Abstract
El Niño southern oscillation (ENSO) events drive profound global impacts on marine environments. These events may result in contrasting conditions in the Southern Ocean, with differing effects on euphausiid species because of their diverse life histories, habitats, and feeding ecologies. We conducted oceanographic surveys during winter (2012–2016) around the northern Antarctic Peninsula and examined the dietary carbon sources, trophic position, and body condition of five euphausiid species (Euphausia crystallorophias, E. frigida, E. superba post-larvae and larvae, E. triacantha, and Thysanoessa macrura) in relation to environmental conditions each year. In addition to general patterns among taxa, we focused on how contrasting conditions during an ENSO-neutral year (2014) and an ENSO-positive year (2016) affected the type, quality, and distribution of food resources each year, as well as the body condition of each species. We observed high chlorophyll-a, low salinity, and shallow upper mixed-layer depths in 2014, and low chlorophyll-a, high salinity, and deep upper mixed-layer depths in 2016. Carbon sources varied among years, with most species enriched in δ13C when ENSO conditions were dominant. Trophic position and body condition also varied among years, with different responses among species depending on conditions; inter-annual variation in δ15N was minimal, while E. triacantha was the only species with notably lower body condition in 2016. We conclude that ENSO conditions around the northern Antarctic Peninsula may result in a more favorable feeding environment for all euphausiid species except E. triacantha, which may be the most negatively impacted by the predicted increase in ENSO conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last several decades, the Southern Ocean has undergone rapid environmental changes, including increases in air (Jones et al. 2019) and seawater temperatures (Verona et al. 2018), and decreases in the duration of annual sea-ice cover (Henley et al. 2019). Changes predicted for the future include increases in the frequency and/or intensity of extreme climate events like the El Niño southern oscillation (ENSO) (Cai et al. 2014; Morley et al. 2020), that may further modulate sea-ice dynamics in this region. Because changes to the physical environment heavily influence ecological and biological interactions in polar marine ecosystems, understanding how such change affects these ecosystems is critical for predicting how they may respond to future change.
As mostly primary or secondary consumers sensitive to fluctuations in environmental conditions, Antarctic zooplankton are good indicators of environmental change (Johnston et al. 2022). Shifts in zooplankton distribution, abundance, prey consumption, growth, and reproduction have all been linked to environmental variability (e.g., Siegel and Loeb 1995; Quetin et al. 2007; Mackey et al. 2012; Constable et al. 2014; Driscoll et al. 2015; Richerson et al. 2018; Reiss et al. 2020). Of all species in the Antarctic, Euphausia superba has been the most intensively studied (e.g., Atkinson et al. 2004, 2019; Meyer et al. 2010; Flores et al. 2012). However, understanding the effects of environmental variability on other euphausiid species may provide valuable insight into how future environmental change may reshape the ecological landscape of the pelagic waters around the Antarctic Peninsula.
The five euphausiid species most common in the marine ecosystem around the northern Antarctic Peninsula include Euphausia crystallorophias, Euphausia frigida, E. superba, Euphausia triacantha, and Thysanoessa macrura. These species are distinct with respect to habitat and feeding ecology (Table 1), and therefore may be affected differently by environmental change. Ongoing environmental trends around the Antarctic Peninsula over the last several decades include strengthened westerly winds associated with the positive phase of the Southern annular mode (SAM), a poleward shift in the Antarctic circumpolar current (ACC), increased water temperatures, shoaled upper mixed layer (UML) depths, an increased presence of warm circumpolar deep water (CDW) on the continental shelf, and decreased sea-ice extent and duration (Sen Gupta et al. 2009; Constable et al. 2014). Among the environmental trends predicted to continue are ocean warming coupled with decreased salinity resulting from ice melt, which may enhance water-column stratification, potentially reduce nutrient availability in surface waters, and could favor phytoplankton communities dominated by small-celled species (Finkel et al. 2010; Deppeler and Davidson 2017). Current and predicted environmental changes may be compounded by a projected increase in the positive trend of the SAM (Zhang et al. 2020) and an increase in the frequency and intensity of extreme El Niño southern oscillation (ENSO) events (Cai et al. 2014), which have pronounced impacts on sea-ice variability along the Antarctic Peninsula (Yuan 2004) and may further disrupt ecological relationships in this region.
The extent to which these changes may affect the life cycles of Antarctic euphausiid species is known to some degree for E. superba (Quetin et al. 2007; Loeb et al. 2009; Meyer et al. 2010; Flores et al. 2012) and to a lesser degree for T. macrura (Richerson et al. 2018), but not for other euphausiid species. Responses to environmental change will depend on biogeographical distributions, physiological tolerances, and prey requirements of each species, and may vary based on the intersection of trends in biotic (e.g., food availability, competition, predation) and abiotic (e.g., ocean–atmosphere interactions, physical forcing) factors that shape the pelagic ecosystem. There is general agreement that current environmental trends will have negative consequences for most euphausiid species, manifested mostly as loss of preferred habitats or geographical and/or species shifts in the prey field (Johnston et al. 2022). Both consequences have implications for predator–prey interactions as phytoplankton community structure is forecasted to favor smaller species, sea-ice cover is projected to decline further, and competition for reduced resources may increase (Montes-Hugo et al. 2009; Constable et al. 2014; Johnston et al. 2022). The paucity of data for all species except E. superba preclude detailed predictions of future interspecific interactions around the northern Antarctic Peninsula.
As part of its decades-long ecosystem monitoring program around the northern Antarctic Peninsula, the U.S. Antarctic Marine Living Resources (AMLR) Program conducted five consecutive winter surveys from 2012 to 2016. We collected samples of the five common euphausiid species to determine sources of dietary carbon (carbon stable isotopes), trophic position (nitrogen stable isotopes), and body condition (lipid content) to compare their responses to winter environmental variability. Here we focus on contrasting environmental conditions between 2014 and 2016, which were largely the result of a “failed” ENSO event in 2014 (McPhaden 2015; Levine and McPhaden 2016) and an extreme ENSO event in 2015/16. During austral summer and fall of 2014, the formation of an anomalous warm water volume in the tropical Pacific Ocean and multiple westerly wind bursts signaled the development of a strong ENSO event (Levine and McPhaden 2016). However, during austral winter 2014, a large easterly wind burst halted the development, but the remaining warm water volume set the stage for the development of an extreme ENSO event during the first half of 2015 that persisted into 2016 (McPhaden 2015).
In this paper, we examine how contrasting oceanographic conditions around the Antarctic Peninsula between 2014 and 2016 likely affected sources of dietary carbon, trophic position, and body condition of five important euphausiid species with different habitats, diets, and overwintering life history strategies. We address the following hypotheses: (1) ENSO-positive conditions around the northern Antarctic Peninsula may enhance the quality of phytoplankton prey by increasing sea-ice cover and altering ocean circulation patterns to bring micronutrient-rich water into the study site; and (2) euphausiid sources of dietary carbon, trophic level, and body condition will have species-specific, non-uniform responses to ENSO-positive conditions. We conclude by discussing the potential effects of predicted ENSO extremes on Antarctic euphausiid species in the future.
Materials and methods
Survey methods for the U.S. AMLR Program Antarctic winter studies have been published previously (Reiss et al. 2015, 2017; Walsh et al. 2020; Walsh and Reiss 2020). All station and biological data for E. superba post-larvae and larvae can be found in Walsh et al. (2020), while the dataset for all other euphausiid species can be found in Walsh and Reiss (2020).
Survey area and general survey design
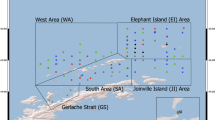
The U.S. AMLR Program conducted winter surveys (August and September 2012–2016) around the northern Antarctic Peninsula aboard the research vessel/ice breaker (RVIB) Nathaniel B. Palmer (Fig. 1a). We attempted to sample a historical grid of 110 fixed stations that were categorized into four sampling areas: the Elephant Island Area (EI; 43,865 km2), the Joinville Island Area (JI; 18,151 km2), the South Area (SA, or Bransfield Strait; 24,479 km2), and the West Area (WA, or area immediately north of Livingston and King George Islands; 38,524 km2). In 2016, we also sampled the Gerlache Strait (GS).
a Map of the U.S. AMLR Program study area around northern Antarctic Peninsula. Red circles indicate stations where temperature-salinity properties were compared between 2014 and 2016. Dashed circle: Bransfield Current; solid circle: Bransfield Basin. Red rectangle represents the Elephant Island (EI) transect, where temperature and salinity properties were compared between years. b Map of where each species was collected for measurements of trophic position, dietary source carbon, and body condition each year
At-sea sampling
At each station, we deployed a conductivity, temperature, depth (CTD) rosette equipped with 24 Niskin bottles (10 L) to 750 m or to within 10 m of the bottom (SBE911; Sea-Bird Scientific). We determined the upper mixed layer (UML; m) depth at each station from CTD profiles by calculating the depth at which the water density changed by 0.05 kg m−3 relative to the average density of the upper 10 m of the water column (Mitchell and Holm-Hansen 1991). UML temperature and salinities were defined to be means over the depth range of the UML. We calculated integrated chl a (to 100 m, mg chl a m−2) using concentrations at the discrete depths of each Niskin bottle sample from 5 to 100 m and averaging between depths to calculate the intermediate depth concentrations (Holm-Hansen et al. 1965; Holm-Hansen and Riemann 1978).
To examine differences in hydrographic structure between 2014 and 2016, we identified a transect extending from 60oS in the EI sampling area directly south to 62.75oS in the JI sampling area (hereafter “the EI transect”) (red rectangle on Fig. 1a). Along the EI transect, we determined the position of the intersection of the 27.6 kg m−3 (σt) isopycnal with the surface each year that has been used as an indicator of the presence and extent of CDW from the Drake Passage in the survey area at depth (Reiss et al. 2009), and the amount of cold, high-salinity water in the Bransfield Strait. We also compared depth profiles of temperature and salinity at two locations in the SA sampling area (the Bransfield Current and the Bransfield Basin) to further examine hydrographic variability in relation to the presence of CDW in the Bransfield Strait between these two years (dashed and solid red circles on Fig. 1a).
At each station, we towed an Isaacs-Kidd Midwater Trawl (IKMT) net with 505 µm mesh to 170 m or to within 10 m of the bottom. We determined the volume of seawater filtered using a calibrated flow meter mounted on the net’s frame (Model 2030 R, General Oceanics, Inc.). We identified large zooplankton, including euphausiid species, using a microscope when more detailed identification was required.
Stable isotope analysis
Stable isotopes provide a record of diet over several weeks to months (Frazer et al. 1997). Higher ratios of heavy to light nitrogen isotopes (15N/14N, denoted as δ15N) reflect higher trophic position, with consumer tissue enriched in δ15N by 3–4 ‰ relative to prey (Post 2002). Ratios of heavy to light carbon isotopes (13C/12C, denoted as δ13C) reflect different sources of dietary carbon.
We used the same samples for stable isotope analysis that we used for lipid analysis (Fig. 1b). Freeze-dried samples of lipid-free zooplankton were analyzed for δ13C and δ15N at the University of California Davis Stable Isotope Facility (UCD SIF) using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon). We report ratios in parts per thousand (‰) compared to standards (PeeDee limestone for δ13C and atmospheric N2 for δ15N).
We did not acid-treat euphausiid samples prior to stable isotope analysis because zooplankton contain few, if any, calcified structures, and acidification may skew δ15N values (Schlacher and Connolly 2014). We did remove lipid from samples prior to stable isotope analysis because it is depleted in 13C relative to other tissues and may result in biased values of δ13C. However, lipid removal can also skew δ15N values (Post et al. 2007). When lipid is extracted using the technique we used (Folch et al. 1957), mean fractionation of δ15N is only 0.25 ‰, which is close to typical analytical error (Post et al. 2007).
Lipid analysis
Compared to stable isotopes, fluctuations in lipid content are more rapid and may reflect changes in diet over days to weeks (Meyer et al. 2002). We extracted lipid from aliquots of 0.5–1.5 g of homogenized animals in a 2:1 chloroform:methanol solution with 0.01% butylated hydroxytoluene as a preservative (Folch et al. 1957; Budge et al. 2006). We filtered lipid dissolved in the chloroform fraction through anhydrous sodium sulfate into pre-weighed glass tubes to remove all traces of methanol and water, and then evaporated the chloroform solvent under nitrogen to dryness. We then re-weighed the tubes to the nearest 0.001 g on the same calibrated analytical balance (Model PA214, Ohaus, Inc.) to determine percent lipid of the dry mass of each sample. We determined dry mass of samples by freeze-drying all 2016 lipid-extracted samples for 16 h (VirTis Benchtop K Lyophilizer, SP Scientific) and calculating the mean percent mass loss for each species. Then, for each sample, we used the mean percent mass loss for that species to calculate dry mass from the sample’s wet mass, and we added back the mass of the removed lipid to obtain the final dry mass of each sample.
Data analysis
In 2012, we did not survey all sampling areas due to time constraints. We present environmental and biological data from 2012 in figures for visual comparisons, but we omitted 2012 data from statistical analyses.
Environmental variables (water temperature, salinity, UML, and chl a) failed the Shapiro–Wilk normality test. We compared environmental variables among years using the Kruskal–Wallis test and the Dunn’s test for post-hoc comparisons using the Bonferroni correction (Ogle et al. 2022).
We calculated three-month averages (December–January–February, March–April–May, June–July–August, September–October–November) of the Bivariate ENSO index (BEST, Smith and Sardeshmukh 2000) for all 5 years of sampling to show the progression of ENSO conditions throughout the entire study period. To summarize environmental variables potentially resulting from the progression of the ENSO and to better describe the observed patterns between 2014 and 2016, we performed a principal component analysis (PCA) using chl a, water temperature, salinity, and UML depth at each sampling station. The first and second principal components (PC1 and PC2, respectively) failed the Shapiro–Wilk normality test and were compared among years using the Kruskal–Wallis test and the Dunn’s test for post-hoc comparisons using the Bonferroni correction (Ogle et al. 2022).
We acquired monthly easterly and northerly surface wind data (10 m) from the National Center for Environmental Prediction (NCEP) (https://psl.noaa.gov/data/gridded/data.ncep.reanalysis2.html accessed 31 August 2018) and averaged wind speed for autumn (April, May, June (AMJ)) and winter (July, August, September (JAS)) of each year to describe wind patterns for the period of three to five months preceding each survey period through the end of each survey period. Data were obtained for a 2.5° grid between − 65oS, − 59.5oS, − 65oW, and − 52oW.
To identify differences in body condition within species among years, we arcsine-square-root transformed percent lipid data to approximate normal distributions. We compared species-specific differences in body condition between years using ANOVA. To identify differences in δ13C and δ15N within species among years, we used ANOVA and the Tukey HSD Test with a 95% confidence level for post-hoc comparisons when the variable was normally distributed. When the variable failed the Shapiro–Wilk normality test, we used the Kruskal–Wallis test and the Dunn’s test for post-hoc comparisons using the Bonferroni correction (Ogle et al. 2022). Results of all statistical tests are presented in Table 2. Post-hoc p values are reported in the text following between-year comparisons.
When means are reported, the dispersion of values around the mean is reported as standard deviation. We performed all statistical tests in R, version 4.1.2 (R Core Team 2021) and created all graphics using QGIS (v 3.4.7-Madeira) and the R package “ggplot2” (Wickham 2016).
Results
Water column properties along the Elephant Island transect and the Bransfield Strait
We compared water-column properties in 2014 and 2016 as representative of an ENSO-neutral year and an ENSO-positive year, respectively. In 2014, the water column along the EI transect was more stratified than in 2016. The 27.6 σt isopycnal intersected the surface approximately 250 km from the northernmost stations, within the SA sampling area (Fig. 2). In 2016, the 27.6 σt isopycnal intersected the surface approximately 100 km farther north compared to 2014, suggesting that colder, saltier water from the Weddell Sea was present in greater proportions over the study area in 2016 (Fig. 2).
Contour plots of salinity (S), temperature (T, oC), and density (σt) along the EI transect in 2014 (a, b, c) and 2016 (d, e, f). Section distance starts at “0” with the northernmost station and ends 300 km south at the southernmost station of the transect. The 27.6 σt isopycnal is highlighted in bold to show the differences in water-column stratification between years of different circumpolar deep water (CDW) and Weddell sea shelf water (WSSW) influence
Depth profiles of temperature and salinity show that the extent of CDW intrusion into the SA sampling area varied between 2014 and 2016 (Fig. 3). Depth profiles of temperature at the Bransfield Current station show that in 2014, warm CDW was a strong feature between 200 and 400 m, suggesting that CDW flowed into the SA sampling area from the southwest (Fig. 3a). CDW was also present at the Bransfield Basin station in 2014, although the range of temperatures among years at this station was smaller (Fig. 3c). CDW was not a strong feature in the SA sampling area in 2016; water at both stations was colder and saltier, indicating that Weddell sea shelf water (WSSW) was more prevalent in the region during 2016.
Environmental patterns among years
Environmental patterns varied among years, with differences between ENSO-neutral and ENSO-positive years (2014 and 2016 presented in Fig. 4). Both chl a and salinity were not different between 2013 and 2014 or between 2015 and 2016. Chl a was higher in 2013 and 2014 than in 2015 and 2016 (p < 0.0001 for all between-year comparisons) while salinity was higher in 2015 and 2016 than in 2013 and 2014 (p < 0.0001 for all between-year comparisons except 2014–2016 (p = 0.0004)). Although UML depth among stations and years was highly variable and annual differences were not always significant, we observed a general trend of shallower depths in 2013 and 2014. UML depth in 2015 was deeper than in 2013 (p = 0.0058), 2014 (p < 0.0001), and 2016 (p = 0.0206). Water temperature differed little among years and was warmer in 2013 than in 2016 (p = 0.0215).
The first principal component (PC1) of the PCA discriminated between environmental conditions during ENSO-neutral and ENSO-positive years and explained 42.5% of the variance in environmental conditions at sampling stations (Table 3). Salinity and UML depth were negatively correlated with PC1 in all years and trended in the opposite direction as chl a, which was positively correlated with PC1 in all years. Water temperature showed little correlation with PC1 among all years. PC1 scores were not different between 2013 and 2014 or between 2015 and 2016, and were higher in 2013 and 2014 (p < 0.0001 for all between-year comparisons). 2013 and 2014 were associated with positive PC1 scores (2013: 0.50 ± 0.96 (n = 84); 2014: 0.48 ± 1.29 (n = 108)), while 2015 and 2016 were associated with negative PC1 scores (2015: − 0.60 ± 1.24 (n = 88); 2016: − 0.43 ± 1.34 (n = 93)).
The second principal component (PC2) was less discerning with respect to ENSO-neutral and ENSO-positive years and explained 25.1% of the variance in environmental conditions at sampling stations (Table 3). PC2 scores were lower in 2013 than in 2015 (p = 0.0042) and 2016 (p = 0.0005). Out of all environmental variables, water temperature was correlated with PC2 and increased as PC2 scores increased in all years.
El Niño southern oscillation indices among years
Because ENSO indices are reported as single values by month and this study’s sampling windows each year spanned two months in 2013 and 2014 (mid-August through mid-September) and one month in 2015 and 2016 (August), statistical tests to explore relationships between ENSO indices and environmental variables were not feasible. Visual inspection of ENSO indices averaged over three-month windows throughout the five study years showed that ENSO conditions began to develop around the time of sampling in 2014, but PC1 scores in 2014 did not reflect ENSO-positive conditions because of a temporal lag between the shift in the ENSO signal and when environmental conditions reflected the shift (Fig. 5). The ENSO index peaked during spring 2015, and although the signal declined over the next several months and was negative by the month of sampling in 2016, PC1 scores in 2016 reflected a temporal lag between the shift in the ENSO signal and when environmental conditions reflected the shift, and remained lower than 2013 and 2014 scores (Fig. 5).
Three-month averages (December–January–February (djj), March–April–May (mam), June–July–August (jja), September–October–November (son)) of the Bivariate ENSO index for all five years of sampling (black line). First principal component scores from a principal component analysis of environmental variables (chl a, water temperature, salinity, and UML depth) are shown as red boxplots for the sampling windows between 2013 and 2016 to summarize environmental responses to ENSO conditions
Atmospheric conditions among years
NCEP-derived autumn and winter surface wind speeds showed a distinct pattern over the five winters (Fig. 6). From 2012 through 2014, winds were relatively weak, with average east–west speeds of 1.88 and 1.22 m sec−1 (from west to east) during autumn and winter, respectively. South-north wind speeds during these winters were also relatively weak, averaging − 2.37 and − 2.44 m sec−1 (from north to south) during autumn and winter, respectively. In contrast, during 2015 and 2016, south-north wind speeds averaged 5.14 and 4.43 m sec−1 (from south to north) during autumn and winter, respectively. East–west wind speeds were low compared to 2012–2014 and averaged − 0.05 and − 0.64 m sec−1 (from east to west) during autumn and winter, respectively.
Stable isotopes
Isotopic turnover rates vary among species and are linked to temperature (Frazer et al. 1997), with poikilotherms in cold environments having slower turnover rates than homeotherms with higher metabolic rates (Ventura and Catalan 2008). Turnover rates have also been linked to size, with faster turnover rates for larger organisms (Post 2002). Data on turnover rates in Antarctic euphausiid species is limited; however, Frazer et al. (1997) determined that turnover in E. superba larvae during winter may be negligible, while turnover rates during spring and summer may be higher. For this study, we assume that turnover rates among euphausiid species of similar sizes are comparable and that isotopic signatures largely reflect diets consumed weeks to months prior to sampling.
Dietary carbon sources (δ13C)
Euphausia crystallorophias did not differ in δ13C among years (Fig. 7) and was enriched in δ13C relative to all other species. Euphausia superba larvae also formed a distinct group with respect to δ13C, between E. crystallorophias and the other euphausiid species. Values of δ13C in E. superba larvae differed among years and were higher in 2016 than in 2014 (p < 0.0001) and 2015 (p = 0.0175). Values in 2015 were higher than in 2014 (p = 0.0436). The rest of the euphausiid species demonstrated considerable overlap in δ13C. Euphausia frigida was more enriched in δ13C in 2015 than in 2013 (p = 0.008) and 2014 (p = 0.005). Values of δ13C in E. superba post-larvae were not different between 2013 and 2015 and between 2014 and 2016, and were higher in 2014 (2013–2014: p = 0.0474; 2014–2015: p = 0.002) and 2016 (2013–2016 and 2015–2016: p < 0.0001). Values of δ13C in E. triacantha were lower in 2013 relative to 2014 (p = 0.0008), 2015 (p = 0.0051), and 2016 (p = 0.0001). Thysanoessa macrura was enriched in δ13C in 2016 relative to 2013 (p = 0.0203), 2014 (p < 0.0001), and 2015 (p = 0.0167).
δ13C and δ15N means (± 1 SD) for all euphausiid species in all sampling years. Sample sizes each year are reported in Table 2. Euphausia superba larvae were not analyzed for stable isotopes in 2012. Stable isotope data have been published previously (Euphausia superba post-larvae and larvae: Walsh et al (2020) https://doi.org/10.3354/meps13325; all other euphausiids: Walsh and Reiss (2020) https://doi.org/10.5061/dryad.hhmgqnkdx)
Trophic position (δ15N)
Euphausia frigida, E. superba post-larvae and larvae, and T. macrura differed in δ15N among years (Fig. 7). Values of δ15N in E. frigida were higher in 2016 relative to 2013 (p = 0.0120), 2014 (p = 0.0220), and 2015 (p = 0.0095). Values of δ15N in E. superba post-larvae were higher in 2016 than in 2015 (p = 0.0008). Values of δ15N in E. superba larvae were lower in 2015 than in 2014 (p = 0.0016) and 2016 (p = 0.0021). Values of δ15N in T. macrura were lower in 2015 than in 2013 (p = 0.0014) and 2016 (p = 0.0073). Euphausia crystallorophias and E. triacantha did not differ in δ15N among years.
Body condition (lipid content)
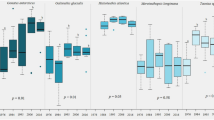
All species except E. crystallorophias differed in lipid content among years (Fig. 8). Euphausia frigida had lower lipid content in 2014 than in 2013 (p = 0.0001) and 2015 (p = 0.0016). Euphausia superba post-larvae had lower lipid content in 2014 than in 2013 (p = 0.0028), 2015 (p = 0.0001), and 2016 (p < 0.0001). Euphausia superba larvae had higher lipid content in 2013 than in 2014 (p < 0.0001), 2015 (p = 0.0028), and 2016 (p = 0.0017). Euphausia triacantha had higher lipid content in 2013 than in 2014 (p = 0.0051) and 2016 (p < 0.0001), and higher lipid content in 2015 than in 2016 (p = 0.0432). Thysanoessa macrura had higher lipid content in 2016 than in 2013 (p = 0.0277) and 2014 (p = 0.0007).
Means (± 1 SD) of lipid content for all euphausiid species in all sampling years. E. c.: Euphausia crystallorophias; E. f.: Euphausia frigida; E. s. l: Euphausia superba larvae; E. s. p-l: Euphausia superba post-larvae; E. t.: Euphausia triacantha; T. m.: Thysanoessa macrura. Sample sizes each year are reported in Table 2. E. superba larvae and E. triacantha were not analyzed for lipid content in 2012. Lipid data have been published previously (E. superba post-larvae and larvae: Walsh et al. (2020) https://doi.org/10.3354/meps13325; all other euphausiids: Walsh and Reiss (2020) https://doi.org/10.5061/dryad.hhmgqnkdx)
Discussion
The Antarctic Peninsula ecosystem has been disproportionately affected by regional warming trends over the last several decades (Jones et al. 2019), with cascading effects for organisms that depend on a suite of ideal conditions for optimal feeding, growth, and reproduction (Quetin et al. 2007). We observed inter-annual differences in body condition in five species of Antarctic euphausiids that cannot be fully explained by changes in trophic position or sources of dietary carbon. While differences in annual lipid content were not always significant for all species among years (likely due to small sample sizes for some species), we observed a general pattern of low lipid content in 2014 relative to other study years for all euphausiid species. Lipid content rebounded in 2015 for all species and then diverged in 2016, most notably in E. triacantha, which had the lowest lipid content of all years in 2016. Because the SAM phase was variable and mostly positive in the six months leading up to the survey period each year (https://legacy.bas.ac.uk/met/gjma/sam.html accessed 11 August 2021) and sea-ice cover was relatively low and similar in spatial coverage in both 2014 and 2016 (Walsh et al. 2020), we propose that environmental conditions generated by the neutral 2014 ENSO event and the extreme 2016 ENSO event affected prey quality and availability for each euphausiid species and were responsible for the observed trends in euphausiid lipid content between 2014 and 2016.
Annual differences in environmental conditions and the phytoplankton community
We observed shallow UML depths and highly stratified surface layers with low salinity in 2014. The position of the 27.6 σt isopycnal and the temperature-salinity profiles in the Bransfield Strait indicate the prevalence of warm CDW in the survey area at depth and a lack of WSSW influence, which are typical of ENSO-neutral conditions. The predicted ENSO-positive event failed to develop in 2014 largely because the strong coupling usually present between the atmosphere and the ocean during ENSO events was absent (McPhaden 2015), which was evident around the northern Antarctic Peninsula because of weak winds relative to 2015 and 2016. Calm conditions, along with the absence of substantial sea-ice cover (Walsh et al. 2020), likely resulted in the high levels of chl a observed in 2014.
While we did not determine phytoplankton species composition for this study, other studies have shown that environmental conditions like those observed in the survey area in 2014 facilitate phytoplankton blooms dominated by smaller species, primarily cryptophytes (Moline et al. 2004; Mendes et al. 2013). For example, Mendes et al. (2018) found that cryptophytes were the dominant phytoplankton species in the surface waters of Gerlache Strait in summer 2014, when UML depths were shallow, salinity was low, surface waters were highly stratified, and chl a was high. While grazing efficiencies of all euphausiid species on small-celled phytoplankton are unknown, E. superba do not graze efficiently on most assemblages of crytophytes, even when chl a concentrations are high (Haberman et al. 2003). Winter phytoplankton cell abundance in the survey area was higher in 2014 than in 2016 (J Iriarte pers. comm.), suggesting that grazing pressure was low. Because phytoplankton communities dominated by small cells are grazed at low rates, these ecosystem conditions may sustain high chl a concentration and may negatively impact primary and secondary consumers (Finkel et al. 2010; Mendes et al. 2013).
Atmospheric and ocean circulation patterns were different in 2016 as a result of the extreme ENSO event. The position of the 27.6 σt isopycnal and the temperature-salinity profiles in the Bransfield Strait indicate the prevalence of WSSW in the survey area and a lack of CDW at depth on the shelf. East–west winds during austral autumn were nearly three times stronger than winds during 2014. These observations are consistent with previous observations from the survey area of deep UML depths and low chl a concentrations associated with ENSO-positive conditions and WSSW intrusion (Reiss et al. 2009). Although 2016 was unusual in that sea-ice cover was anomalously low around Antarctica (Wang et al. 2019), spatial shifts in the subtropical and polar front jets during ENSO-positive events usually result in colder, icier conditions in the Atlantic sector of the Southern Ocean and warmer, less icy conditions in the Pacific sector (Yuan 2004). The dip in the subpolar jet toward the tip of the Antarctic Peninsula during ENSO-positive conditions intensifies the Weddell gyre, causing WSSW to flow into the northern Antarctic Peninsula region (Loeb et al. 2009; Reiss et al. 2009).
WSSW is iron-enriched (Reiss et al. 2009; Ardelan et al. 2010), which favors the growth of larger phytoplankton cells (Helbling et al. 1991) with high cellular fatty acid concentrations (Chen et al. 2011). Chl a concentration has been shown to be inversely related to phytoplankton biovolume, with low concentrations of chl a related to high concentrations of larger-celled phytoplankton (Felip and Catalan 2000). Although we did not measure water-column iron in this study, the observed environmental conditions and low chl a concentrations in 2016 are consistent with the massive diatom bloom that occurred around the northern Antarctic Peninsula in spring and summer of 2015/16 (Costa et al. 2021).
Annual differences in source carbon
To determine whether annual variability in δ13C was the result of differences in the carbon signatures of phytoplankton, we performed compound-specific stable isotope analyses on two fatty acid markers for diatoms and flagellates in E. superba post-larvae (Walsh et al. 2020). Compound-specific δ13C did not vary among years, suggesting that annual differences in δ13C were the result of differences in prey consumed each year.
Although we observed significant differences in δ13C in all species except E. crystallorophias among years, the ranges of values were small (differences in mean annual values were < 2 ‰ for all species except E. superba larvae (difference of 3.16 ‰ between 2014 and 2016) and T. macrura (difference of 2.11 ‰ between 2014 and 2016)) and tended to be elevated in 2016 relative to other years. In polar environments, higher values of δ13C generally indicate increased feeding on sea-ice resources compared to water-column resources (Frazer 1996), but may also indicate increased benthic feeding (France 1995) or feeding in highly productive areas (Fischer 1991).
Given that sea-ice cover was similar between 2014 and 2016, elevated values of δ13C in most species in 2016 is likely a reflection of either directly consuming diatoms or consuming diatom-dependent prey during the bloom that occurred around the northern Antarctic Peninsula during the spring and summer of 2015/2016 (Costa et al. 2021), prior to our surveys. Ratios of fatty acids indicative of diatom consumption were elevated in E. superba post-larvae in 2016 relative to the other study years, indicating that diatoms were abundant in the study area (Walsh et al. 2020). Despite high diatom availability, E. superba larvae, which had the largest difference in δ13C between 2014 and 2016, did not appear to rely on diatoms for nutrition in 2016 and instead may have been enriched in δ13C either from consuming benthic resources in the absence of extensive sea-ice resources (Walsh et al. 2020), or possibly by consuming sea-ice resources in the weeks and months prior to sampling. Euphausia superba post-larvae may perform deep vertical migrations (> 1000 m) and feed on benthic resources (Schmidt et al. 2011). Euphausia superba larvae have been found at depths exceeding 900 m (Daly 2004), and while they have been described as efficient scavengers (Daly 2004), the extent to which they exploit benthic resources is not well understood and warrants further study.
Annual differences in trophic position
As with δ13C, we observed significant differences in δ15N in all species except E. crystallorophias and E. triacantha among years, and again the ranges of mean annual values were small (< 1.3 ‰ for all species). A difference in δ15N of less than 3–4 ‰ does not reflect a full trophic level shift (Post 2002), indicating that differences in environmental conditions and in the phytoplankton community among years had a negligible effect on trophic position.
Annual differences in body condition
Limited data are available on the overwinter lipid utilization strategies of most Antarctic euphausiid species except for E. superba. Most studies on E. superba (e.g., Meyer 2012) cite reduced feeding and metabolic rates in combination with increased mobilization of stored lipid to survive winter months when food availability is low. Studies on E. crystallorophias (Kattner and Hagen 1998) and T. macrura (Hagen and Kattner 1998) also cite increased mobilization of stored lipid during winter. However, since these early studies, feeding activity during winter has been shown to be regional, with E. superba in the Bransfield Strait showing more evidence of overwinter feeding than E. superba in other regions of Antarctica (Schmidt et al. 2014). Data on lipid content collected during U.S. AMLR Program summer surveys show that lipid content in E. superba and T. macrura is higher in winter than in summer (U.S. AMLR Program, unpubl.), suggesting that euphausiid species continue to feed to some extent in the survey area during autumn and winter, and that euphausiid lipid content in this study reflected the feeding environment around the time samples were collected.
In 2014, all euphausiid species had low lipid content relative to 2013 and 2015. ENSO-neutral conditions and the presence of CDW over the continental shelf in 2014 likely resulted in an ecosystem-wide shift in the phytoplankton community to small species, leading to less-efficient grazing and bottom-up effects on the body condition of all euphausiid species, despite their varied habitats, feeding strategies, and life histories.
In 2016, all species except E. triacantha had higher lipid content than in 2014. While high lipid content in E. crystallorophias and T. macrura in 2016 suggests that these species fed to some extent during winter, their improved body condition may reflect more favorable feeding conditions in their respective habitats during the previous spring and summer. Both species may not feed consistently throughout winter and instead store more than half their lipid as wax esters, which are a long-term form of energy storage and which allow them to maintain high levels of lipid throughout winter in preparation for spawning prior to the spring phytoplankton bloom (Hagen and Kattner 1998; Ju and Harvey 2004). Source carbon and trophic position were nearly identical in E. crystallorophias among years, while T. macrura varied in source carbon between 2014 and 2016 but trophic position only varied by 0.40 ‰. Lipid content in both species nearly doubled in 2016 compared to 2014 despite higher chl a and phytoplankton cell abundance in 2014 (J Iriarte pers. comm.), suggesting that prey quality was the driving factor in lipid accumulation.
Euphausia frigida and E. superba store lipid as triacylglycerol (Phleger et al. 1998), which is a short-term form of energy storage (Nicol et al. 2004), and therefore cannot rely on lipid accumulated during spring and summer to survive winters with low productivity. The nearly two-fold increase in lipid content in E. superba post-larvae from 2014 to 2016 was likely the result of directly feeding on diatoms throughout the winter. In contrast to E. superba post-larvae, which aggregate in the Bransfield Strait during winter (Reiss et al. 2017), E. frigida are considered an oceanic, “cold water” species (Mackey et al. 2012; Loeb and Santora 2015) and are generally found over the northern area of the continental shelf. Diatoms may have been less abundant in areas where E. frigida are common because of less influence of WSSW and an onshore-to-offshore gradient in large to small phytoplankton cells (Montes-Hugo et al. 2008). Little is known about the diet and overwintering strategies of E. frigida; however, this species was enriched in both δ13C and δ15N in 2016 compared to 2014, yet had only a moderate increase in lipid content, suggesting that while overall prey quality was higher in 2016, offshore areas may not have been as optimal for feeding compared to nearshore areas.
While E. superba larvae may have fed on benthic resources in 2016, the difference in lipid content between 2014 and 2016 was small compared to E. crystallorophias, E. superba post-larvae, and T. macrura. Sea ice plays an important role in benthic communities, with ice algae known to enhance the nutritional quality of benthic food resources (McMahon et al. 2006; Michel et al. 2019). Although ENSO conditions promote sea-ice cover around the northern Antarctic Peninsula (Yuan 2004), sea-ice extent was anomalously low in 2016 because of unusual atmospheric and oceanic conditions (Stuecker et al. 2017; Wang et al. 2019). Without a large contribution of ice algae to benthic food webs in 2016, benthic resources may have been suboptimal compared to pelagic resources, although more extreme ENSO events in the future may benefit benthic communities and the organisms that feed there.
Euphausia triacantha was the only euphausiid species to break from the pattern of increased lipid content in 2016 relative to 2014. Euphausia triacantha are distinct among the five species with respect to habitat and diet. They are considered an oceanic, “warm water” species and are distributed across the most northern areas of the continental shelf and slope and are found mostly in the Drake Passage (Mackey et al. 2012), where they perform deep diel vertical migrations (Loeb and Santora 2015). Euphausia triacantha are mostly carnivorous and have coarse mesh-size filter baskets for feeding (Loeb and Santora 2015), and may be unable to graze efficiently on small phytoplankton cells. While other omnivorous or carnivorous species may be secondary consumers of diatoms, E. triacantha gain lipid independent of diatom availability (Stübing and Hagen 2003).
Euphausia triacantha also differ from other euphausiid species in their phenology. Euphausia triacantha are the only euphausiids with considerable temporal overlap of their adult stage with E. superba larvae (Loeb and Santora 2015). Positive correlations between abundance anomalies of E. triacantha and E. superba larvae have been observed over 17 years of U.S. AMLR Program summer surveys, suggesting that E. superba larvae, primarily furcilia stages, may be a primary prey item for E. triacantha during late winter (Loeb and Santora 2015).
Patterns of lipid accumulation in E. triacantha between 2014 and 2016 may have been related to the degree of vertical habitat overlap between E. triacantha and E. superba larvae, as both species had similar horizontal distributions between years (Online Resource 1). In 2014, these species had a high degree of overlap in dietary source carbon, suggesting that both fed in the water column on suboptimal resources and that E. superba larvae were an available prey item for E. triacantha. However, in 2016, benthic feeding in E. superba larvae may have reduced their availability as a prey item for E. triacantha. Little is known about whether E. triacantha feed on benthic resources, but they are generally found closer to the surface at night, where they feed to compensate for the metabolic cost of crossing a broad temperature range while vertically migrating (Liszka et al. 2021). While E. triacantha are known to feed on copepods and store a small proportion of their lipid as wax esters (Phleger et al. 1998), the sharp decline in lipid between 2014 and 2016 suggests that their prey field may have been limited in 2016, possibly by the absence of E. superba larvae in the water column. The degree to which predation by E. triacantha on E. superba larvae may impact E. superba larval populations during winter around the northern Antarctic Peninsula is unknown, but may be substantial and warrants further investigation.
Implications
Sediment core studies of diatom assemblages from the late Holocene show a consistent pattern of enhanced sea-ice cover in the Bransfield Strait during ENSO-positive events over the last several thousand years (Nie et al. 2022), suggesting that if ENSO-positive events increase in frequency and intensity in the future (Cai et al. 2014), sea-ice cover may respond more strongly to this driver. While sea-ice extent may be driven more by climate events rather than seasonal cooling in the future, regional sea-ice cover around the peninsula may remain highly variable in response to the interplay between other atmospheric and oceanic drivers, particularly ENSO and the SAM (Stuecker et al. 2017). Although sea-ice cover may increase with more frequent ENSO-positive events, sea-ice drift driven by strong winds may redistribute sea ice and contribute to its variability around the northern Antarctic Peninsula (Wang et al. 2019). Therefore, predictions about how euphausiid species may respond to future ENSO-positive events are cautious.
Improved body condition in E. crystallorophias, E. frigida, E. superba post-larvae, and T. macrura during ENSO-positive events suggests that future ENSO events may benefit these species by increasing sea-ice extent around the northern Antarctic Peninsula and by favoring phytoplankton communities composed of larger cells with high nutritional value. Extensive winter sea ice and a favorable feeding environment may result in earlier maturation and spawning, followed by high larval survival and recruitment for E. superba (Siegel and Loeb 1995). Euphausia crystallorophias and T. macrura may also exploit sea-ice resources to a greater degree than once thought (Kohlbach et al. 2019), suggesting that these species may also benefit from increased winter sea-ice extent. Both species spawn in late winter/early spring, and for T. macrura, high lipid content in autumn and early winter is linked to multiple spawning events per season (Wallis et al. 2018). More studies are needed to evaluate whether E. frigida will benefit from ENSO-positive conditions in the future.
In contrast, because of its northern range and mostly carnivorous diet, E. triacantha may be the most negatively affected by an increase in ENSO-positive events around the northern Antarctic Peninsula. Under warming conditions, both E. triacantha and E. superba are expected to shift their distributions south, although not to the same extent (Mackey et al. 2012; Atkinson et al. 2019). The center of distribution of E. triacantha will likely remain farther north (Mackey et al. 2012) and thus this species is projected to have reduced habitat overlap with E. superba in the future.
In addition to reduced habitat overlap, changes in sea-ice extent in more southern areas along the Antarctic Peninsula during winter may affect prey availability for E. triacantha if E. superba larvae are an important prey item. Winter sea ice is strongly related to E. superba recruitment (Siegel and Loeb 1995; Loeb et al. 1997, 2009; Atkinson et al. 2004; Saba et al. 2014); however, extensive winter sea ice does not necessarily translate into high abundances of E. superba larvae around the northern Antarctic Peninsula (Walsh et al. 2020). Therefore, although ENSO events result in colder, icier conditions in this region, the strong influence of the positive SAM may mean that ice cover will be more variable and perhaps more mobile in the future. Abundant E. superba larvae may not be available to E. triacantha every winter because of the less predictable nature of sea ice around the northern Antarctic Peninsula. Additionally, a substantial proportion of E. superba larvae around the northern Antarctic Peninsula may not be the result of local production, but rather may be transported to this region from “upstream” areas farther southwest along the peninsula (Siegel et al. 2003; Siegel 2005; Conroy et al. 2020). Larvae may advect northeast in ocean currents, and increasing evidence suggests that larvae are also transported within sea ice (Meyer et al. 2017; Veytia et al. 2021). While ENSO events increase sea-ice extent around the northern Antarctic Peninsula, they have the opposite effect along the western Antarctic Peninsula because of the weakened polar front jet and the anomalous heat flux to this area from the north (Yuan 2004). Increased presence of CDW on the western peninsula shelf resulting from other modes of climate forcing (i.e., the SAM) may further reduce sea-ice cover in southern areas along the peninsula (Vaughan et al. 2003). Decreased sea-ice cover in these upstream regions may have negative consequences in the future for E. triacantha by reducing the flux of E. superba larvae to the northern Antarctic Peninsula, although the supply of larval E. superba to this region may be augmented by intrusions of WSSW during ENSO-positive events (Reiss et al. 2020).
Conclusion
Current and projected environmental changes around the Antarctic Peninsula may have substantial bottom-up effects on zooplankton populations. While many of these changes are poised to negatively affect euphausiid species around the northern Antarctic Peninsula (e.g., continued ocean warming, increased CDW over the continental shelf, decreased sea-ice cover, and phytoplankton communities dominated by small cells), the projected increase in the frequency of ENSO-positive events may benefit the adult stages of some euphausiid species by increasing both the proportion of WSSW and sea-ice extent around the northern peninsula. Iron-rich WSSW has the potential to generate phytoplankton blooms dominated by large cells (i.e., diatoms), resulting in improved body condition for both primary and secondary consumers, and while 2016 was unusual with respect to sea-ice cover, increased sea-ice extent during ENSO-positive years may enhance the nutritional quality of benthic communities. Of the five euphausiid species in this study, E. triacantha may be negatively affected by ENSO-positive conditions, as reductions in sea-ice cover in more southern areas along the peninsula may reduce the supply of E. superba larvae to the northern Antarctic Peninsula in the future. Additionally, recent evidence suggests that extreme ENSO events may increase ocean warming over the continental shelf, but may slow down surface warming and sea-ice decline (Cai et al. 2023), which may confound the ability to predict with reasonable confidence how euphausiid species will ultimately respond during future ENSO-positive years. Euphausia triacantha are poorly studied compared to other euphausiid species, and they are often excluded from ecosystem models seeking to project how future environmental change will alter the structure and function of Antarctic pelagic ecosystems (Johnston et al. 2022). Future studies are necessary to gain better insight into E. triacantha’s role as a predator, a prey item (e.g., for squid) (Cherel and Duhamel 2003), and a contributor to the Southern Ocean carbon cycle as climate change continues to shape the future of the Antarctic pelagic ecosystem.
Data availability
The data that support the findings of this study on E. superba post-larvae and larvae, and on environmental conditions at each sampling station in each year, are included in the supplementary files of the published article by Walsh et al. 2020 (https://doi.org/10.3354/meps13325). The data that support the findings of this study on all other euphausiid species are available in the Data Dryad repository (https://doi.org/10.5061/dryad.hhmgqnkdx).
References
Ardelan MV, Holm-Hansen O, Hewes CD et al (2010) Natural iron enrichment around the Antarctic Peninsula in the Southern Ocean. Biogeosciences 7:11–25. https://doi.org/10.5194/bg-7-11-2010
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103. https://doi.org/10.1038/nature02996
Atkinson A, Siegel V, Pakhomov EA et al (2008) Oceanic circumpolar habitats of Antarctic krill. Mar Ecol Prog Ser 362:1–23. https://doi.org/10.3354/meps07498
Atkinson A, Hill SL, Pakhomov EA et al (2019) Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat Clim Chang. https://doi.org/10.1038/s41558-018-0370-z
Budge SM, Iverson SJ, Koopman HN (2006) Studying trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Mar Mammal Sci 22:759–801. https://doi.org/10.1111/j.1748-7692.2006.00079.x
Cai W, Borlace S, Lengaigne M et al (2014) Increasing frequency of extreme El Niño events due to greenhouse warming. Nat Clim Chang 4:111–116. https://doi.org/10.1038/nclimate2100
Cai W, Jia F, Li S et al (2023) Antarctic shelf ocean warming and sea ice melt by projected El Niño changes. Nat Clim Chang. https://doi.org/10.1038/s41558-023-01610-x
Chen X, Wakeham SG, Fisher NS (2011) Influence of iron on fatty acid and sterol composition of marine phytoplankton and copepod consumers. Limnol Oceanogr 56:716–724. https://doi.org/10.4319/lo.2011.56.2.0716
Cherel Y, Duhamel G (2003) Diet of the squid Morotheuthis ingens (Teuthoidea: Onychoteuthidae) in the upper slope waters of the Kerguelen Islands. Mar Ecol Prog Ser 250:197–203
Conroy JA, Reiss CS, Gleiber MR et al (2020) Linking Antarctic krill larval supply and recruitment along the Antarctic Peninsula. Integr Comp Biol 60:1386–1400. https://doi.org/10.1093/icb/icaa111
Constable AJ, Melbourne-Thomas J, Corney SP et al (2014) Climate change and Southern Ocean ecosystems I: How changes in physical habitats directly affect marine biota. Glob Chang Biol 20:3004–3025. https://doi.org/10.1111/gcb.12623
Costa RR, Mendes CRB, Ferreira A et al (2021) Large diatom bloom off the Antarctic Peninsula during cool conditions associated with the 2015/2016 El Niño. Commun Earth Environ 252:1–11. https://doi.org/10.1038/s43247-021-00322-4
Daly KL (1990) Overwintering development, growth, and feeding of larval Euphausia superba in the Antarctic marginal ice zone. Limnol Oceanogr 35:1564–1576
Daly KL (2004) Overwintering growth and development of larval Euphausia superba: an interannual comparison under varying environmental conditions west of the Antarctic Peninsula. Deep-Sea Res II 51:2139–2168. https://doi.org/10.1016/j.dsr2.2004.07.010
Deppeler SL, Davidson AT (2017) Southern Ocean phytoplankton in a changing climate. Front Mar Sci 4:40. https://doi.org/10.3389/fmars.2017.00040
Donnelly J, Sutton TT, Torres JJ (2006) Distribution and abundance of miconekton and macrozooplankton in the NW Weddell Sea: Relation to a spring ice-edge bloom. Polar Biol 29:280–293. https://doi.org/10.1007/s00300-005-0051-z
Driscoll RM, Reiss CS, Hentschel BT (2015) Temperature-dependent growth of Thysanoessa macrura: Inter-annual and spatial variability around Elephant Island, Antarctica. Mar Ecol Prog Ser 529:49–61. https://doi.org/10.3354/meps11291
Falk-Petersen S, Sargent JR, Lønne OJ et al (1999) Functional biodiversity of lipids in Antarctic zooplankton: Calanoides acutus, Calanus propinquus, Thysanoessa macrura, and Euphausia crystallorophias. Polar Biol 21:37–47
Felip M, Catalan J (2000) The relationship between phytoplankton biovolume and chlorophyll in a deep oligotrophic lake: decoupling in their spatial and temporal maxima. J Plankton Res 22:91–105
Finkel ZV, Beardall J, Flynn KJ et al (2010) Phytoplankton in a changing world: cell size and elemental stoichiometry. J Plankton Res 32:119–137. https://doi.org/10.1093/plankt/fbp098
Fischer G (1991) Stable carbon isotope ratios of plankton carbon and sinking organic matter from the Atlantic sector of the Southern Ocean. Mar Chem 35:581–596
Flores H, Atkinson A, Kawaguchi S et al (2012) Impact of climate change on Antarctic krill. Mar Ecol Prog Ser 458:1–19. https://doi.org/10.3354/meps09831
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
France RL (1995) Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar Ecol Prog Ser 124:307–312
Frazer TK (1996) Stable isotope composition (δ13C and δ15N) of larval krill, Euphausia superba, and two of its potential food sources in winter. J Plankton Res 18:1413–1426
Frazer TK, Ross RM, Quetin LB et al (1997) Turnover of carbon and nitrogen during growth of larval krill, Euphausia superba Dana: a stable isotope approach. J Exp Mar Biol Ecol 212:259–275
Haberman KL, Quetin LB, Ross RM (2003) Diet of the Antarctic krill (Euphausia superba Dana): I. Comparisons of grazing on Phaeocystis antarctica (Karsten) and Thalassiosira antarctica (Comber). J Exp Mar Bio Ecol 283:79–95
Hagen W, Kattner G (1998) Lipid metabolism of the Antarctic euphausiid Thysanoessa macrura and its ecological implications. Limnol Oceanogr 43:1894–1901
Hagen W, Kattner G, Terbrüggen A et al (2001) Lipid metabolism of the Antarctic krill Euphausia superba and its ecological implications. Mar Biol 139:95–104
Helbling EW, Villafañe V, Holm-Hansen O (1991) Effect of iron on productivity and size distribution of Antarctic phytoplankton. Limnol Oceanogr 36:1879–1885
Henley SF, Schofield OM, Hendry KR et al (2019) Variability and change in the west Antarctic Peninsula marine system: research priorities and opportunities. Prog Oceanogr 173:208–237. https://doi.org/10.1016/j.pocean.2019.03.003
Holm-Hansen O, Riemann B (1978) Chlorophyll a determination: improvements in methodology. Oikos 30:438–447
Holm-Hansen O, Lorenzen CJ, Holmes RW et al (1965) Fluorometric determination of chlorophyll. J Cons Perm Int Explor Mer 30:3–15
Johnston NM, Murphy EJ, Atkinson A et al (2022) Status, change, and futures of zooplankton in the Southern Ocean. Front Ecol Evol 9:624692. https://doi.org/10.3389/fevo.2021.624692
Jones ME, Bromwich DH, Nicolas JP et al (2019) Sixty years of widespread warming in the southern middle and high latitudes (1957–2016). J Clim 32:6875–6898. https://doi.org/10.1175/JCl-D-18-0565.1
Ju SJ, Harvey HR (2004) Lipids as markers of nutritional condition and diet in the Antarctic krill Euphausia superba and Euphausia crystallorophias during austral winter. Deep-Sea Res II 51:2199–2214. https://doi.org/10.1016/j.dsr2.2004.08.004
Kattner G, Hagen W (1998) Lipid metabolism of the Antarctic euphausiid Euphausia crystallorophias and its ecological implications. Mar Ecol Prog Ser 170:203–213
Kattner G, Hagen W, Falk-Petersen S et al (1996) Antarctic krill Thysanoessa macrura fills a major gap in marine lipogenic pathways. Mar Ecol Prog Ser 134:295–298
Kohlbach D, Lange BA, Graeve M et al (2019) Varying dependency of Antarctic euphausiids on ice algae- and phytoplankton-derived carbon sources during summer. Mar Biol 166:79. https://doi.org/10.1007/s00227-019-3527-z
Levine AFZ, McPhaden MJ (2016) How the July 2014 easterly wind burst gave the 2015–2016 El Niño a head start. Geophys Res Lett 43:6503–6510. https://doi.org/10.1002/2016GL069204
Liszka CM, Robinson C, Manno C et al (2021) Diel vertical migration of a Southern Ocean euphausiid, Euphausia triacantha, and its metabolic response to consequent short-term temperature changes. Mar Ecol Prog Ser 660:37–52. https://doi.org/10.3354/meps13618
Loeb VJ, Santora JA (2015) Climate variability and spatiotemporal dynamics of five Southern Ocean krill species. Prog Oceanogr 134:93–122. https://doi.org/10.1016/j.pocean.2015.01.002
Loeb V, Siegel V, Holm-Hansen O et al (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Loeb VJ, Hofmann EE, Klinck JM et al (2009) ENSO and variability of the antarctic peninsula pelagic marine ecosystem. Antarct Sci 21:135–148. https://doi.org/10.1017/S0954102008001636
Mackey AP, Atkinson A, Hill SL et al (2012) Antarctic macrozooplankton of the southwest Atlantic sector and Bellingshausen Sea: Baseline historical distributions (Discovery Investigations, 1928–1935) related to temperature and food, with projections for subsequent ocean warming. Deep-Sea Res II 59–60:130–146. https://doi.org/10.1016/j.dsr2.2011.08.011
McMahon KW, Ambrose WG Jr, Johnson BJ et al (2006) Benthic community response to ice algae and phytoplankton in Ny Alesund, Svalbard. Mar Ecol Prog Ser 310:1–14
McPhaden MJ (2015) Playing hide and seek with El Niño. Nat Clim Change 5:791–795. https://doi.org/10.1038/nclimate2775
Mendes CRB, Tavano VM, Leal MC et al (2013) Shifts in the dominance between diatoms and cryptophytes during three late summers in the Bransfield Strait (Antarctic Peninsula). Polar Biol 36:537–547. https://doi.org/10.1007/s00300-012-1282-4
Mendes CRB, Tavano VM, Dotto TS et al (2018) New insights on the dominance of cryptophytes in Antarctic coastal waters: a case study in Gerlache Strait. Deep-Sea Res II 149:161–170. https://doi.org/10.1016/j.dsr2.2017.02.010
Meyer B (2012) The overwintering of Antarctic krill, Euphausia superba, from an ecophysiological perspective. Polar Biol 35:15–37. https://doi.org/10.1007/s00300-011-1120-0
Meyer B, Atkinson A, Stübing D et al (2002) Feeding and energy budgets of Antarctic krill Euphausia superba at the onset of winter—I. Furcilia III. Larvae Limnol Oceanogr 47:943–952. https://doi.org/10.4319/lo.2002.47.4.0943
Meyer B, Auerswald L, Siegel V et al (2010) Seasonal variation in body composition, metabolic activity, feeding, and growth of adult krill Euphausia superba in the Lazarev Sea. Mar Ecol Prog Ser 398:1–18. https://doi.org/10.3354/meps08371
Meyer B, Freier U, Grimm V et al (2017) The winter pack-ice zone provides a sheltered but food-poor habitat for larval Antarctic krill. Nat Ecol Evol. https://doi.org/10.1038/s41559-017-0368-3
Michel LN, Danis B, Dubois P et al (2019) Increased sea ice cover alters food web structure in East Antarctica. Sci Rep 9:8062. https://doi.org/10.1038/s41598-019-44605-5
Mitchell BG, Holm-Hansen O (1991) Observations and modeling of the Antarctic phytoplankton crop in relation to mixing depth. Deep-Sea Res A 38:981–1007. https://doi.org/10.1016/0918-0149(91)90093-U
Moline MA, Claustre H, Frazer TK et al (2004) Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Glob Chang Biol 10:1973–1980. https://doi.org/10.1111/j.1365-2486.2004.00825.x
Montes-Hugo MA, Vernet M, Martinson D et al (2008) Variability on phytoplankton size structure in the western Antarctic Peninsula (1997–2006). Deep-Sea Res II 55:2106–2117. https://doi.org/10.1016/j.dsr2.2008.04.036
Montes-Hugo M, Doney SC, Ducklow HW et al (2009) Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science 323:1470–1473. https://doi.org/10.1126/science.1164533
Morley SA, Abele D, Barnes DKA et al (2020) Global drivers on Southern Ocean ecosystems: changing physical environments and anthropogenic pressures in an Earth system. Front Mar Sci 7:547188. https://doi.org/10.3389/fmars.2020.547188
Nicol S, Virtue P, King R et al (2004) Condition of Euphausia crystallorophias off East Antarctica in winter in comparison to other seasons. Deep-Sea Res II 51:2215–2224. https://doi.org/10.1016/j.dsr2.2004.07.002
Nie S, Xiao W, Wang R (2022) Mid-Late Holocene climate variabilities in the Bransfield Strait, Antarctic Peninsula driven by insolation and ENSO activities. Palaeogeogr Palaeoclimatol Palaeoecol 601:111140. https://doi.org/10.1016/j.palaeo.2022.111140
Ogle DH, Doll JC, Wheeler P, et al. (2022) FSA: fisheries stock analysis. R package version 0.9.3, https://github.com/fishR-Core_team/FSA
Pakhomov EA, Perissinotto R, Froneman PW (1998) Abundance and trophodynamics of Euphausia crystallorophias in the shelf region of the Lazarev Sea during austral spring and summer. J Marine Syst 17:313–324
Phleger CF, Nichols PD, Virtue P (1998) Lipids and trophodynamics of Antarctic zooplankton. Comp Biochem Phys B 120:311–323
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Post DM, Layman CA, Arrington DA et al (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. https://doi.org/10.1007/s00442-006-0630-x
Quetin L, Ross R, Fritsen C, Vernet M (2007) Ecological responses of Antarctic krill to environmental variability: can we predict the future? Antarct Sci 19:253–266. https://doi.org/10.1017/S0954102007000363
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reiss CS, Hewes CD, Holm-Hansen O (2009) Influence of atmospheric teleconnections and upper circumpolar deep water on phytoplankton biomass around Elephant Island, Antarctica. Mar Ecol Prog Ser 377:51–62. https://doi.org/10.3354/meps07840
Reiss CS, Walsh J, Goebel ME (2015) Winter preconditioning determines feeding ecology of Euphausia superba in the Antarctic Peninsula. Mar Ecol Prog Ser 519:89–101. https://doi.org/10.3354/meps11082
Reiss CS, Cossio A, Santora JA et al (2017) Overwinter habitat selection by Antarctic krill under varying sea-ice conditions: implications for top predators and fishery management. Mar Ecol Prog Ser 568:1–16. https://doi.org/10.3354/meps12099
Reiss CS, Hinke JT, Watters GM (2020) Demographic and maturity patterns of Antarctic krill (Euphausia superba) in an overwintering hotspot. Polar Biol. https://doi.org/10.1007/s00300-020-02704-4
Richerson K, Driscoll R, Mangel M (2018) Increasing temperature may shift availability of Euphausiid prey in the Southern Ocean. Mar Ecol Prog Ser 588:59–70. https://doi.org/10.3354/meps12460
Saba GK, Fraser WR, Saba VS et al (2014) Winter and spring controls on the summer food web of the coastal West Antarctic Peninsula. Nat Commun 5:4318. https://doi.org/10.1038/ncomms5318
Schlacher TA, Connolly RM (2014) Effects of acid treatment on carbon and nitrogen stable isotope ratios in ecological samples: a review and synthesis. Methods Ecol Evol 5:541–550. https://doi.org/10.1111/2041-210X.12183
Schmidt K, Atkinson A, Steigenberger S et al (2011) Seabed foraging by Antarctic krill: implications for stock assessment, bentho-pelagic coupling, and the vertical transfer of iron. Limnol Oceanogr 56:1411–1428. https://doi.org/10.4319/lo.2011.56.4.1411
Schmidt K, Atkinson A, Pond DW et al (2014) Feeding and overwintering of Antarctic krill across its major habitats: the role of sea ice cover, water depth, and phytoplankton abundance. Limnol Oceanogr 59:17–36. https://doi.org/10.4319/lo.2014.59.1.0017
Sen Gupta A, Santoso A, Taschetto AS et al (2009) Projected changes to the Southern Hemisphere ocean and sea ice in the IPCC AR4 climate models. J Clim 22:3047–3078. https://doi.org/10.1175/2008JCLI2827.1
Siegel V (2005) Distribution and population dynamics of Euphausia superba: summary of recent findings. Polar Biol 29:1–22. https://doi.org/10.1007/s00300-005-0058-5
Siegel V, Loeb V (1995) Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Mar Ecol Prog Ser 123:45–56
Siegel V, Ross RM, Quetin LB (2003) Krill (Euphausia superba) recruitment indices from the western Antarctic Peninsula: are they representative of larger regions? Polar Biol 26:672–679. https://doi.org/10.1007/s00300-003-0537-5
Siegel V, Reiss CS, Dietrich KS et al (2013) Distribution and abundance of Antarctic krill (Euphausia superba) along the Antarctic Peninsula. Deep-Sea Res I 77:63–74. https://doi.org/10.1016/j.dsr.2013.02.005
Smith CA, Sardeshmukh P (2000) The effect of ENSO on the intraseasonal variance of surface temperature in winter. Int J Climatol 20:1543–1557
Stübing D, Hagen W (2003) Fatty acid biomarker ratios—suitable trophic indicators in Antarctic euphausiids? Polar Biol 26:774–782. https://doi.org/10.1007/s00300-003-0550-8
Stuecker MF, Bitz CM, Armour KC (2017) Conditions leading to the unprecedented low Antarctic sea ice extent during the 2016 austral spring season. Geophys Res Lett 44:9008–9019. https://doi.org/10.1002/2017GL074691
Tarling GA, Stowasser G, Ward P et al (2012) Seasonal trophic structure of the Scotia Sea pelagic ecosystem considered through biomass spectra and stable isotope analysis. Deep-Sea Res II 59–60:222–236. https://doi.org/10.1016/j.dsr2.2011.07.002
Vaughan DG, Marshall GJ, Connolley WM et al (2003) Recent rapid regional warming on the Antarctic Peninsula. Clim Change 60:243–274
Ventura M, Catalan J (2008) Incorporating life histories and diet quality in stable isotope interpretations of crustacean zooplankton. Freshwater Biol 53:1453–1469. https://doi.org/10.1111/j.1365-2427.2008.01976.x
Verona L, Noro M, Barbosa E et al (2018) Western Antarctic ocean surface variability during the 20th century in the NCAR-CCSM4. Deep-Sea Res II 149:47–57. https://doi.org/10.1016/j.dsr2.2017.12.007
Veytia D, Bestley S, Kawaguchi S et al (2021) Overwinter sea-ice characteristics important for Antarctic krill recruitment in the southwest Atlantic. Ecol Indic 129:107934. https://doi.org/10.1016/j.ecolind.2021.107934
Wallis JR, Kawaguchi S, Swadling KM (2018) Sexual differentiation, gonad maturation, and reproduction of the Southern Ocean euphausiid Thysanoessa macrura (Sars, 1883) (Crustacea: Euphausiacea). J Crust Biol 38:107–118. https://doi.org/10.1093/jcbiol/rux091
Walsh J, Reiss C (2020) Lipid content and stable isotopes of zooplankton during five winters around the northern Antarctic Peninsula. Sci Data 7:380. https://doi.org/10.1038/s41597-020-00722-9
Walsh J, Reiss C, Watters G (2020) Flexibility in Antarctic krill (Euphausia superba) decouples diet and recruitment from overwinter sea-ice conditions in the northern Antarctic Peninsula. Mar Ecol Prog Ser 642:1–19. https://doi.org/10.3354/meps13325
Wang Z, Turner J, Wu Y et al (2019) Rapid decline of total Antarctic sea ice extent during 2014–2016 controlled by wind-driven sea ice drift. J Clim 32:5381–5395. https://doi.org/10.1175/JCLI-D-18-0635.1
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York
Yuan X (2004) ENSO-related impacts on Antarctic sea ice: a synthesis of phenomenon and mechanisms. Antarct Sci 16:415–425. https://doi.org/10.1017/S0954102004002238
Zhang Z, Hofmann EE, Dinniman MS et al (2020) Linkage of the physical environments in the northern Antarctic Peninsula region to the Southern Annular Mode and the implications for the phytoplankton production. Prog Oceanogr 188:102416. https://doi.org/10.1016/j.pocean.2020.102416
Acknowledgements
We thank the crew and the scientific support staff of the U.S. National Science Foundation vessel RVIB Nathaniel B. Palmer, as well as all of the technicians over our five winter surveys for their hard work, enthusiasm, and dedication while collecting and processing samples at sea. We especially thank Anthony Cossio, Kim Dietrich, Ryan Driscoll, and Rachel Pound. We would also like to thank Drs. Douglas Krause and Jefferson Hinke for helpful feedback and advice during manuscript preparation.
Funding
The authors have no financial or proprietary interests in any material discussed in this article.
Author information
Authors and Affiliations
Contributions
JW collected samples, conducted laboratory analyses, analyzed data, and wrote the manuscript. CR assisted in statistical analyses, generated figures related to CDW, and provided feedback. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walsh, J., Reiss, C. Extreme El Niño southern oscillation conditions have contrasting effects on the body condition of five euphausiid species around the northern Antarctic Peninsula during winter. Polar Biol 46, 319–338 (2023). https://doi.org/10.1007/s00300-023-03129-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03129-5