarctica (Monteiro et al. 2022). Lake Hoare located 15 km from the coast, profiles mean annual air temperatures of -14.8 °C and an average mean relative humidity of 66% (Doran et al. 2002). It has the lowest mean yearly solar flux in the Dry Valleys due to its position adjacent to the Aasgard Range (Doran et al. 2002). Samples were stored at − 20 °C for 1 day, transported to Scott Base, back to New Zealand on dry ice, and stored at − 60 °C until processed.
Polar deserts environmental chamber
The experimental design was conducted in a modified Contherm Global5000 Environmental Chamber (CAT 5400/RHS) explicitly designed to emulate key environmental attributes and processes that affect soil microbial communities in ice-free polar deserts (PDEC–Polar Deserts Environmental Chamber) (Fig. S1) (Contherm Scientific Limited, New Zealand). Permafrost is a major controlling factor in the dynamics of Antarctic terrestrial ecosystems, and it is ubiquitously present in ice-free regions (Bockheim et al. 2007). As such, it was fundamental to emulate permafrost conditions in our experimental design. The construction of the underlying permafrost layer started using soils sampled in depth increments in the Miers Valley in 2013. The bulk dry soil was autoclaved and reconstructed inside an insulated acrylic box (54 × 49 × 54 cm) by mixing it with sterile water until a mud-like consistency was achieved (Fig. 1). The permafrost container has a low-temperature cooling coil set to − 15 °C to freeze the mixture (Fig. 1, Fig. S1). Four large open-ended master cylindric containers (12.5 × 35.5 cm) were placed to a depth of 10 cm inside the soil mixture. Once the soil mixture froze, we filled the large master cylinders to the top using the same autoclaved Miers Valley soil. To emulate permafrost ablation processes, which are dynamic processes influenced by air relative humidity, temperature, and wind, the PDEC has the capacity to constantly remove moisture from the chamber through refrigeration. The reduction of relative humidity was assisted by placing a set of fans inside the chamber, which increases air circulation close to the surface of the soil and disturbs the boundary layer. The latter creates an imbalance between the surface of the ice in the permafrost and the percentage of vapour water inside the chamber, causing moisture to move upward and creating a gradient in humidity within the overlying soil (Fig. S2) (Law and Van Dijk 1994). The gradient in humidity was recorded by placing six humidity sensors (HOBO Micro Station Logger) 5 cm apart from the bottom to the top 2 cm in one master cylinder. Humidity changes were recorded in 5 min intervals during the 8 weeks of the experiment (Fig. 1).
a Polar desert environmental chamber (PDEC) designed to emulate temperature, relative humidity, light, and permafrost observed in the McMurdo Dry Valleys. A digital thermostat shows the permafrost container’s current temperature, and an LCD on the door shows the current temperature and relative humidity values inside the chamber; b–d the different phases involved in the reconstruction of the permafrost; b mixing of the second layer of autoclaved dry soil collected in Miers Valley with sterile water inside the permafrost container; c/d insertion of four master cylinders 10 cm down the soil mixture; d soil freezing; e filling the cylinders with additional sterile dry soil; f view of the permafrost container with the master cylinders in place and the small open-ended test cylinders on top filled with Lake Hoare dry soils. Humidity loggers were set on the front right cylinder; g view of the small cylinders containing Lake Hoare soil on top of the master cylinders and the light system above; h experimental setup after the soils were wet
Above the permafrost container, we set a light sourcing system (FUTURELED, Berlin) specifically constructed using LEDs emitting light at all wavelengths across the visible spectra and into the near UV. The light system was set 30 cm above the soils and calibrated to provide similar quality spectra and 50% of the incoming light intensity experienced in the MDVs during the Austral summer (please refer to the methods in the supplementary material) (Fig. S2). The temperature inside the chamber was set to be maintained at 5 °C, as required to maintain the relative humidity inside the chamber of 50%, a value within the range observed in the field (Doran et al. 2002).
Experimental setup
Before the start of the experiment, the entire chamber was cleaned with 80% (vol/vol) ethanol. All incubation cylinders and materials used during the experiment were cleaned with 80% (vol/vol) ethanol and sterilized with UV light for 30 min before setup. On top of the three large master cylinders, we placed ten small open-ended test cylinders containing 40 g of untreated, dry soil collected from Lake Hoare in 2018. Each set of ten test cylinders was subjected to one of the three different treatments for a total of 8 weeks: in the dry control, the soils remained dry throughout the experiment (dry control); in the wetting treatment, the soils were sprayed with 8 ml of sterile Milli-Q water twice a day to stay wet; in the wetting/re-drying treatment, the soils were sprayed with 8 ml of sterile Milli-Q water twice a day for the first 4 weeks and then, they were left to dry gradually for the following 4 weeks of the experiment (Fig. S1). Samples were incubated under 24 h daylight conditions. The chamber conditions were monitored daily, and samples were collected from each treatment before the start of wetting, when all the soils were dry (T0), and after 24 h (T1), 1 week (T2), 4 weeks (T3), 5 weeks (T4), and 8 weeks (T5) from the start of the experiment. After T3, the soils from the wetting/re-drying treatment stopped being wetted. The time points T4 and T5 mark five and 8 weeks, respectively, since the start of the experiment and one and 4 weeks since the soils in the wetting/re-drying treatment stopped being wet. At T0, before the experiment started, one small cylinder placed on top of each master cylinder, representative of each treatment, was removed, and one soil sample from each small cylinder was extracted and stored at − 60 °C until processed. Considering that at T0, all soils were still dry and that all soil samples used in this experiment came from the same field sample, these samples were called the T0 control (n = 3). From T1 until T5, we aseptically removed one of the small cylinders from each treatment and collected three replicates of soil per treatment (n = 3). These samples were stored at − 60 °C until processed. Two additional field samples were used as a field control to ensure that the starting chamber-based community structure reflected what was found in the field. Soil moisture content within each experimental treatment was determined at T5, according to Monteiro et al. (2022).
DNA extraction, sequencing, and data processing
Forty-eight experimental samples (16 from each treatment) were collected from the manipulation experiment and processed alongside two additional samples from the field. Total DNA extraction, the PCR amplification of the 16S rRNA gene, and Next Generation Sequencing library preparation were conducted according to Monteiro et al. (2022). Amplicon sequencing was performed using the Ion PGM™ System for Next Generation Sequencing (ThermoFisher Scientific) at the Waikato DNA Sequencing Facility (University of Waikato, New Zealand).
Sequencing primers and indexing barcodes were removed with cutadapt (v2.3) (Martin 2011). A total of 1,387,788 reads across 50 samples were processed using DADA2 (v1.14.1) (Callahan et al. 2016) to generate amplicon variants (ASVs) in R (v3.6.2) (R Development Core Team 2010). Taxonomical identity was assigned to each variant using the SILVA database (v138) (Quast et al. 2012). ASVs classified as mitochondria, chloroplast, eukaryotes, or without any taxonomical assignment at the Phylum level were removed from the dataset. ASVs not present at T0 or having less than 100 reads across the entire dataset (relative abundance threshold of 0.007%) were removed from our analysis as a control for potential contamination and to help to reduce the noise across the dataset (Bokulich et al. 2013).
Statistical analysis
All statistical and visualization analyses were computed in R (v4) (R Development Core Team 2010) using the following packages: phyloseq (v1.26.1) (McMurdie and Holmes 2013), vegan (v2.5) (Oksanen et al. 2012), Picante (v1.8) (Kembel et al. 2010), ggplot2 (v3.2.1) (Wickham 2016), and DeSeq2 (Love et al. 2014). Alpha diversity using richness and phylogenetic diversity indexes were calculated using a non-rarefied dataset. Diversity differences among groups were tested using one-way ANOVA. Normality assumptions were tested using Shapiro–Wilk test, and the assumption of homogeneity of variances was tested using the Levene’s test. Beta diversity was calculated on a transformed dataset using a total sum normalization and log-ratio transformation (Coenen et al. 2020) and visualized in a principal coordinates analysis (PCoA) using a weighted UniFrac phylogenetic pairwise distance (Lozupone and Knight 2015). Calculated pairwise distances between samples of each treatment relative to the dry control treatment were visualized using boxplots generated using ggpubR (Kassambara and Kassambara 2020) (v0.4.0). PERMANOVA was applied to identify differences in beta diversity between different treatments at each time point. The test was perfomed using adonis from vegan R package (Oksanen et al. 2012). We used betadisp to test for the homogeneity of group dispersions, included in the same package. For differential analysis, raw read counts of ASVs were log2 transformed and modelled using a negative binomial generalized linear model implemented in the DESeq2 package in R (Love et al. 2014). ASVs identified as significantly different resulted from comparisons between wetting treatment and wetting/re-drying treatment relative to the dry control at each time point. An adjusted p-value below 0.01 was considered significant. To evaluate what ecological factors underly the microbial composition with changes in water availability, we calculated phylogenetic community assembly among species within each sample using the mean nearest taxon distance (MNTD) and the nearest taxon index (NTI) using “taxa.labels” null model, with 999 iterations, using the function ‘ses.mntd’ from the R package Picante (v1.8.2). The NTI was quantified as the number of standard deviations that the observed MNTD was from the mean of the MNTD null distribution, multiplying by -1. For a single community, observed NTI values > + 2 or < − 2 indicate phylogenetic clustering of species or phylogenetic overdispersion, respectively. NTI values between − 2 and 2 usually indicate the influence of a stochastic assembly (Stegen et al. 2012).
Results
Chamber monitoring
The chamber maintained a constant 5 °C, 50% relative humidity, and -15 °C in the lower permafrost. Before starting the experiment, Lake Hoare soils presented a moisture content of 0.4 ± 0.3%, pH of 8.75 ± 0.2, and electrical conductivity of 268 ± 267 µS. A power failure affected the chamber and interrupted the permafrost freezing process 29 days into the experiment leading to an increase in the chamber’s temperature and consequent alteration of the relative humidity and defrosting of the permafrost layer for three consecutive days (Fig. S2). This event was recorded after T3 sampling, and likely contributed to the variance observed at T4 and T5 in the dry control samples. We did not identify other impacts in samples submitted to wetting (Fig. 2).
Sequencing analysis
After quality control steps, we identified 2779 ASVs. Chimera check identified 431 bimeric sequences, which were subsequently removed, generating 2348 unique ASVs and 1,339,818 reads (average length 215 bp) across 50 samples. Filtering out low abundant ASVs (< 0.007% cutoff, representative of less than 100 reads across the entire dataset) removed 59% of the initial ASVs. The additional removal of ASVs classified as mitochondria (0 ASVs), chloroplast (2 ASVs), eukaryotes (7 ASVs), not classified at the Phylum level (204 ASVs), not present or with only one sequence at T0 (48 ASVs), removed an additional 27% of the ASVs. The remaining dataset comprised 1,108,012 sequences representing 82% of the initial sequencing data and 706 ASVs.
Temporal dynamics of community diversity during the wetting treatment
We observed a significant decrease in α-diversity, measured using Faith’s Phylogenetic Diversity, after 2 months of wetting (T5) (one-way ANOVA, F-value: 114.91, p-value = 2.58e-08, Figure S3a, Table S1). Differences in α-diversity remained significant in relation the control at the same time point (one-way ANOVA, F-value: 112.84, p-value = 1.74e-05, Figure S3b, Table S1). Nonetheless, no significant differences were encountered among the dry controls throughout the experiment (Figure S3). Additional α-diversity measures supported this trend: the observed number of ASVs, Chao1 richness, and Shannon index (data not shown).
Microbial community β-diversity changed with time and treatment (Fig. 2). Samples were clustered by sampling time point across the primary axis (55% of the variability within the dataset) and treatment across the secondary axis (22% of the variability within the dataset) (Fig. 2). During the 4 weeks of constant wetting (T0-T3), β-diversity increased between wetted samples and the dry controls, for each respective time (Fig. 3). Once the wetting ceased (T3), β-diversity decreased between the control and communities submitted to the re-drying process (T5) (Fig. 3). For each time point, we observed significant differences in β -diversity between the respective control samples and the treated samples (T1-PERMANOVA: F model = 9.40, p-value = 0.03; T2-PERMANOVA: F model = 20.61, p-value = 0.02; T3-PERMANOVA: F model = 28.17, p-value = 0.03; T4-PERMANOVA: F model = 66.44, p-value = 0.01; T5-PERMANOVA: F model = 118.97, p-value = 0.01). The assumptions to conduct a PERMANOVA test were tested and met.
Weighted UniFrac distance values representative of each pairwise comparison calculated between a wetting or wetting/re-drying sample versus the control, and pairwise comparisons between controls, at each respective time point identified by the panel. After T3, the soils from the wetting/re-drying treatment stopped being wetted
Communities from the dry control samples were compositionally and structurally similar to field samples at T0 (Fig. 2). However, we observed variability within the control samples during the experiment (Fig. S4a). While sources for this variability could be related to initial acclimatization to the conditions in the chamber, the highest variation occurred after the chamber breakdown (between T3 and T4), which would have been in response to an increase in temperature and changes in relative humidity (Fig. S2). While changes in temperature and relative humidity may have caused a response from dry communities or communities in the early days of the re-drying step (Fig. S4a/c), the degree of such response was still less pronounced than the response observed to wetting (Fig. S4d). Additionally, we do not expect that such an event had a significant effect on communities that remained wet since the dissimilarity distances between wet samples and the control continuously increased in the same direction (either in relation to the T0 control or in relation to the respective time point control) (Fig. S4b; Fig. 3). This observation suggests the effects of changes in moisture dominates microbial communities from ice-free regions over alterations in temperature. Nonetheless, it also highlights the sensitivity of these communities to subtle environmental changes, and the importance of accurately emulating conditions in manipulative laboratory experiments to avoid generating incorrect interpretations of the results and inaccurate predictions.
Microbial compositional changes during the wetting treatment
Temporal changes in the relative abundances of microbial taxa were analyzed by aggregating all taxa at the phylum (Fig. 4) and family (Fig. 5) levels. Four major bacterial phyla dominated dry soil communities alongside the field samples: Actinobacteriota (39%), mainly composed of the family Solirubrobactereaceae; Bacteroidota (20%), primarily consisting of the family Chitinophagaceae; Acidobacteriota (11%), mainly represented by the family Blastocatellaceae, and Proteobacteria (10%) primarily consisting of the Alphaproteobacteria family Sphingomonadaceae. This structure remained consistent in the dry control soils throughout the entirety of the experiment (Fig. 4).
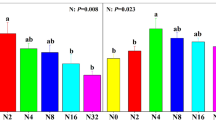
Temporal dynamics of the eight most dominant phyla during the control, wetting, and wetting/re-drying treatments. Panels show the relative sequence abundance (%) changes of the eight most abundant phyla during the 8 weeks of the PDEC manipulative experiment. T0 was taken before the start of the experiment with T1 taken 24 h after, T2 1 week after, and T3 4 weeks after. At T3, soils submitted to the wetting/re-drying treatment stopped being wet. At T4, soils submitted to the wetting treatment were wet for 5 weeks, and soils submitted to the wetting/re-drying treatment haven’t received any water input for 1 week. The last time point, T5, represents 8 weeks of daily wetting and 4 weeks of the drying period
During the wetting treatment and the initial 4-week wet phase of the wetting/re-drying treatment, the phylum Proteobacteria (representing 10% of the initial community) doubled in relative sequence abundance to 23% from T0 to T3. By the end of the experiment (T5), the relative sequence abundance of this phyla was three times higher (from 10 to 31%) compared to the initial sample (T0) in the wetting treatment (Fig. 4). The Proteobacteria family Sphingomonadaceae (Alphaproteobacteria) dominated the community apart from T3 when it was succeeded by taxa belonging to the Oxalobacteraceae family (Fig. 5a). After 1 week of constant wetting (T2 onwards), we observed a continuous increase in the Comamonadaceae and Xanthomonadacea, both belonging to the subdivision Gammaproteobacteria, which became significant after 5 weeks of wetting (Kruskal–Wallis: chi-squared = 13.5, df = 4, p-value = 0.001; chi-squared = 12.9, df = 4, p-value = 0.01, respectively) (Fig. 5a). Alongside Proteobacteria, members of the low abundant phylum Planctomycetota increased steadily by 2.5% in their relative sequence abundance throughout the wetting treatment (Fig. 4). Bacteroidota phylum had an overall increase of 5% with constant wetting treatment compared to the relative abundance at the beginning of the experiment (T0) (Fig. 4). However, throughout the wetting treatment, different families showed an asynchronous response toward this perturbation (Fig. 5d). In contrast, the relative sequence abundance of the Actinobacteriota phylum declined from 39 to 11% in response to wetting (Fig. 4). A 23% reduction in relative sequence abundance across all detected Actinobacteriota families signs the negative impact of wetting in this phylum (Fig. 5b). Taxa within the Acidobacteriota and Chloroflexi also decreased relative sequence abundance by 3% after 8 weeks of continuous wetting (Fig. 4). Such decline was more pronounced in the Blastocatellaceae family (Fig. 5c). Low abundant phyla, such as Deinococcota, despite an initial increase by 0.02% in relative sequence abundance with wetting, decreased by 0.05% from T2 to T5 (Fig. 4).
Microbial compositional changes during the wetting/re-drying treatment
Four weeks after the wetting ceased, the relative sequence abundance of Proteobacteria decreased by 3%. By the end of the experiment (T5), it was 10% less abundant than the samples that were kept wet (Fig. 4). The response to the re-establishment of dry conditions was particularly evident for the dominant families Sphingomonadaceae and Comamonadaceae, which decreased their relative sequence abundance, and for Oxalobacteraceae family which increased their relative sequence abundance during the first week of re-drying (Fig. 5a). In contrast, after 4 weeks of drying (T5), the relative sequence abundance of Actinobacteriota was 14% higher than those in the wetting treatment but still comparatively 9% lower than the dry control samples (Fig. 4). The family Solirubrobactereacea in particular, showed a slow recovery after the drying process started (Fig. 5b). Acidobacteriota were 5% more abundant than those in the wetting treatment and reached a similar relative sequence abundance to those observed in the dry control samples (15%) (Fig. 4). Blastocatellaceae and Pyrinomonadaceae families showed signs of a quick recovery 1 week after the drying process started (T4) (Fig. 5c). Despite the positive response to wetting, the Bacteroidota phylum increased in relative sequence abundance in 1 week after the drying process started (T4), being 3% more abundant than the dry control, by the end of the experiment (T5) (Fig. 4). The latter was mainly driven by the 8% increase in relative sequence abundance of the family Chitinophagaceae compared to the beginning of the experiment (T0). All other Bacteroidota families declined in relative sequence abundance with the re-establishment of dry soil conditions (Fig. 5d).
Differentially abundant ASVs during wetting and wetting/re-drying periods
We used DESeq2 to identify the ASVs that were differentially abundant between the treated samples and the control after 24 h (T1), 1 week (T2), 4 weeks (T3), 5 weeks (T4), and 8 weeks (T5) (Fig. 6). At T1, T2, and T3, we identified 96, 144, and 116 ASVs significantly different between the wetting treatment and the control. The number of ASVs continued to increase at T4 and T5, with 202 and 194 ASVs significantly different from the control. Of the identified ASVs, those that became less abundant than the control (log2 fold change, LFC < 0) continuously increased with the exposure to wet conditions, from 36% (T1) to 58% (T5), while those that became more abundant with wetting decreased from 64% (T1) to 42% (T5) (Fig. S5, Table S2).
a–e Heatmaps indicating the number of significant amplicon sequence variants (ASVs) identified by DeSeq2 analysis, for each time point. Comparisons were made between each treatment (wetting and wetting/re-drying) and the control at (a) T1, (b) T2, (c) T3, (d) T4 and (e) T5. Cells were coloured using the normalized read counts (log + 1) from DeSeq2 analysis, with a posterior scale by row transformation. Different coloured cells in the leftmost columns indicate the phylum affiliation for each ASV identified. Coloured cells in the highest part of the heatmap identify dataset characteristics such as the type of treatment
In the wetting/re-drying treatment, the number of ASVs significantly different from the control was similar to those identified in the wetting treatment, with 105, 139, and 119 ASVs found at T1, T2, and T3, respectively. However, 1 week after the wetting ceased (T4), we only identified 54 ASVs as significantly different from the control, decreasing to 48 ASVs 1 month after (T5) (Fig. 6, Table S2). By the end of the experiment (T5), communities submitted to an initial wetting disturbance followed by a re-drying event resembled the dry control communities more than the wet counterparts (Fig. 3 and Fig. 6). Of the identified ASVs, those that became less abundant than the control during the wetting phase (T1 to T3) represented 41% of the identified ASVs. During the re-drying phase, the percentage of ASVs that became more abundant than the control increased from 59% (T3) to 65% (T5) (Fig. S5, Table S2).
Most ASVs affiliated to the phyla Actinobacteriota, Bacteroidota (family Chitinophagacea), Chloroflexi, and Acidobacteriota decreased relative sequence abundance with wetting. In contrast, the relative sequence abundance of ASVs affiliated with Proteobacteria, Bacteroidota, and Planctomycetota increased during wetting (Fig. 6, Table S3).
Phylogenetic structure of the communities during the wetting treatment
Microbial communities displayed significant phylogenetic clustering in dry soils (dry control NTI mean values: 2.18 ± 0.18) during the experiment. This pattern was significantly affected by prolonged wetting (one-way ANOVA: F-value = 76.08, p-value = 1.9e-07), depicted by a shift towards a stochastic assembly of the community after 4 weeks of daily wetting (T3) (wetting treatment NTI mean values: 1.34 ± 0.68; wetting/re-drying treatment mean values: 1.70 ± 0.29) (Fig. 7). We observed a second shift after T3 once wetting ceased and the re-drying phase started (Fig. 7).
NTI values for every community sampled from each treatment and control over the time course of the experiment. Dashed grey lines at the − 2 and + 2 values delimitate the significance thresholds from the null expectation. NTI values < − 2 or > + 2 suggest that phylogenetic turnover is less or greater than the null expectation and is related to deterministic processes. NTI values ranging between − 2 and 2 are usually considered to signify the influence of stochastic assembly
Discussion
The climate is changing in Antarctica, resulting in impacts that will likely affect the diversity and stability of terrestrial communities (Lee et al. 2017). Long-term observations are invaluable for documenting ecosystem changes, providing more comprehensive reports of ecosystem processes that occur over prolonged periods. However, the demands of budgets, the logistical constraints associated with long-term monitoring programs challenge the maintenance of these programs. Moreover, the time required to resolve patterns of change and draw inferences can take years to decades (McKnight et al. 2007; Tiao et al. 2012), which can delay the applicability of the results within rapidly evolving decision-making processes.
Several studies have looked at the impact of wetting on ecosystems and microbial life (Evans and Wallenstein 2014; Barnard et al. 2013, 2015, 2020). However. Antarctic research is held under a strict code of conduct to help reduce or eliminate the human impact on the continent. As a result, research conducted in the field requires permits, which can limit the type and the number of experimental manipulations undertaken in situ. In this study, we aimed to demonstrate the ability to perform controlled experiments outside the continent as a complementary approach to field observations to study the response of the Antarctic biota to environmental change. The ability to accelerate changes predicted to occur in the ecosystem under realistic controlled conditions of temperature, relative humidity, and light in the laboratory, with replication, will help identify the tipping point and thresholds of current stable communities under climate change scenarios (Lee et al. 2017). Moreover, the ability to precisely emulate permafrost conditions and physical processes, such as the ablation of ice from the underlying permafrost that may influence below-ground communities, is an essential requirement to reliably simulate the physical and chemical environment that regulates the Antarctic terrestrial microbiome. For instance, the unfortunate event of the chamber breakdown within this study and the subsequent response of the microbial community observed in the control samples further emphasizes the need to accurately emulate key environmental attributes in a manipulative laboratory experiment, and the importance of the inclusion of controls to enhance the internal validity of the experiment. The effectiveness of our approach was further validated by comparing our results with previous field observations similarly focused on wetting events conducted in the McMurdo Dry Valleys. Our primary reference experiment was performed in the Miers Valley, where community changes were monitored for 7 weeks after a stream was re-diverted into historically dry soil (Niederberger et al. 2019).
We observed significant structural and compositional changes in the microbial communities after 1 day of wetting, which persisted throughout the 8 weeks of constant wetting (Fig. 3). Those were consistent with the compositional shifts and turnover rates reported by Niederberger et al. (2019) and reflected, to some extent, the variation in community composition observed along wetness gradients in the Wright and Taylor valleys (Monteiro et al. 2022). Proteobacteria exhibited a positive response to wetting, with relative sequence abundance tripling after 8 weeks of constant wetting compared to the dry control, becoming the most dominant phylum under wet conditions. The increase, during the first month of wetting, was mainly driven by the Alphaproteobacteria family Sphingomonadacea and Gammaproteobacteria families Oxalobactereaceae, Comamonadaceae, and Xanthomonadaceae (Fig. 5a), which have been previously associated with wet soils in the dry valleys (Monteiro et al. 2022). Conversely, we observed an overall decline in the relative sequence abundance of Actinobacteriota, Acidobacteriota, and Deinococcota during the 8 weeks of constant wetting compared to the dry control (Fig. 4). Particularly for Actinobacteriota, the most dominant phylum in dry soils, the sensitivity to wetting persisted at the family level (Fig. 5b). These clear taxa-specific abundance-changes hints at the possibility that a phylogenetic coherence might exist within Actinobacteriota to their sensitivity to wetting (Evans and Wallenstein 2014), which has been observed in previous wetting/dry-down experiments (Barnard et al. 2013, 2015). The latter, implies that these taxa should share traits that are key to adapting to extreme aridity. Part of these drought tolerance traits can be related to the capability to sporulate, the thickness of cell walls, and the ability to produce and accumulate organic osmolytes (e.g. proline, glycine, trehalose) to help adjust to the osmotic stress (Barnard et al. 2020).
Although the rapid responsiveness of microbial communities to wetting events has been well described or predicted in the MDV (Buelow et al. 2016; Van Horn et al. 2014; Lee et al. 2018; Monteiro et al. 2022; Niederberger et al. 2015, 2019; Tiao et al. 2012), evidence for subsequent recovery is still scarce. Our results indicate that microbial communities from historically dry soils can recover from short-term wetting events (Figs. 3, 6). However, it is possible that long-term exposure to wet conditions alters the structure and dominance patterns of the community to a point that the legacy effect of the disturbance could affect the ability to recover (Monteiro et al. 2022). These observations are relevant since climate predictions suggest that MDV soils will likely become more frequently wet during the summer due to warming (Fountain et al. 2014). The shift in community structure once wetting ceased was mainly driven by the rapid increase in relative sequence abundance of Acidobacteriota and Bacteroidetes family Chitinofagaceaea to values identical to the dry control, the relative decline in Proteobacteria and Bacteroidota families (apart from Chitinofagaceaea) and the much slower relative increase of Actinobacteriota, a pattern associated with drought-resistors (Figs. 4 and 6) (Brangarí et al. 2021). The potential conservation of drought-resistant taxa during the wetting period and fast relative increase during the drying period are essential traits for community resilience and stability of the ecosystem (Manzanera 2021). These attributes have been associated with the historical legacy of the harsh conditions that preceded the disturbance (Jiang and Patel 2008; Evans and Wallenstein 2014; Hawkes et al. 2017; Brangarí et al. 2021).
The Niederberger et al. (2019) field experiment discussed whether dispersal mechanisms could have driven early compositional changes in the community. Our PDEC-controlled study demonstrates that community changes could be attributed to the fast responsiveness of resident members of dry soil communities to wetting (Fig. 4). Since ecological processes such as dispersion cannot be accounted for during our laboratory experiment, the asynchronous changes in the relative abundance of dominant phyla and families to quick alterations in water availability indicate that dry soils communities are composed of coexisting taxa with different preference for environmental conditions. Ultimately, it could suggest that dry soil communities maintain different physiological aptitudes and metabolisms which are relevant attributes to increase the ecosystem’s stability during short wetting disturbances. Concurrently, these highly responsive taxa could be used as biological indicators for recent changes in water availability in Antarctic terrestrial systems (Monteiro et al. 2022), adding further evidence on how sensitive communities inhabiting polar deserts are to those changes.
Following the observed asynchronous response at the phylum and family levels with changes in water availability, we examined which ecological factor best explained the structuring of the community during the experiment. In dry soil communities and during the initial exposure to wetness (1 week), we observed the prevalence of phylogenetic clustering through selection (NTI values > 2) (Fig. 7). This result is consistent with previous in situ observations using wetness gradients (Monteiro et al. 2022), and provides further evidence that communities emerge through strong environmental filtering leading to niche segregation in dry or recently wet soils. Under historical aridity conditions, taxa are likely selected based on traits related to stress mitigation or efficient resource utilization under ultra oligotrophy and extreme aridity. For instance, recent genomic analyses suggest that the highly abundant Actinobacteriota can use atmospheric trace gases (e.g. H2, CO) to support aerobic respiration and carbon fixation in desert soils (Bay et al. 2021; Ortiz et al. 2021). Likewise, Acidobacteriota are better adapted to compete when resources are limited (Fierer et al. 2007), with some members of this phylum capable of oxidizing hydrogen from the atmosphere to sustain energy requirements (Greening et al. 2015). However, we observed that quick changes in water availability could compromise the survival of well-adapted taxa to extreme aridity. For instance, species historically adapted to low water potential have less tolerance to immediate hydrological shocks during wetting, resulting in cell lysis (Schimel et al. 2007). In contrast, the consequent release of cellular material from sensitive taxa into the soils may benefit opportunistic heterotrophic taxa, which rely on rapid and efficient growth, rather than investing significant resources in stress mitigation strategies.
After 1 week of continuous exposure to wetness, the prevalence of phylogenetic clustering started to decrease, and neutral processes began to determine community composition (NTI between − 2 and 2) (Fig. 7). We believe this could result from increased stochasticity coincident with the decline in selective pressures associated with desiccation/moisture stress and rapid increase in newly available carbon sources from the death and lysis of more sensitive taxa (Dini-Andreote et al. 2015). At this stage, we postulate that the relative increase in resource availability from cell lysis of sensitive taxa enabled a broad range of taxa to utilize newly available resources (e.g. different carbon substrates) (Martiny et al. 2013), thereby decreasing the competition among coexisting taxa and leading to a more stochastic assembly of the community (Chase 2010). The increase in the relative abundance of Gammaproteobacteria, associated with higher productivity soils in the MDV (Niederberger et al. 2008), provides evidence for a potential increase in resource availability.
We identified two significant differences between the PDEC experiment and the analogous field wetting experiment (Niederberger et al. 2019). Firstly, we did not observe a significant increase in Cyanobacteria during our wetting treatment, which contrasts with the 18% increase reported after 7 weeks of wetting in the field experiment (Niederberger et al. 2019). Cyanobacteria are major primary producers in Antarctic ice-free regions, exhibiting an excellent capacity to persist under arid conditions and considerable dispersal abilities (McKnight et al. 2007; Wood et al. 2008). As such, one explanation for the absence of Cyanobacteria increase in our experiment could be related to the difficulty of simulating dispersion processes in close systems such as the PDEC, which otherwise would naturally occur in the field, particularly during a natural wetting event (Niederberger et al. 2019). Alternatively, it is also possible that the conditions in PDEC were not ideal for Cyanobacteria to thrive or the storing conditions pre-manipulation (− 60 °C) impaired these taxa from growing. Secondly, we observed a significant decrease in alpha diversity relative to the dry control during the wetting treatment, particularly after 2 months of continuous wetting (Fig. S3). This observation contrasts with the twofold increase in species richness observed during the 7 weeks field experiment (Niederberger et al. 2019) and the positive associations between water availability and diversity observed in the field (Niederberger et al. 2008; Lee et al. 2018; Monteiro et al. 2022). Yet, the positive associations between wetness and diversity aren’t always consistent. For instance, 3 years after a mummified seal was moved to a new location in the MDV, the authors observed significant structural changes in the soil microbiome underneath the seal, correlated with changes in relative humidity and a significant reduction in diversity (Tiao et al. 2012). We then hypothesize that under wet conditions and in the absence of primary producers or biomass imports through wind or water dispersion, the potential decrease in resource availability over time could have increased resource competition affecting community diversity.
While in this study, we described community changes from a compositional and structural standpoint, further studies are necessary to address the association between composition and functional attributes of microbial communities during wetting events. Ultimately, a better understanding of the functional attributes underpinning the response of dry soil microbial taxa and how those are affected during wetting events will improve our ability to predict the ecological consequences of hydrological changes in the MDV ecosystem and other Antarctic ice-free regions.
Conclusion and significance
Overall the similarities in the results obtained through our laboratory experiment, emulating the microbial response to a wetting event, with those previously observed in the field validates the use of a PDEC as a complementary tool to study the temporal response and resilience of communities from Antarctic ice-free regions to climate change. We demonstrated that coexisting taxa with different environmental preferences initially drive fast structural shifts in dry soils. The latter offers insights into the diversity of metabolic functions and strategies inherent in the MDV terrestrial microbiome. We further demonstrated that compositional changes during 4 weeks of wetting were not permanent, with the conservation of drought-resistant taxa underpinning resilient communities that oscillates in response to a periodic wetting/re-drying event.
Despite the limitations associated with laboratory-controlled experiments, such as the simulation of dispersal mechanisms (e.g. wind and water) which are relevant for the distribution of biomass in ice-free regions (Wood et al. 2008), the ability to adequately simulate a naturally low diversity system while maintaining the benefits of a controlled setting offers unlimited opportunities for hypothesis testing. When supported by field studies, such observations will help define the tipping points of community response to environmental disturbances in the Antarctic ice-free regions. Future experiments could be expanded to include single or synergetic physical, chemical, and biological disturbances, predicted to become more frequent in the system due to climate change (Hughes and Convey 2010), to understand how these will impact ecosystem functions and resilience. Lastly, the human presence associated with fieldwork activities in the continent can leave long-lasting disturbances in the ice-free soils affecting Antarctica’s wilderness and aesthetic values as well as habitat suitability for its biota (Convey 2010; O’Neill et al. 2015). As such, efforts to develop infrastructure that enables remote and more sustainable research practices on ice-free regions should be encouraged and part of frameworks aimed to conserve and manage Antarctica’s unique biodiversity and environment, one of the founding principles of the Antarctic Treaty.
Data availability
The raw DNA sequences have been deposited in SRA under the BioProject accession number PRJNA912229.
References
Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J 7:2229–2241. https://doi.org/10.1038/ismej.2013.104
Barnard RL, Osborne CA, Firestone MK (2015) Changing precipitation pattern alters soil microbial community response to wet-up under a Mediterranean-type climate. ISME J 9:946–957
Barnard RL, Blazewicz SJ, Firestone MK (2020) Rewetting of soil: revisiting the origin of soil CO2 emissions. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2020.107819
Bay SK, Waite DW, Dong X et al (2021) Chemosynthetic and photosynthetic bacteria contribute differentially to primary production across a steep desert aridity gradient. ISME J 15:3339–3356. https://doi.org/10.1038/s41396-021-01001-0
Bockheim JG, Campbell IB, McLeod M (2007) Permafrost distribution and active-layer depths in the McMurdo Dry Valleys. Antarct, Permafr Periglac Process 18:217–227. https://doi.org/10.1002/ppp.588
Bokulich NA, Subramanian S, Faith JJ et al (2013) Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. https://doi.org/10.1038/nmeth.2276
Bottos EM, Laughlin DC, Herbold CW et al (2020) Abiotic factors influence patterns of bacterial diversity and community composition in the Dry Valleys of Antarctica. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiaa042
Brangarí AC, Manzoni S, Rousk J (2021) The mechanisms underpinning microbial resilience to drying and rewetting–A model analysis. Soil Biol Biochem 162:108400. https://doi.org/10.1016/j.soilbio.2021.108400
Buelow HN, Winter AS, Van Horn DJ et al (2016) Microbial community responses to increased water and organic matter in the arid soils of the mcmurdo dry valleys, Antarctica. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01040
Bütikofer L, Anderson K, Bebber DP et al (2020) The problem of scale in predicting biological responses to climate. Glob Chang Biol 26:6657–6666. https://doi.org/10.1111/gcb.15358
Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Cary S, Mcdonald I, Barrett J, Cowan D (2010) On the rocks: the microbiology of Antarctic dry valley soils. Nat Rev Microbiol 8:129–138. https://doi.org/10.1038/nrmicro2281
Chase JM (2010) Stochastic community assembly causes higher biodiversity in more productive environments. Science 328:1388–1391. https://doi.org/10.1126/science.1187820
Coenen AR, Hu SK, Luo E et al (2020) A primer for microbiome time-series analysis. Front Genet. https://doi.org/10.3389/fgene.2020.00310
Convey P (2010) Terrestrial biodiversity in Antarctica–recent advances and future challenges. Polar Sci 4:135–147. https://doi.org/10.1016/j.polar.2010.03.003
Convey P, Peck LS (2019) Antarctic environmental change and biological responses. Sci Adv. https://doi.org/10.1126/sciadv.aaz0888
Convey P, Chown SL, Clarke A et al (2014) The spatial structure of Antarctic biodiversity. Ecol Monogr 84:203–244. https://doi.org/10.1890/12-2216.1
Dini-Andreote F, Stegen JC, van Elsas JD, Salles JF (2015) Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1414261112
Doran PT, McKay CP, Clow GD et al (2002) Valley floor climate observations from the McMurdo dry valleys, Antarctica, 1986–2000. J Geophys Res Atmos. https://doi.org/10.1029/2001JD002045
Evans SE, Wallenstein MD (2014) Climate change alters ecological strategies of soil bacteria. Ecol Lett 17:155–164. https://doi.org/10.1111/ele.12206
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364. https://doi.org/10.1890/05-1839
Fountain AG, Levy JS, Gooseff MN, Van Horn D (2014) The McMurdo Dry Valleys: a landscape on the threshold of change. Geomorphology 225:25–35
Greening C, Carere CR, Rushton-Green R et al (2015) Persistence of the dominant soil phylum Acidobacteria by trace gas scavenging. Proc Natl Acad Sci USA 112:10497–10502. https://doi.org/10.1073/pnas.1508385112
Hawkes CV, Waring BG, Rocca JD, Kivlin SN (2017) Historical climate controls soil respiration responses to current soil moisture. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1620811114
Hughes KA, Convey P (2010) The protection of Antarctic terrestrial ecosystems from inter-and intra-continental transfer of non-indigenous species by human activities: a review of current systems and practices. Glob Environ Chang 20:96–112
Hughes KA, Pertierra LR, Molina-Montenegro MA, Convey P (2015) Biological invasions in terrestrial Antarctica: what is the current status and can we respond? Biodivers Conserv 24:1031–1055
Jiang L, Patel SN (2008) Community assembly in the presence of disturbance: a microcosm experiment. Ecology 89:1931–1940. https://doi.org/10.1890/07-1263.1
Kassambara A, Kassambara MA (2020) Package ‘ggpubr.’ R Packag version 0.4.0
Kembel SW, Cowan PD, Helmus MR et al (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464. https://doi.org/10.1093/bioinformatics/btq166
Law J, Van Dijk D (1994) Sublimation as a geomorphic process: a review. Permafr Periglac Process 5:237–249. https://doi.org/10.1002/ppp.3430050404
Lee JR, Raymond B, Bracegirdle TJ et al (2017) Climate change drives expansion of Antarctic ice-free habitat. Nature 547:49–54. https://doi.org/10.1038/nature22996
Lee KC, Caruso T, Archer SDJ et al (2018) Stochastic and Deterministic effects of a moisture gradient on soil microbial communities in the McMurdo Dry Valleys of Antarctica. Front Microbiol 9:2619. https://doi.org/10.3389/fmicb.2018.02619
Lee CK, Laughlin DC, Bottos EM et al (2019) Biotic interactions are an unexpected yet critical control on the complexity of an abiotically driven polar ecosystem. Commun Biol 2:62. https://doi.org/10.1038/s42003-018-0274-5
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:1–21
Lozupone CA, Knight R (2015) The unifrac significance test is sensitive to tree topology. BMC Bioinformatics 16:211. https://doi.org/10.1186/s12859-015-0640-y
Manzanera M (2021) Dealing with water stress and microbial preservation. Environ Microbiol 23:3351–3359. https://doi.org/10.1111/1462-2920.15096
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17:10–12
Martiny AC, Treseder K, Pusch G (2013) Phylogenetic conservatism of functional traits in microorganisms. ISME J 7:830–838. https://doi.org/10.1038/ismej.2012.160
McKnight DM, Tate CM, Andrews ED et al (2007) Reactivation of a cryptobiotic stream ecosystem in the McMurdo Dry Valleys, Antarctica: a long-term geomorphological experiment. Geomorphology 89:186–204. https://doi.org/10.1016/j.geomorph.2006.07.025
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. https://doi.org/10.1371/journal.pone.0061217
Monteiro MR, Marshall AJ, Hawes I et al (2022) Geochemically defined space-for-time transects successfully capture microbial dynamics along lacustrine chronosequences in a Polar Desert. Front Microbiol. https://doi.org/10.3389/fmicb.2021.783767
Niederberger TD, McDonald IR, Hacker AL et al (2008) Microbial community composition in soils of Northern Victoria Land, Antarctica. Environ Microbiol 10:1713–1724. https://doi.org/10.1111/j.1462-2920.2008.01593.x
Niederberger TD, Sohm JA, Gunderson TE et al (2015) Microbial community composition of transiently wetted Antarctic Dry Valley soils. Front Microbiol 6:9. https://doi.org/10.3389/fmicb.2015.00009
Niederberger TD, Bottos EM, Sohm JA et al (2019) Rapid microbial dynamics in response to an induced wetting event in Antarctic Dry Valley Soils. Front Microbiol 10:621. https://doi.org/10.3389/fmicb.2019.00621
O’Neill TA, Aislabie J, Balks MR (2015) Human impacts on soils. In: Bockheim JG (ed) The soils of Antarctica. Springer, Cham, pp 281–303
Oksanen J, Blanchet FG, Kindt R, et al (2012) Vegan: Community Ecology Package. R package version 2.0–2
Ortiz M, Leung PM, Shelley G et al (2021) Multiple energy sources and metabolic strategies sustain microbial diversity in Antarctic desert soils. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2025322118
Quast C, Pruesse E, Yilmaz P et al (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596
R Development Core Team (2010) R: A language and environment forstatistical computing. R Foundation for Statistical Computing, Vienna
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394. https://doi.org/10.1890/06-0219
Stegen JC, Lin X, Konopka AE, Fredrickson JK (2012) Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J 6:1653–1664. https://doi.org/10.1038/ismej.2012.22
Tiao G, Lee CK, McDonald IR et al (2012) Rapid microbial response to the presence of an ancient relic in the Antarctic Dry Valleys. Nat Commun. https://doi.org/10.1038/ncomms1645
Van Horn D, Okie JG, Buelow HN et al (2014) Soil Microbial responses to increased moisture and organic resources along a salinity gradient in a Polar Desert. Appl Environ Microbiol 80:3034–3043. https://doi.org/10.1128/AEM.03414-13
Wickham H (2016) ggplot: Elegant Graphics for Data Analysis Springer
Wood SA, Rueckert A, Cowan DA, Cary SC (2008) Sources of edaphic cyanobacterial diversity in the Dry Valleys of Eastern Antarctica. ISME J 2:308–320. https://doi.org/10.1038/ismej.2007.104
Acknowledgements
We would like to thank Antarctica New Zealand for the critical logistical support needed to carry out soil sampling in the MDV and Jacinda Challis for helping with the setup of the PDEC experiment, monitoring the PDEC, sample collection, and nucleic acids extractions.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was conducted as part of the Resilience in Antarctic Biota and Ecosystems Project funded by the New Zealand Antarctic Research Institute to SCC and CKL (NZARI 2016–2). SCC and CKL were partially supported by the Dry Valley Ecosystem Resilience (DryVER) Programme funded by the New Zealand Ministry of Business, Innovation and Employment (UOWX1401) and the New Zealand Antarctic Science Platform (ANTA1801). AM was supported by the Rutherford Foundation Royal Society Te Aparangi Postdoctoral Fellowship (20-UOW006). MM was supported by the University of Waikato Doctoral Scholarship.
Author information
Authors and Affiliations
Contributions
SCC, CKL and IRM conceived the Polar Desert Environmental Chamber. MRM, SCC, CKL and IRM designed the experiment. MRM performed the sample collection and laboratorial analyses. MRM and AJM analysed the data. MRM wrote the manuscript. All authors read, reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monteiro, M.R., Marshall, A.J., Lee, C.K. et al. Bringing Antarctica to the lab: a polar desert environmental chamber to study the response of Antarctic microbial communities to climate change. Polar Biol 46, 445–459 (2023). https://doi.org/10.1007/s00300-023-03135-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03135-7