Abstract
Arctic zooplankton develop large energy reserves, as an adaptation to strong seasonality, making them valuable prey items. We quantified the energy content (kJ g−1 dry weight) of abundant krill (arcto-boreal, Thysanoessa inermis and boreal, Meganyctiphanes norvegica) and amphipods (Arctic, Themisto libellula and sub-Arctic-boreal, Themisto abyssorum) in the Barents Sea in late summer (August) and early winter (December). Variation in energy content was attributed to species-specific traits and body size categories, the latter in part as a proxy for ontogeny. T. inermis had the highest energy content, (Aug: 26.8 ± 1.5 (SD) kJ g−1) and remained similar from summer to winter. Energy content increased in M. norvegica and decreased in both amphipod species, with the lowest energy content being in T. abyssorum (Dec: 17.8 ± 0.8 kJ g−1). The effect of body size varied between species, with energy content increasing with size in T. inermis and T. libellula, and no change with size in M. norvegica and T. abyssorum. The reproductive stages of T. libellula differed in energy content, being highest in gravid females. Energy content varied with species’ dependence on energy storage. Our findings highlight how phylogenetically and morphologically similar prey items cannot necessarily be considered equal from a predator´s perspective. Energetically, the northern T. inermis was higher quality compared to the more southern M. norvegica, and mostly so during summer. Ecological models and management strategies should consider such variation in prey quality, especially as Arctic borealization is expected to change species composition and the energetic landscape for predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energy flux is a key component in an ecosystem’s functioning (Barnes et al. 2018). Inherent to this flux, from primary producers to apex predators, is the energy content of an organism (Brown et al. 2004; Van de Putte et al. 2006). Prey quality typically increases with energy content and thereby impacts the foraging behaviour and prey choice in consumers (Lawson et al. 1998; Houston and McNamara 1999; Spitz et al. 2010a). Thus, quantifying the energy content of key species in an ecosystem is an important step in understanding food web dynamics, energy flux and overall ecosystem functioning.
However, an organism does not exist in a constant state, but rather experiences physical, physiological and behavioural changes throughout its lifetime, and through the year. This variability is apparent with changes in body size and developmental stage, where trade-offs in the energy allocated to growth and reproduction occur over an annual cycle and throughout a lifetime (Perrin and Sibly 1993; McNamara and Houston 1996; Stearns 2000). Seasonal environmental variables, such as food availability, shape life history and foraging strategies, which in turn impact selection pressures on energy storage and allocation (Varpe 2017).
Arctic marine ecosystems have strong seasonality, in particular through changing light conditions, sea ice cover and primary production (Ji et al. 2013). Arctic zooplankton species have evolved adaptations to this seasonal environment, notably through their use of energy reserves. Many Arctic species develop prominent lipid stores to survive periods of low food availability, as well as use reserves to fuel reproduction ahead of the productive period (i.e. capital breeding), rather than depend on concurrent food intake (i.e. income breeding) (Hagen 1999; Varpe et al. 2009; Daase et al. 2013).
In Arctic and sub-Arctic seas, macrozooplankton such as krill and amphipods are an important dietary link between primary and secondary producers and higher trophic levels, being prey items of fish (Cusa et al. 2019; Eriksen et al. 2021), seabirds (Mehlum and Gabrielsen 1993; Dalpadado 2001) and marine mammals (Falk-Petersen et al. 2004). The Barents Sea is highly productive and one of the most biodiverse marine ecosystems in the Arctic (Sakshaug et al. 1994; Michel et al. 2012; Dalpadado et al. 2020). Both Arctic and Atlantic water influenced, the Barents Sea hosts Arctic and boreal species of macrozooplankton, including krill, such as the arcto-boreal Thysanoessa inermis and the boreal Meganyctiphanes norvegica, as well as amphipods, such as the Arctic Themisto libellula and the sub-Arctic-boreal Themisto abyssorum. These species make for interesting comparisons of species-specific traits related to feeding and reproductive strategies, and how these differences may affect energy content. However, there are only a few studies that explore the energetic dynamics of these important prey species, as outlined below.
To our knowledge, only two studies so far report energy content of these krill and amphipod species for the Barents Sea region (Mårtensson et al. 1996; Weslawski et al. 1999). More so, a key seasonal transition in the Arctic is the shift from summer to winter, from the productive to the relatively unproductive period. However, most of the existing studies for these species take place almost exclusively in the summer months (Percy and Fife 1981; Kulka and Corey 1982; Wolowicz and Szaniawska 1986; Weslawski et al. 1999, 2000; Walkusz et al. 2012) excluding energetic changes occurring in the lead up and during winter. Indeed, we are not aware of winter energy content data for these macrozooplankton species from the Barents Sea region. Borealization, with sub-Arctic species already seen to be moving northwards into warming Arctic waters (Fossheim et al. 2015), is also expected to alter current species composition in the Barents Sea and may impact the quality of prey available for predators (CAFF 2017; Griffith et al. 2019). It is important to understand the energetic dynamics of key prey species, such as krill and amphipods, to predict the effects such changes will have on the functioning of a future Arctic system.
The aim of this study is to quantify the energy content of abundant macrozooplankton species, making comparison between Arctic and boreal species, namely the krill T. inermis and M. norvegica, and the amphipods T. libellula and T. abyssorum, in the late summer and early winter in the Barents Sea. We investigate how energetic changes from the productive to unproductive period are related to the feeding and life history strategies and annual routines of these species. We categorise individuals into size classes, to investigate the effect of different developmental stages on changes in energy allocation. We also compare the early winter energy content of male and female T. libellula. Additionally, we summarise energy content estimates from the literature for these species and review our results in the context of these works.
Methods
Study site, species, and sample collection
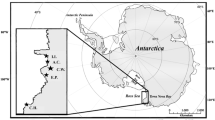
The target macrozooplankton species were sampled at six stations along a transect from 76° N to 82° N in the Barents Sea during late summer (August 4–27, 2019, SQ3, Nansen Legacy project) and at nine stations in early winter (November 28–December 17, 2019, SQ4, Nansen Legacy project) on-board RV Kronprins Haakon (Fig. 1).
Map showing wider European Arctic (inlay) and the Barents Sea (main) with summer (August) and winter (December) transects and sampling stations indicated for each cruise. The ice edge, as defined by the limit between “very open drift ice” and open water, is indicated by the white dashed lines for August and December. Ice edge is based on satellite data from the 12th day of each month. Map provided by Bernt Bye, Norwegian Polar Institute (2021)
At the sampling stations in open water, a Methot Isaac Kidd (MIK) net (13 m length, 2 m diameter, 1.5-mm mesh, with rear 1.5 m 500-µm mesh) was towed as an oblique haul, or a macrozooplankton trawl was used. In ice covered water, the MIK net was towed vertically. At each station, the entire water column was sampled, from 20 m above the bottom depth to the surface. At the northernmost station, where bottom depth exceeded 3000 m, nets were taken from 1000 m and 500 m to the surface, due to limitations of wire time.
Macrozooplankton were sorted to species level in cold seawater on ice, to keep individuals alive and prevent degradation. Using millimetre paper, individuals from each species were measured to the nearest millimetre and sorted into size classes: 0–10 mm, 11–20 mm, 21–30 mm and 31–40 mm. Amphipods, T. libellula and T. abyssorum, were measured from the front of the head to the tip of the longest uropod (Dunbar 1957). Krill, T. inermis and M. norvegica, were measured from the anterior edge of the eye to the tip of the telson, excluding the setae (Mauchline and Fisher 1969). The size classes represent a rough estimation of life stage. Within each size class, individuals that were collected at the same sampling station, or sometimes at two consecutive stations, were pooled together to achieve a bulk sample with a minimum wet weight of 1 g. This minimum weight is required to have enough biological material for energetic analyses. In cases where bulk samples were less than 1 g, then multiple samples would be pooled together to have enough material for analysis. If bulk samples were large enough, multiple subsamples were taken and used to make replicate pellets for bomb calorimetry. The number of individuals per sample varied depending on species and life stage. The samples were weighed and stored in aluminium foil at -20ºC until analysis. The number of samples for each species, season and size class combination varied, due to sampling effort and the abundance of krill and amphipods.
During the winter cruise, sexually mature individuals of T. libellula were observed and sampled. Males were identified by the presence of long antennae and gravid females were identified by the presence of eggs in the brood pouch. Therefore, winter samples of this species were classified as undetermined sex, male or gravid female.
Details on the distribution within the Barents Sea, average adult body size, feeding strategy, and reproductive strategy and breeding season were summarised for the four species of macrozooplankton in this study (Table 1). Capital breeding is when energy reserves are used to fuel reproduction, as opposed to income breeding which is when reproduction is dependent on concurrent energy intake (Varpe et al. 2009). Capital and income breeding are extremes on a gradient, and many species combine elements of the two, which can be referred to as mixed breeding (Varpe and Ejsmond 2018). References in Table 1 do not explicitly use capital-income terminology, and the capital-income terms we apply in Table 1 are approximations of where they lie on this reproductive spectrum.
Energy content
Energy content was measured using a Parr® 6725 Semi-micro Oxygen Bomb Calorimeter. Frozen samples were freeze-dried for a minimum of 24 h, after which the dry weight of each sample was determined. Dried samples were homogenised using a pestle and mortar. Using the Parr® 2812 Pellet Press, subsamples were taken from the bulk homogenised sample and pressed into pellets roughly weighing between 0.1 and 0.3 g (Table 2). Energy content (kJ g−1 dry weight) was measured for each pellet. Where possible, two replicate pellets of a single sample were run to ensure the calorimeter output was consistent. In some cases, more than two replicate pellets were run from a single sample (see data DOI for information regarding the number of replicates per sample). An average was taken of the replicates per sample (i.e. each sample produced a single calorimetric value), for use in further data analysis. Benzoic acid tablets (0.2 g) were used for calibration. Error margin for benzoic acid was ± 0.9%, while the error margin for biological material is slightly higher depending on homogeneity of the sample.
Data treatment and statistical analysis
Due to the unbalanced data set, the effects of season, species and size class were tested using a combination of Two-Way ANOVAs: To test for intra-specific differences in energy content from summer to winter, data were grouped by species and effects of season and size were estimated. To test for inter-specific differences, data were grouped by season and tested for the effect of species and size class on differences in energy content in both summer and winter. The effect of reproductive stage and size class on energy content was also tested for in winter T. libellula samples. Post-hoc Tukey’s HSD tests were used where ANOVAs were significant (p value < 0.05). For statistical testing, the midpoint of the allocated size classes was used (i.e. 15 mm for the 10–20 mm class). All statistical testing was performed with R software (version 4.0.2) (R Core Team 2020).
Results
Intra-specific energy differences, based on seasons and size classes
The energy content of the krill T. inermis was similar from summer to winter (ANOVA, F = 0.10, p = 0.76 (Table 3; Fig. 2a, b). The only seasonal energetic differences occurred when comparing different size classes, with the largest size class, 21–30 mm, having the highest energy content in both seasons (Tukey’s HSD, both seasons p < 0.001) (Fig. 2c, d). For M. norvegica, the average energy content increased 8.3%, by 1.89 kJ g−1, from summer to winter (ANOVA, F = 14.36, p < 0.001) (Table 3; Fig. 2a, b). However, there was no difference in the energy content between size classes (ANOVA, F = 1.38, p = 0.27) (Fig. 2c, d).
Boxplots showing energy content (Energy content kJ g−1 dry weight (DW)) across species in a late summer and b early winter and the distribution of energy content across size classes in each species in c late summer and d early winter. Boxes represent the interquartile range (IQR), with the horizontal line representing the median and vertical bars representing range, up to 1.5 × IQR. Points beyond these lines are considered outliers. In a and b the number of samples represented by each box plot is indicated by “n = ”, and diamonds inside boxes represent the mean
The amphipod species showed greater seasonal variation in energy content compared to the krill species. T. libellula showed the largest difference, from summer to winter, with a 18.6% decrease, of 4.3 kJ g−1, whilst T. abyssorum saw a 15.6% decrease of 3.3 kJ g−1 (ANOVA, F = 11.47, p = 0.001 and F = 9.44, p = 0.01, respectively) (Table 3, Fig. 2a, b). The average energy content for T. libellula was higher in summer compared to winter across all size classes. Within both summer and winter, the energy content of T. libellula increased with size, from 11–20 to 31–40 mm (ANOVA, F = 18.28, p < 0.001), but remained similar in the 0–10 mm and 11–20 mm size classes (Tukey’s HSD, p = 0.73) (Fig. 2c, d). For T. abyssorum, energy content did not differ between size classes within either season (ANOVA, F = 0.79, p = 0.40) (Fig. 2c, d).
Inter-specific energy differences in summer and winter
Summer
T. inermis had the highest energy content of all study species in summer, whilst T. abyssorum had the lowest (ANOVA, F = 17.01, p < 0.001) (Table 3). The Arctic counterpart in krill, T. inermis, had a higher energy content compared to the boreal M. norvegica (Tukey’s HSD, p < 0.001), whilst both the amphipod species were similar in summer (Tukey’s HSD, p = 0.1) (Fig. 2a). There was no difference in energy content between M. norvegica and both T. libellula and T. abyssorum (Tukey’s HSD, p = 0.95 and p = 0.39, respectively) (Fig. 2a).
Winter
Differences in energy content between species were dependent on size class in winter, for all species (ANOVA, F = 3.93, p = 0.005). T. inermis in the 21–30 mm size class had the highest energy content compared to other study species and sizes (Tukey’s HSD, ranging from p = 0.001–0.04, depending on species and size) (Fig. 2d). T. abyssorum had the overall lowest energy content in winter but was similar in value to the smaller size classes (0–30 mm) of T. libellula (Tukey’s HSD, p > 0.5) (Fig. 2d).
Energetic differences between T. libellula reproductive stages in winter
In winter, the energy content of T. libellula varied depending on reproductive stage (ANOVA, F = 6.72, p < 0.001). The average energy content of gravid females was 2.57 kJ g−1 higher than males (Fig. 3a). The highest energy content was in a single 31–40 mm gravid female, at 24.9 kJ g−1. Males had a lower energy content than both undetermined (Tukey HSD, p = 0.01) and gravid (Tukey HSD, p = 0.006) samples (Fig. 3a). Differences in energy content varied between size classes, with the 30–40 mm size class having higher energy contents than both the 11–20 mm (Tukey HSD, p < 0.001) and 21–30 mm (Tukey HSD, p = 0.003) size classes (Fig. 3b).
Boxplot showing energy content (Energy content kJ g−1 dry weight (DW)) across a reproductive stages and b size classes, of winter T. libellula samples. Boxes represent the interquartile range (IQR), with the horizontal line representing the median and vertical bars representing range, up to 1.5 × IQR. Points beyond these lines are considered outliers. In panel a the number of samples represented by each box plot is indicated by “n = ”, and diamonds inside boxes represent the mean
Discussion
Energetics and feeding strategies
As expected, the highest energy content in both summer and winter was in the herbivorous Arctic krill, T. inermis. This species is known to have large lipid stores, primarily consisting of energy-rich wax esters (Falk-Petersen et al. 1981), a trait it shares with other predominantly herbivorous Arctic zooplankton, such as the copepods Calanus hyperboreus and Calanus glacialis (Hüenerlage et al. 2016; Cabrol et al. 2019a). Wax esters are associated with long-term energy storage in zooplankton (Lee et al. 2006). The relative stability in T. inermis energy content estimates coincide with the need to conserve energy throughout the winter, both to survive low food availability and fuel reproduction in the spring (Falk-Petersen et al. 2000). This conservation is achieved through a range of adaptations, such as sexual regression and negative growth, however metabolism through the winter is not reduced (Hüenerlage et al. 2015). There is also evidence of some carnivory in T. inermis (Hüenerlage et al. 2015; Cabrol et al. 2019a), which could subsidize energy stores.
The Atlantic krill M. norvegica had a lower energy content than T. inermis, likely due to lower lipid content, as well as its primary storage lipid being triacylglycerol, which is less energy dense than wax esters (Falk-Petersen et al. 1981). Furthermore, triacylglycerol is associated with short-term storage in marine zooplankton (Lee et al. 2006), with M. norvegica likely relying on feeding throughout the year, compared to the stronger reliance T. inermis has on energy stores (Cabrol et al. 2019b). M. norvegica was the only species in our study to show an increase in energy content from summer to winter. Inhabiting an extensive geographic range, occurring across the north Atlantic and reaching south to the Mediterranean, M. norvegica shows great plasticity in its feeding depending on region and season (Schmidt 2010; Pond et al. 2012). In northern regions, this Atlantic krill shifts from a relatively herbivorous to a Calanus spp.-based diet in autumn (Båmstedt and Karlson 1998; Schmidt 2010; Cabrol et al. 2019a) which could explain higher winter energy contents that reflect preying on lipid rich copepods.
Contrastingly, the two amphipod species we studied showed lower energy content than either krill species, as well as a greater difference between summer and winter. These amphipods are known to be predominantly carnivorous, with less dependence on energy stores to withstand the non-productive period (Auel et al. 2002). Despite showing a large decrease in energy content from summer to winter, both amphipod species have been found to feed during the polar night in some Svalbard fjords (Kraft et al. 2013). However, more than half of the Themisto spp. guts sampled in Rijpfjorden, a fjord characterised by Arctic waters, were less than 25% full (Kraft et al. 2013), indicating that hunting during the dark winter months might be less effective for these predominantly visual predators. Although there will be differences between fjord and Barents Sea populations, this potentially less effective winter feeding may, in part, explain the decrease in energy content from summer to winter in Themisto spp.
Energetics, body size and life history strategies
There was a strong positive relationship between size and energy content for T. inermis and T. libellula. However, M. norvegica and T. abyssorum, did not show variation in energy content based on size. For M. norvegica, this was likely a result of small sample sizes per size class in summer, with no samples at all for the 11–20 mm size class (Table 2), therefore making it difficult to discuss ontogenetic energetic variation. For T. abyssorum, the lack of energetic variation with size may be due to applying size classes that were too broad for this small species, as T. abyssorum has a maximum size of about 18 mm (Koszteyn et al. 1995; Table 1).
The increase in energy content with size in T. inermis is likely due to reallocation of energy from growth to reproductive efforts upon reaching maturity. T. inermis spawns from May to June, fuelling reproduction with energy reserves (Dalpadado and Skjoldal 1995; Table 1). Therefore, the increase in energy content with increasing body size is likely a result of energy being allocated to reserves for reproduction in the spring, versus being used for growth (Dalpadado and Ikeda 1989; Falk-Petersen et al. 2000; Pinchuk and Hopcroft 2006). This dependence on building energy stores for reproduction aligns with the stability in the energy content from summer to winter seen in this krill species.
T. libellula showed the most pronounced energy increase with size. Thus, energy content, from small to large individuals, is likely driven by ontogenetic changes in energy allocation, from somatic growth to energy reserves, for survival and reproduction. Total lipids increase from juveniles to adults (Noyon et al. 2011), correlating with the increase in energy content we see with increasing size in our study. The observed decrease in energy content, from summer to winter, aligns with lipid content being seen to decrease from summer to winter in T. abyssorum and T. libellula, due to reproduction (Kraft et al. 2015). This suggests there is an increase in reproductive investment towards the winter, which aligns with a mixed breeding approach (Table 1). This is further supported by the presence of mature males and gravid females of T. libellula in winter samples and although sample sizes were small, there were some differences between these reproductive categories. T. libellula is a brooder, with females carrying developing eggs in a brood pouch until they are released as juveniles (Percy 1993). However, eggs were not removed from the brood pouch in the analysed females, and therefore the estimates from these female samples “retain” the energetic content allocated to reproduction, through lipid rich eggs. This is one explanation for the highest energy content in winter T. libellula samples being a 31–40 mm gravid female, as well as higher energy content in 11–20 mm gravid females compared to males. From a predator perspective, gravid females could offer a highly nutritious prey option compared to males.
Present understanding and energetics in a future Arctic
We summarised energy content estimates in the existing literature (Table 4), and here compare our estimates with previous works. Where seasons and body sizes were comparable, the energy content estimates between our study and previous studies were mostly similar (e.g. energy content for T. libellula in our study and Walkusz et al. 2012). Energetic variation between seasons and body sizes has been shown to some extent in previous studies, with varying degrees of resolution (Table 4). One caveat of the present study is its restriction to only two seasons, namely late summer and early winter. Many past studies focused only on a single season, or a few months, but some studies sampled over the course of a full year (e.g. Tyler 1973; Table 4). Although, compiling studies gives additional insight into seasonal variation, in some cases over almost the entire year (e.g. for T. inermis, Table 4).
However, lack of differentiation between size classes of the individuals used for calometric analyses, or in some cases complete lack of size determination, is a barrier to comparisons between studies. In such cases, it cannot be known if variation in energy content is due to seasonal differences, or simply differences in the size classes used for analyses. The present study has shown that size is an important factor in energy content variation, both within and between species, due to changes (i.e. in diet or developmental stage) occurring over an organism’s annual and life cycle. More so, size is known to impact a predator´s choice of prey (Gill 2003). Therefore, energetic values for a range of size classes will improve our knowledge of the energy content of prey available to predators. Such size dependencies are amplified in visually searching predators that more easily detect large individuals, and in the Arctic, sea ice declines may provide a more beneficial light environment for such visual search (Langbehn and Varpe 2017). Future studies, with frequent seasonal sampling and a high resolution of size classes, would provide an important understanding of the energetic landscape available to predators over an annual cycle.
Energy content is another important factor in prey choice (Houston and McNamara 1999; Spitz et al. 2010b, 2012). For endothermic predators, such as seabirds and marine mammals, living costs and the resultant metabolisms are high. More so, they can experience periods of particularly high energy demands, such as when seabirds are feeding their chicks (Gabrielsen et al. 1987). Therefore, changes in prey quality, from energy-rich to energy poor, can have major consequences. Coined the “Junk-food hypothesis”, the phenomena of changing prey quality, from high to low, resulting in adverse effects on predators, has been well-documented (Österblom et al. 2008). Studies have suggested that lack of energetically rich fish species has resulted in decreased body condition and population decline in stellar sea lions (Eumetopias jubatus) (Alverson 1992) and reduced breeding success in black-legged kittiwakes (Rissa tridactyla) and tufted puffins (Fratercula cirrhata) (Romano et al. 2006), cape gannets (Morus capensis) (Grémillet et al. 2008) and common guillemots (Uria aalge) (Wanless et al. 2005). These negative outcomes were all a result of changing prey composition, where there was a reduced availability of high-quality prey. In a future Arctic, changes in the species composition of prey items could end up impacting predators in similar ways.
Like the rest of the Arctic, the Barents Sea is experiencing the effects of climate change at a faster rate and a greater magnitude than elsewhere on the planet, predominantly through increasing temperatures and reduced sea ice cover (CAFF 2017; Lind et al. 2018; AMAP 2021). This warming climate is leading to borealization, with sub-Arctic species of zooplankton and fish becoming more abundant in Arctic regions (Fossheim et al. 2015; Vihtakari et al. 2018). The present study shows that phylogenetically and morphologically similar prey items cannot necessarily be considered equal, and that feeding and reproductive strategies influence the energy content of prey. This is shown in the comparison of Arctic and Atlantic krill in the present study, whose energetic discrepancies are also seasonally dependent. Spitz et al. (2010b) categorised the energetic quality of prey (kJ g−1 of wet weight (WW)) as follows: Low Quality (< 4 kJ g−1), Moderate Quality (4–6 < kJ g−1) and High Quality (> 6 kJ g−1). For the 20–30 mm size class of Thysanoessa inermis and Meganyctiphanes norvegica, the average percentage dry weights (%DW) were then taken for each species. The dry weight of the total sample was divided by the wet weight, for each species in each season. In some cases, the dry weight or wet weight had not been taken, and therefore these samples were excluded from the %DW calculation. The existing average energy content (kJ g−1 dry weight) for each species in each season was divided by the average %DW, to produce energy content estimates in kJ g−1 wet weight. When the summer and winter averages for T. inermis and M. norvegica in the size class 20–30 mm are converted into energy content based on wet weight, T. inermis can be considered a high-quality prey item in both summer and winter, 8.6 kJ g−1 (WW) and 8.3 kJ g−1 (WW), respectively. Contrastingly, M. norvegica shows an energy content almost half of its Arctic counterpart in summer, 4.5 kJ g−1 (WW), categorising it as moderate quality, and rising to a high-quality standard in winter, 6.6 kJ g−1 (WW). If M. norvegica were to replace T. inermis as a dominant prey item in Arctic waters, it would prove a lower quality replacement in the summertime.
Indeed, the complexity of prey energetics shown in this study needs to be fully realised, to make informed decisions on the sustainable development of Arctic marine ecosystems. The Norwegian Ministry of Climate and Environment (2006; referenced in 2020 report) explicitly states the need for “more knowledge about energy flow and interactions between species if we are to develop a sound management regime” in the Integrated Management Plan for the Barents Sea. The energy content estimates in the present study takes a step towards this understanding.
This study has shown that energy content, and hence the quality of prey, can vary seasonally and so cannot be considered a constant value over an annual cycle. More so, this study shows that energy content can vary between even seemingly similar species and is associated with traits related to feeding and energy storage. Size differences, within and across species, are also an important factor in the energy content. Realising the energetic variation of key prey species is paramount in our understanding of the functioning of Arctic marine ecosystems, particularly when considering the effects to higher trophic levels. Hence, the dynamics shown in this study should be considered when incorporating energetics into ecological models and management strategies.
Data availability
Data to be deposited in as a Darwin Core Archive on the National Infrastructure for Research Data (NIRD). https://doi.org/10.11582/2023.00001.
References
Alverson D (1992) A review of commercial fisheries and the Steller sea lion (Eumetopias jubatus): the conflict arena. Rev Aquat Sci 6:203–256
AMAP (2021) Arctic Climate Change Update 2021: Key Trends and Impacts. Summary for Policy-makers. Arctic Monitoring and Assessment Programme (AMAP), Tromsø, Norway
Auel H, Werner I (2003) Feeding, respiration and life history of the hyperiid amphipod Themisto libellula in the arctic marginal ice zone of the Greenland Sea. J Exp Mar Biol Ecol 296:183–197. https://doi.org/10.1016/S0022-0981(03)00321-6
Auel H, Harjes M, da Rocha R, Stübing D, Hagen W (2002) Lipid biomarkers indicate different ecological niches and trophic relationships of the arctic hyperiid amphipods Themisto abyssorum and T. libellula. Polar Biol 25:374–383. https://doi.org/10.1007/s00300-001-0354-7
Båmstedt U, Karlson K (1998) Euphausiid predation on copepods in coastal waters of the Northeast Atlantic. Mar Ecol Prog Ser 172:149–168. https://doi.org/10.3354/meps172149
Barnes AD, Jochum M, Lefcheck JS, Eisenhauer N, Scherber C, O’Connor MI, de Ruiter P, Brose U (2018) Energy flux: the link between multitrophic biodiversity and ecosystem functioning. Trends Ecol Evol 33:186–197. https://doi.org/10.1016/j.tree.2017.12.007
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Cabrol J, Nadalini J-B, Tremblay R, Galbraith PS, Nozais C, Starr M, Plourde S, Winkler G (2019a) Seasonal and large-scale spatial variability of the energy reserves and the feeding selectivity of Meganyctiphanes norvegica and Thysanoessa inermis in a sub-arctic environment. Prog Oceanogr 179:102203. https://doi.org/10.1016/j.pocean.2019.102203
Cabrol J, Trombetta T, Amaudrut S, Aulanier F, Sage R, Trembla R, Nozais C, Starr M, Plourde S, Winkler G (2019b) Trophic niche partitioning of dominant North-Atlantic krill species, Meganyctiphanes norvegica, Thysanoessa inermis, and T. raschii. Limnol Oceanogr 64:165–181. https://doi.org/10.1002/lno.11027
CAFF (2017) State of the Arctic Marine Biodiversity Report: Key Findings and Advice for Monitoring. Conservation of Arctic Flora and Fauna (CAFF). Akureyri, Iceland
Cusa M, Berge J, Varpe Ø (2019) Seasonal shifts in feeding patterns: individual and population realized specialization in a high arctic fish. Ecol Evol 9:11112–11121. https://doi.org/10.1002/ece3.5615
Daase M, Falk-Petersen S, Varpe Ø, Darnis G, Søreide JE, Wold A, Leu E, Berge J, Philippe B, Fortier L (2013) Timing of reproductive events in the marine copepod Calanus glacialis: a pan-arctic perspective. Can J Fish Aquat Sci 70:871–884. https://doi.org/10.1139/cjfas-2012-0401
Dalpadado P (2001) Distribution of Themisto (Amphipoda) spp. in the Barents Sea and predator-prey interactions. ICES J Mar Sci 58:876–895. https://doi.org/10.1006/jmsc.2001.1078
Dalpadado P (2002) Inter-specific variations in distribution, abundance and possible life-cycle patterns of Themisto spp. (Amphipoda) in the Barents Sea. Polar Biol 25:656–666. https://doi.org/10.1007/s00300-002-0390-y
Dalpadado P, Ikeda T (1989) Some observations on moulting, growth and maturation of krill (Thysanoessa inermis) from the Barents Sea. J Plankton Res 11:133–139. https://doi.org/10.1093/plankt/11.1.133
Dalpadado P, Skjoldal HR (1991) Distribution and life history of krill from the Barents Sea. Polar Res 10:443–460. https://doi.org/10.3402/polar.v10i2.6758
Dalpadado P, Skjoldal HR (1995) Distribution and life cycle of krill north of 73° N in the Barents Sea. Havforskningsinstituttet, Tromsø, Norway
Dalpadado P, Skjoldal HR (1996) Abundance, maturity and growth of the krill species Thysanoessa inermis and T. longicaudata in the Barents Sea. Mar Ecol Prog Ser 144:175–183
Dalpadado P, Arrigo KR, van Dijken GL, Skjoldal HR, Bagøien E, Dolgov AV, Prokopchuk IP, Sperfeld E (2020) Climate effects on temporal and spatial dynamics of phytoplankton and zooplankton in the Barents Sea. Prog Oceanogr 185:102320. https://doi.org/10.1016/j.pocean.2020.102320
Dunbar MJ (1957) The determinants of production in northern seas: a study of the biology of Themisto libellula mandt. Can J Zool 35:797–819. https://doi.org/10.1139/z57-067
Eriksen E, Skjoldal HR, Dolgov AV, Strand E, Keulder-Stenevik F, Prokopchuk IP, Prokhorova TA, Prozorkevich D, Benzik AN (2021) Diet and trophic structure of fishes in the Barents Sea: seasonal and spatial variations. Prog Oceanogr 197:102663. https://doi.org/10.1016/j.pocean.2021.102663
Falk-Petersen S, Gatten RR, Sargent JR, Hopkins CCE (1981) Ecological investigations on the zooplankton community in Balsfjorden, Northern Norway: seasonal changes in the lipid class composition of Meganyctiphanes norvegica (M. Sars), Thysanoessa raschii (M. Sars), and T. inermis (Krøyer). J Exp Mar Biol Ecol 54:209–224. https://doi.org/10.1016/0022-0981(81)90158-1
Falk-Petersen S, Hagen W, Kattner G, Clarke A, Sargent J (2000) Lipids, trophic relationships, and biodiversity in arctic and Antarctic krill. Can J Fish Aquat Sci 57:178–191. https://doi.org/10.1139/f00-194
Falk-Petersen S, Haug T, Nilssen KT, Wold A, Dahl TM (2004) Lipids and trophic linkages in harp seal (Phoca groenlandica) from the eastern Barents Sea. Polar Res 23:43–50. https://doi.org/10.3402/polar.v23i1.6265
Fossheim M, Primicerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV (2015) Recent warming leads to a rapid borealization of fish communities in the arctic. Nat Clim Change 5:673–677. https://doi.org/10.1038/nclimate2647
Gabrielsen GW, Mehlum F, Nagy K (1987) Daily energy expenditure and energy utilization of free-ranging black-legged kittiwakes. The Condor 89:126–132. https://doi.org/10.2307/1368766
Gill AB (2003) The dynamics of prey choice in fish: the importance of prey size and satiation. J Fish Biol 63:105–116. https://doi.org/10.1111/j.1095-8649.2003.00214.x
Grémillet D, Pichegru L, Kuntz G, Woakes AG, Wilkinson S, Crawford RJM, Ryan PG (2008) A junk-food hypothesis for gannets feeding on fishery waste. Proc Biol Sci 275:1149–1156. https://doi.org/10.1098/rspb.2007.1763
Griffith GP, Hop H, Vihtakari M, Wold A, Kalhagen K, Gabrielsen GW (2019) Ecological resilience of arctic marine food webs to climate change. Nat Clim Change 9:868–872. https://doi.org/10.1038/s41558-019-0601-y
Hagen W (1999) Reproductive strategies and energetic adaptations of polar zooplankton. Invertebr Reprod Dev 36:25–34. https://doi.org/10.1080/07924259.1999.9652674
Harvey HR, Pleuthner RL, Lessard EJ, Bernhardt MJ, Shaw TC (2012) Physical and biochemical properties of the euphausiids Thysanoessa inermis, Thysanoessa raschii, and Thysanoessa longipes in the eastern Bering Sea. Deep-Sea Res Pt II 65–70:173–183. https://doi.org/10.1016/j.dsr2.2012.02.007
Houston AI, McNamara JM (1999) Models of adaptive behaviour: an approach based on state. Cambridge University Press, Cambridge
Hüenerlage K, Graeve M, Buchholz C, Buchholz F (2015) The other krill: overwintering physiology of adult Thysanoessa inermis (Euphausiacea) from the high-arctic Kongsfjord. Aquat Biol 23:225–235. https://doi.org/10.3354/ab00622
Hüenerlage K, Graeve M, Buchholz F (2016) Lipid composition and trophic relationships of krill species in a high arctic fjord. Polar Biol 39:1803–1817. https://doi.org/10.1007/s00300-014-1607-6
Ji R, Jin M, Varpe Ø (2013) Sea ice phenology and timing of primary production pulses in the arctic Ocean. Glob Change Biol 19:734–741. https://doi.org/10.1111/gcb.12074
Kooka K, Hamatsu T, Yamamura O (2018) Transported zooplankton from the Okhotsk Sea facilitate feeding and growth of juvenile walleye pollock on a continental shelf along the Oyashio Current, western sub-Arctic Pacific. Mar Biol 165:100. https://doi.org/10.1007/s00227-018-3360-9
Koszteyn J, Timofeev S, Węsławski JM, Malinga B (1995) Size structure of Themisto abyssorum Boeck and Themisto libellula (Mandt) populations in European arctic seas. Polar Biol 15:85–92. https://doi.org/10.1007/BF00241046
Kraft A, Berge J, Varpe Ø, Falk-Petersen S (2013) Feeding in arctic darkness: mid-winter diet of the pelagic amphipods Themisto abyssorum and T. libellula. Mar Biol 160:241–248. https://doi.org/10.1007/s00227-012-2065-8
Kraft A, Graeve M, Janssen D, Greenacre M, Falk-Petersen S (2015) Arctic pelagic amphipods: lipid dynamics and life strategy. J Plankton Res 37:790–807. https://doi.org/10.1093/plankt/fbv052
Kulka DW, Corey S (1982) Length and weight relationships of Euphausiids and caloric values of Meganyctiphanes norvegica (M. Sars) in the Bay of Fundy. J Crustacean Biol 2:239–247. https://doi.org/10.2307/1548004
Langbehn TJ, Varpe Ø (2017) Sea-ice loss boosts visual search: fish foraging and changing pelagic interactions in polar oceans. Glob Change Biol 23:5318–5330. https://doi.org/10.1111/gcb.13797
Lawson JW, Magalhães AM, Miller EH (1998) Important prey species of marine vertebrate predators in the northwest Atlantic: proximate composition and energy density. Mar Ecol Prog Ser 164:13–20. https://doi.org/10.3354/meps164013
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306. https://doi.org/10.3354/meps307273
Lind S, Ingvaldsen RB, Furevik T (2018) Arctic warming hotspot in the northern Barents Sea linked to declining sea-ice import. Nat Clim Change 8:634–639. https://doi.org/10.1038/s41558-018-0205-y
Mårtensson PE, Lager Gotaas AR, Nordøy ES, Blix AS (1996) Seasonal changes in energy density of prey of northeast Atlantic seals and whales. Mar Mammal Sci 12:635–640. https://doi.org/10.1111/j.1748-7692.1996.tb00080.x
Mauchline J, Fisher LR (1969) The biology of euphausiids. In: Russell FS, Yonge M (eds) Advances in marine biology, vol 7. Academic Press, London
McNamara JM, Houston AI (1996) State-dependent life histories. Nature 380:215–221. https://doi.org/10.1038/380215a0
Mehlum F, Gabrielsen GW (1993) The diet of High-arctic seabirds in coastal and ice-covered, pelagic areas near the Svalbard archipelago. Polar Res 12:1–20. https://doi.org/10.3402/polar.v12i1.6698
Michel C, Bluhm B, Gallucci V, Gaston AJ, Gordillo FJL, Gradinger R, Hopcroft R, Jensen N, Mustonen T, Niemi A, Nielsen TG (2012) Biodiversity of arctic marine ecosystems and responses to climate change. Biodiversity 13:200–214. https://doi.org/10.1080/14888386.2012.724048
Norrbin F, Båmstedt U (1984) Energy contents in benthic and planktonic invertebrates of Kosterfjorden, Sweden. A comparison of energetic strategies in marine organism groups. Ophelia 23:47–64. https://doi.org/10.1080/00785236.1984.10426604
Norwegian Ministry of Climate and Environment (2006) Integrated Management of the Marine Environment of the Barents Sea and the Sea Areas off the Lofoten Islands. Report No. 8 to the Storting. Stoltenberg II Government.
Norwegian Ministry of Climate and Environment (2020) Norway’s integrated ocean management plans Barents Sea–Lofoten area; the Norwegian Sea; and the North Sea and Skagerrak. Meld. St. 20 (2019 – 2020) Report to the Storting. Solberg Government.
Noyon M, Narcy F, Gasparini S, Mayzaud P (2011) Growth and lipid class composition of the arctic pelagic amphipod Themisto libellula. Mar Biol 158:883–892. https://doi.org/10.1007/s00227-010-1615-1
Österblom H, Olsson O, Blenckner T, Furness RW (2008) Junk-food in marine ecosystems. Oikos 117:967–969. https://doi.org/10.1111/j.0030-1299.2008.16501.x
Percy JA (1993) Reproduction and growth of the arctic hyperiid amphipod Themisto libellula Mandt. Polar Biol 13:131–139. https://doi.org/10.1007/BF00238546
Percy JA, Fife FJ (1981) The biochemical composition and energy content of arctic marine macrozooplankton. Arctic 34:307–313
Perrin N, Sibly RM (1993) Dynamic models of energy allocation and investment. Annu Rev Ecol Syst 24:379–410. https://doi.org/10.1146/annurev.es.24.110193.002115
Pinchuk AI, Hopcroft RR (2006) Egg production and early development of Thysanoessa inermis and Euphausia pacifica (Crustacea: Euphausiacea) in the northern Gulf of Alaska. J Exp Mar Biol Ecol 332:206–215. https://doi.org/10.1016/j.jembe.2005.11.019
Pond DW, Tarling GA, Schmidt K, Everson I (2012) Diet and growth rates of Meganyctiphanes norvegica in autumn. Mar Biol Res 8:615–623. https://doi.org/10.1080/17451000.2011.653366
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Romano MD, Piatt JF, Roby DD (2006) Testing the junk-food hypothesis on marine birds: effects of prey type on growth and development. Waterbirds 29:407–414. https://doi.org/10.1675/1524-4695(2006)29[407:TTJHOM]2.0.CO;2
Sakshaug E, Bjørge A, Gulliksen B, Loeng H, Mehlum F (1994) Structure, biomass distribution, and energetics of the pelagic ecosystem in the Barents Sea: a synopsis. Polar Biol 14:405–411. https://doi.org/10.1007/BF00240261
Schmidt K (2010) Chapter Five - food and feeding in northern krill (Meganyctiphanes norvegica Sars). In: Tarling GA (ed) Advances in marine biology, vol 57. Academic Press, London
Spitz J, Mourocq E, Leauté J-P, Quéro J-C, Ridoux V (2010a) Prey selection by the common dolphin: fulfilling high energy requirements with high quality food. J Exp Mar Biol Ecol 390:73–77. https://doi.org/10.1016/j.jembe.2010.05.010
Spitz J, Mourocq E, Schoen V, Ridoux V (2010b) Proximate composition and energy content of forage species from the Bay of Biscay: high- or low-quality food? ICES J Mar Sci 67:909–915. https://doi.org/10.1093/icesjms/fsq008
Spitz J, Trites AW, Becquet V, Brind’Amour A, Cherel Y, Galois R, Ridoux V (2012) Cost of living dictates what whales, dolphins and porpoises eat: the importance of prey quality on predator foraging strategies. PLoS ONE 7:e50096. https://doi.org/10.1371/journal.pone.0050096
Stearns SC (2000) Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87:476–486. https://doi.org/10.1007/s001140050763
Tarling GA (2010) Chapter Three - population dynamics of northern krill (Meganyctiphanes norvegica Sars). In: Tarling GA (ed) Advances in marine biology, vol 57. Academic Press, London
Tyler AV (1973) Caloric values of some North Atlantic invertebrates. Mar Biol 19:258–261. https://doi.org/10.1007/BF02097146
Van de Putte A, Flores H, Volckaert F, van Franeker JA (2006) Energy content of Antarctic mesopelagic fishes: implications for the marine food web. Polar Biol 29:1045–1051. https://doi.org/10.1007/s00300-006-0148-z
Varpe Ø (2017) Life history adaptations to seasonality. Integr Comp Biol 57:943–960. https://doi.org/10.1093/icb/icx123
Varpe Ø, Ejsmond MJ (2018) Trade-offs between storage and survival affect diapause timing in capital breeders. Evol Ecol 32:623–641. https://doi.org/10.1007/s10682-018-9961-4
Varpe Ø, Jørgensen C, Tarling GA, Fiksen Ø (2009) The adaptive value of energy storage and capital breeding in seasonal environments. Oikos 118:363–370
Vihtakari M, Welcker J, Moe B, Chastel O, Tartu S, Hop H, Bech C, Descamps S, Gabrielsen GW (2018) Black-legged kittiwakes as messengers of atlantification in the arctic. Sci Rep 8:1178. https://doi.org/10.1038/s41598-017-19118-8
Walkusz W, Williams WJ, Harwood LA, Moore SE, Stewart BE, Kwasniewski S (2012) Composition, biomass and energetic content of biota in the vicinity of feeding bowhead whales (Balaena mysticetus) in the Cape Bathurst upwelling region (south eastern Beaufort Sea). Deep-Sea Res Pt I 69:25–35. https://doi.org/10.1016/j.dsr.2012.05.016
Wanless S, Harris M, Redman P, Speakman J (2005) Low fish quality as a probable cause of a major seabird breeding failure in the North Sea. Mar Ecol Prog Ser 294:1–8. https://doi.org/10.3354/meps294001
Weslawski J, Legeżyńska J (2002) Life cycles of some arctic amphipods. Pol Polar Res 23:253–264
Weslawski JM, Ryg M, Smith TG, Øritsland NA (1994) Diet of Ringed Seals (Phoca Hispida) in a Fjord of West Svalbard. Arctic 47:109–114
Weslawski J, Koszteyn J, Kwasniewski S, Stempniewicz L, Malinga M (1999) Summer food resources of the little auk, Alle alle (L.) in the European arctic seas. Pol Polar Res 20:387–403
Weslawski J, Pedersen G, Falk-Petersen S (2000) Entrapment of macroplankton in an arctic fjord basin, Kongsfjorden, Svalbard. Oceanologia 42:57–69
Wolowicz M, Szaniawska A (1986) Calorific value, lipid content and radioactivity of common species from Hornsund, southwest Spitsbergen. Polar Res 4:79–84. https://doi.org/10.3402/polar.v4i1.6923
Acknowledgements
We gratefully acknowledge the captain and crew of the R/V Kronprins Haakon for their skilled support in our sampling. We thank Anette Wold (NPI) for the sampling organisation and mentorship during fieldwork, Ireen Vieweg (UiT) for her teaching and guidance in calorimetric methodology, Janne Søreide (UNIS) for discussions and advice on zooplankton, and Julia Giebichenstein (UiO) for her invaluable partnership during sampling.
Funding
Open access funding provided by The University Centre in Svalbard. This work was part of The Nansen Legacy project, funded by the Research Council of Norway (RCN # 276730).
Author information
Authors and Affiliations
Contributions
RN, KB, GWG and ØV contributed to study design and conception. RN conducted fieldwork, with help from ØV. RN conducted lab analysis. RN analysed the data, with supervision by ØV. RN wrote the manuscript, with feedback and contributions from KB, GWG and ØV. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nowicki, R.C., Borgå, K., Gabrielsen, G.W. et al. Energy content of krill and amphipods in the Barents Sea from summer to winter: variation across species and size. Polar Biol 46, 139–150 (2023). https://doi.org/10.1007/s00300-023-03112-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03112-0