Abstract
Although Atlantic cod has been observed in Svalbard waters since the 1880s, knowledge about the presence in the Arctic shallow water zone is limited. The regular catch of juvenile Atlantic cod in Kongsfjorden since 2008 is in line with an overall northward shift of boreal fish species toward the Arctic. This is the first study showing the age class composition, growth rates, and stomach content of Atlantic cod in the shallow water zone of Kongsfjorden, Svalbard. From 2012 to 2014 a total of 721 specimens were sampled in 3 to 12 m water depth. The primary age classes were identified as 0+, 1+, and 2+ using otolith age analysis. The different cohorts of these specimens show stable growth rates during the polar day and night. By stomach content analysis, we show that these specimens primarily feed on benthic food sources. These observations support the assumption that the shallow water zone of Kongsfjorden is likely to be a nursery ground for Atlantic cod.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atlantic cod (Gadus morhua) is generally distributed along the continental shelves of the North Atlantic between 40° N and 80° N (Sundby 2000; Neat and Righton 2007). Ottersen et al. (2014) describes the Northeast Arctic cod (NEAC) in the Barents Sea as the currently largest stock. One of the most northern components can be found around Svalbard and its fjords, like Kongsfjorden. Here, Atlantic cod has been observed and to some degree commercially caught in periods since the 1880s, with juveniles regularly caught and documented since 2008 (Berge et al. 2015b). The presence of Atlantic cod in Kongsfjorden is likely connected to a northward shift of marine fish species in the Northern hemisphere (Christiansen et al. 2014; Fossheim et al. 2015; Misund et al. 2016). Kongsfjorden at the west coast of Svalbard at 79°N, 12°E is characterized as a sub-Arctic glacial fjord (Fig. 1). The sub-Arctic character is based on its hydrography, with a strong influx of Atlantic water masses that have increased over the last two decades (Beszczynska-Möller et al. 2012; Payne and Roesler 2019; Hop et al. 2019). The increased temperatures in Kongsfjorden are within the thermal niche of Atlantic cod, which is reported to range from -1.5 to 19 °C, with a narrower range of 1 to 8 °C during the spawning season (Righton et al. 2010).
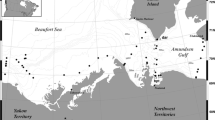
Map of Kongsfjorden at 79°N, 12°E. a The Svalbard archipelago with its primary settlement Longyearbyen and the study site Kongsfjorden. b Kongsfjorden with the settlement Ny-Ålesund and its island Blomstrandhalvøya. Light areas on land represent glacier surfaces. The sampling sites are marked as follows: Sor—Sørvågen, HnN—Hansneset North, HnC—Hansneset Central, HnS—Hansneset South, Lon—London, Bra—Brandal, OPE—Old Pier East, OPC—Old Pier Central, OPW—Old Pier West, and Gas—Gåsebu. At the locations Hansneset and Old Pier, three sampling sites were spaced 100 m apart in a perpendicular orientation to the coastline. The map data were provided by the Norwegian Polar Institute (from Brand and Fischer 2016)
Characteristic for NEAC is an annual long-distance migration between spawning and foraging areas. One foraging area is in the eastern Barents Sea at Novaya Zemlya and the other is on the western continental shelf of the Svalbard archipelago (Brander 2005). The main spawning area of NEAC is located on the west coast of Norway from Møre in the south to Finnmark in the north, with the main spawning grounds at the Lofoten (Godø 1984a, b; Brander 2005; Sundby and Nakken 2008). NEAC spawning occurs from February to May, with the main spawning period in March and April (Brander 2005). Suthers and Sundby (1993) observed post-larval cod with a standard length (SL) of 25.2 mm in the spawning areas and up to 68 km offshore in July. About 10-40% of the total larval abundance is transported to the west coast of Svalbard with the WSC, while the majority (60-90%) drifts with the North Atlantic Current and is transported to the Barents Sea (Ottersen et al. 1998). The settlement of juveniles is known to occur from September to October (Ottersen et al. 2014). After settlement, the juveniles can be referred to as age class 0 +.
All Atlantic cod at the Svalbard archipelago and its associated fjord systems are described in the literature as NEAC (Brander 2005), but a recent study could show that Atlantic cod in Kongsfjorden and other Svalbard fjords not only belong to the NEAC ecotype but also to the Norwegian Coastal cod (NCC) which normally can only be found along the Norwegian coast (Spotowitz et al. 2022).
Atlantic cod find a highly structured shallow water zone in Kongsfjorden, including hard bottom areas that are covered with kelp forests. By their characteristics, these areas are potential nursery areas for Atlantic cod (Seitz et al. 2014). From 2012 to 2014, a combined study of the shallow water zone of Kongsfjorden was conducted. Data were gathered by a permanently deployed underwater observatory (Baschek et al. (2017), Fischer et al. 2017) and an extensive baseline fishing campaign from 2012 to 2014. The first results of the fishing campaigns of 2012 and 2013 showed a high abundance of Atlantic cod with a standard length (SL) ranging from 5 to 20 cm (Brand and Fischer 2016). By literature, it is known that age classes 0+ to 2+ remain in the settlement area and might only perform limited seasonal migrations (Woodhead 1959; Ottersen et al. 1998). Individuals in age class 3 + typically start migrations toward their later spawning habitats on the west coast of Norway (Ottersen et al. 1998).
With this study, we present a first-time report about the life history of Atlantic cod in the shallow water zone (0-12 m water depth) of Kongsfjorden for the years 2012–2014. We used otolith-based age determination to identify age classes and age–length relationships. We show the temporal distribution of different age classes, as well as growth rates in different years and seasons. Furthermore, stomach content analysis identifies the food sources of the specimens. The Arctic is expected to be one of the focal areas facing climate change-induced temperature increases in the coming decades (IPCC 2014). These data may provide a valuable snapshot for comparison with past and future studies of the Arctic coastal ecosystem. Dramatic changes in species distribution in the central Arctic can already be seen today (Snoeijs-Leijonmalm et al. 2022), and it is expected that rising seawater temperatures further foster the establishment of non-Arctic species (Fossheim et al. 2015).
Materials and methods
Sampling
Sampling was conducted in 2012, 2013, and 2014. Two sampling episodes per year were done in the months of June and September. The sampling started in June 2012 at two locations: one at the southern shoreline (Fig. 1, OPC - Old Pier Central) and the other on the shoreline of Blomstrandhalvøya (HnS - Hansneset South). Paired sampling was performed using one fyke net (diameter 40 cm, length 90 cm, bar mesh size 12 mm, deployed in 3m water depth) and a trammel net (inner/outer mesh size 1/15 cm, length 20 m, height 2 m, deployed in a depth of 5-12 m). The net was deployed perpendicular to the shoreline, with recovery and redeployment every 24 h. The 24-h interval was extended to 48 h if bad weather conditions did not allow recovery.
The September 2012 sampling period showed problematic interactions between young seals and the trammel nets. To avoid harming the wildlife, we stopped using trammel nets and relied exclusively on paired sampling using fyke nets. The new configuration comprised two fyke nets (diameter 40 cm, length 90 cm, bar mesh size 12 mm, deployment in 3 and 12m water depth) and one double-fyke net (diameter 60 cm, length 110 cm, bar mesh 12 mm, deployment in5-8-m water depth). The double-fyke net was connected by an 80 cm high steering net (18 mm bar mesh). All three nets were deployed perpendicular to the shoreline and fish tissue was used as bait. This new standard configuration was used for all further sampling. Species-level identification of Atlantic cod specimens was performed based on morphological traits using the methods proposed by Hayward and Ryland (2009) and Klekowski and We̜sławski (1990). The primary features of distinction were the structure of the lateral line, the coloration of the ventral side, and the protruding upper or lower jaw. In the laboratory, the SL and wet weight (WW) of all the sampled fish were measured. A total of 720 Atlantic cod were caught in all sampling periods. For a listing of all other species caught in the sampling periods of 2012 and 2013, see Brand and Fischer (2016). The sagittal otoliths were extracted, cleaned in distilled water, and stored for later analysis. Stomach content samples were weighed and stored in formalin.
After evaluation of the sampling episodes in 2012, sampling in 2013 and 2014 was extended to five sites on Blomstrandhalvøya and five sites on the southern shore (Fig. 1). The exposure time of the nets was by standard 24 h. Owing to logistical and weather constraints, the exposure was extended to a maximum of 96 h. This extended exposure time was deemed reasonable due to the generally low saturation of the fyke nets. As a metric for fish abundance, catch per unit effort (CPUE) was used to standardize fish catch for differences in exposure times. CPUE represents the number of fish per net per 24-h exposure time. Previous analysis by Brand and Fischer (2016) showed no effect of different exposure times on CPUE. Due to the differences in sampling setup, no CPUE of 2012 is compared to 2013 or 2014. All quantitative analyses in this study use exclusively CPUE of 2013 and 2014, where identical sampling strategies and gears were used (Table 1). For comparison of CPUE of the years, 2013 and 2014, a Kruskal-Wallis rank sum test (Kruskal and Wallis 1952) was performed. The results were further analyzed by a post hoc Tukey (Tukey 1949; Driscoll 1996). Data from 2012 are included for qualitative comparison as, e.g., standard length composition. We compared the standard length (SL) distribution of the sampling years 2012, 2013, and 2014 by Kruskal-Wallis rank sum test (Kruskal and Wallis 1952) and Dunn’s test for multiple comparisons using rank sums for post hoc analysis (Dunn 1964). This analysis was later repeated for the age class composition of those years. The analysis was performed in R (R Core Team 2021) using the package PMCMRplus (Pohlert 2021).
Otolith analysis and age–length keys
We performed otolith structure analysis (Stevenson and Campana 1992) on sagittal otoliths to reliably assign age classes based on the SL. From a total of 720 specimens, we sampled the sagittal otoliths of 551 specimens. Of those we successfully processed samples of 533 specimens (see Table 2). Otolith analysis was performed with a Leica M60 binocular zoom microscope with an ACHRO 0.8X lens, zoom level set to 2.0, and binoculars with 10 × magnification. A part of the otoliths was embedded in resin and prepared on slides. Those were read in ‘bright field’ setting. The other otoliths, which were untreated, were broken in two in the transversal plane, and the piece with the core was mounted on a modeling compound, with the fracture side up. The otolith was illuminated from the side so that the light is scattered up through the fracture.
Length classes were binned by centimeters. An age frequency per length class table was created and used to compute an age–length key (ALK), see also Ailloud and Hoenig (2019). ALK were calculated separately for June and September of 2012, 2013, and 2014. The age–length key was used to assign age class information to specimens for which no otolith sample was available (Fig. 3). The full method is presented in Ogle (2016).
Growth rates
Cohorts of fish were tracked over multiple sampling periods to calculate intra- (June to September) and inter-annual (September of actual to June of the following year) growth rates. Per year and season, the average SL per age class and sampling period were calculated. Age information was gathered by otolith analysis. The number of days between the middle of successive sampling periods was calculated (Table 3), and the changes in average SL per age class were standardized to growth per day. Additionally, the standard length and wet weight were plotted against each other to visualize the length-to-weight relationship as a growth parameter (s. SI Fig. 1).
Food sources
The stomach contents were removed and its weight was determined. We calculated a fill index (FI) by the formula FI = Wi*10,000/W (W = bodyweight of fish, Wi= weight of stomach content). The stomach content was stored in formalin (4%). A subset of 47 stomach content samples from the 2013 sampling campaign were analyzed for the presence of different food items. Food items were identified to the lowest possible taxonomic level by an expert taxonomist. We recorded the presence of food categories by sampling season and age class. The food items were categorized as benthic, demersal, pelagic, or fish tissue. In Table 4 the two most common items per group are shown, while the remaining items per category are presented cumulatively. We compared the FI of the sampling years 2012, 2013, and 2014 by Kruskal-Wallis rank sum test (Kruskal and Wallis 1952) and Dunn’s test for multiple comparisons using rank sums for post hoc analysis (Dunn 1964).
Water temperature
The water temperature was recorded at 12 m water depth by the AWIPEV underwater observatory at the Old Pier in Ny-Ålesund (Fischer et al. 2017). The data are published in Fischer et al. (2018a, b, c). We calculated the average water temperature from June17 to October 17 of each year, as this time frame corresponds with the fishery assessments. A corresponding box plot was created in R (R Core Team 2021).
Results
In six sampling campaigns from 2012 to 2014, a total of 721 Atlantic cod were sampled, measured, and weighted (see SI Fig. S1). We removed sagittal otoliths from 552 specimens for age class determination.
Comparison of spatial and temporal differences in species abundance
A significant difference in the overall Catch per Unit Effort (CPUE) was observed between the four sampling campaigns of 2013 and 2014 (Kruskal-Wallis test: H 3 = 10.931, p = 0.015). Post hoc analysis shows only the sampling campaign of June 2013 (0.073 ± 0.055, n = 10) and September 2013 (0.235 ± 0.067, n = 10) are significantly different (Tukey test: p = < 0.03). No significant difference in CPUE could be found between the different sampling sites (Kruskal-Wallis test: H 9 = 5.5327, p = 0.7856).
Length frequency distribution and age class determination
The standard length (SL) frequency distribution in Fig. 2 shows characteristic differences between the six sampling seasons. It is recognizable that in the June campaigns of all years the number of specimens below 10 cm SL was very low. Additionally, it is recognizable that the September season of 2013 had a higher number of specimens above 21 cm SL than the other campaigns. Statistically significant differences regarding SL distribution could be observed between the sampling years 2012, 2013, and 2014 (Kruskal-Wallis test: H 2 = 10.76, p = < 0.01). The post hoc test revealed significant differences between 2012 and 2013 (Dunn’s test: p = 0.019) and 2013 and 2014 (p = 0.014). Additionally, significant differences in age class composition could be detected between the sampling years (Kruskal-Wallis test: H 2 = 25.367, p = < 0.01). Here the post hoc test also revealed significant differences between 2012 and 2013 (Dunn’s test: p < 0.01) and 2013 and 2014 (p < 0.01).
Comparison of length frequency and age class distribution
Regarding length class distribution, a slight overlap between age class 1+ and 2+ is given. Additionally, in September 2013 an overlap between 0+ and 1+ specimens was detected (see Fig. 3). Overall age class 1+ was the dominant fraction of all specimens in all sampling episodes with a total share of 63.8%. Age class 2+ represents overall 22.05% of all specimens. Age class 0+ was only detected in September and represented 10.96% of all specimens. Age classes greater than 2+ accounted for 3.19% of all samples. The details given in Fig. 4 also show that the age class composition in 2013 deviates from 2012 to 2014. For June 2013 the share of age class 2+ specimens was clearly higher in 2013 (46.43%) than in 2012 (23.08%) and 2014 (13.51%). Also, in September 2013 the share of 0+ and 2+ specimens was elevated. Age class 0+ specimens represent 23.40%, which was more than double that observed in 2012 (9.72%) and 2014 (7.26%). Age class 2+ specimens represented 35.82%, a distinctively higher amount than in 2012 (0%) and 2014 (14.52%). In between the sampling campaigns of 2012–2014, we saw per age class no difference in average SL beyond the standard deviation (see Fig. 5).
Detailed age and length distribution of the age classes 0+ to 2 +. The results based on the otolith analysis are shown in center left and right. Specimens without age class (black, NA) had no otolith sample or the sample was unreadable. Specimens with NA were given an age class based on the developed age length key. The results are shown to the very left and right
Growth rate over the years
We detected an overall growth rate of 0.176 mm SL/day for the 2011 cohort, 0.217 mm SL/day for the 2012 cohort, and 0.216 mm SL/day for the 2013 cohort (see SI Fig. S2). Growth in the summer months was higher 0.49 ± 0.10 mm SL/day (n = 5) than in winter months 0.14 ± 0.04 mm SL/day (n = 4) (see Table 3).
Stomach content analysis
Stomach content analysis of 47 samples revealed 35 different food items. These food items were categorized into benthic organisms (n = 14), demersal organisms (n = 14), pelagic organisms (n = 4), and the category fish tissue. Amphipods were present in 97.9% of all samples, with Ischyrocerus spp. and Anonyx sarsi being the most abundant. Further, benthic items were present in 66% of all the samples, and Caprella septentrionalis and Harpacticoida were the most frequent. Prey in the pelagic category was present in 29.8% of all samples with Calanus spp. and Thysanoessa inermis being the most frequent groups in this category. Fish tissue was found in 8.5% of all the samples (Table 4). A more detailed analysis showed that fish tissue was only found in September and only in age classes 1+ (14.3%) and 2+ (37.5%). Items in the benthic and demersal categories were found in all age classes from all sampling episodes.
The fullness indices (FI) in June were significantly lower than in September (Kruskal-Wallis test: H 1 = 4.7985, p = 0.028), see Fig. 6. We also detected differences in the FI between the different sampling years (Kruskal-Wallis test: H 2 = 9.2056, p = 0.01). The highest average FI was for 2012, followed by 2014 and 2013. The post hoc analysis showed significant differences between 2012–2013 (Dunn’s test: p < 0.01) and 2012–2014 (p < 0.02).
Water temperature
At the sampling site Old Pier in Ny-Ålesund we detected the following water average water temperatures for June-—September. In 2012 (4.30 ± 0.93, n = 1750), 2013 (4.70 ± 0.94, n = 1907), and 2014 (5.43 ± 1.05, n = 2107), see SI Fig. S3.
Discussion
Atlantic cod in Kongsfjorden
This study offers the first quantitative and detailed report about the age class composition of Atlantic cod in the shallow water zone (0–12 m water depth) of Kongsfjorden, Svalbard. The specimens with a standard length of 5–20 cm as described in Brand and Fischer 2016 could be identified as belonging to the age class 0+ and 1+ . For 2012 and 2014 these age classes account for more than 88% of all specimens. For 2013 we could detect a high share of age class 2+ in June (47%) and September (36%). According to Ottersen et al. (1998) Atlantic cod will not undertake large seasonal movements in their first 2 years, after settlement which coincides well with the permanent presence of age class 0+ and 1+ . The absence of age class 0+ in June 2012, 2013, and 2014 could be explained by the reported spawning period of Northeast Arctic Cod (NEAC) from February to early May at the Lofoten (Brander 2005) and the subsequent transport by the West Spitsbergen Current (WSC). As the settlement is reported at an age of 5–6 months (Ottersen et al. 2014), it seems reasonable that specimens arrive in Kongsfjorden in August. This assumption is also supported by Mark (2013a, b), who reported that Atlantic cod with a SL ranging from 5.5 to 9.5 cm were observed in Forlandsundet and the mouth of Kongsfjorden in August 2013. The specimens with the lowest SL sampled in this study were 6.5 cm and had a body height of 10 mm. Age class 0+ specimens below 12 mm body height might be underrepresented in this study because the sampling gear had a bar mesh size of 12 mm. Consequently, the average SL shown for age class 0+ specimens could be overestimated, as the smallest specimens might not have been sampled. By year-round observation data from the Kongsfjorden underwater observatory, it is most likely that no age class 0+ specimens were present before August (Fischer et al. 2017). Therefore, the absence of age class 0+ specimens from any June sampling episodes in this study is unlikely to be an artifact of gear selectivity.
Shallow water kelp forests as a foraging ground
The presence of age class 0+ and 1+ in the shallow water zone and its kelp forests opens the question of what the ecological function of this zone is. The analysis of stomach content shows that these age classes feed primarily on benthic and demersal food items. Only a small number of specimens show pelagic food sources, primarily Calanus spp. and Thysanoessa inermis. Both are usually not available in the shallow water zone. Importantly, these zooplankton species are known to conduct diel vertical migration throughout the year, which could explain how shallow water cod are able to prey on these species (Berge et al. 2009). Gotceitas et al. (1995) highlighted that juvenile Atlantic cod use kelp forests as structural protection to avoid active predators. Fish size in relation to the density of the kelp forests seems to be an important factor, as fish that exceed a certain size cannot swim easily through the kelp forest. Depending on the structure and density of the kelp forest, this might facilitate age class separation. The kelp forests between 2.5 m and 15 m depth (Bartsch et al. 2016) might fulfill a dual function by providing structural protection against predators and being a feeding ground. Norderhaug et al. (2005) showed that Atlantic cod is feeding on a large range of kelp-associated invertebrates, as also shown in this study. These kelp-associated species might find food year-round in the kelp forests. Renaud et al. (2015) showed that most benthic taxa feed on a broad mixture of particulate organic matter and macroalgal detritus. During the polar night, the infauna of the decaying kelp beds of Kongsfjorden might be an important energy and food resource for Atlantic cod. This concurs with Berge et al. (2015b), who observed the feeding activity of Atlantic cod during the polar night and a high abundance of fauna associated with Saccharina latissima. This suggests that the polar night is not a time of biological quiescence (Berge et al. 2015a). Also, in this study, we observe a growth of Atlantic cod between September and June. The average growth rate is lower than during the summer months (June–September). This might be connected to lower water temperatures and an overall lower amount of available food. Fittingly we detected that the fullness index (FI) for specimens was highest in September at the end of the summer season. The combination of these facts suggests that Atlantic cod uses kelp forests and subtidal soft bottoms of Kongsfjorden as nursery areas, as also reviewed by Seitz et al. (2014).
Differences in age class separation
Our results showed that age classes 0+ to 1+ are the dominant age classes in the shallow water zone. Specimens of age classes 2+ or greater were very low in abundance, indicating that these might have shifted their habitat as part of their life history. This concurs with Ottersen et al. (1998), who showed that after settlement, fish do not undertake large seasonal movements in their first 2 years. After this period, Atlantic cod in the Barents Sea have been reported to show horizontal migrations, which are connected to predation avoidance and prey availability. Brander (2005) and also this study show a more fish-based diet starting in age class 2 +. On the vertical migrations in the Barents Sea Mallotus villosus is one of the main prey items that is followed.
In this study, it was notable that specimens of age class 2+ and above were sampled only in 2013 in larger quantities in the shallow water zone between 0 and 12 m. The reason therefore could be differences in this year’s class strengths, sampling effects, or differences in the hydrographic regime. While the sampling technique and effort remained comparable, differences in year class strength cannot be entirely excluded, as Kongsfjorden is an open system. Movement of Atlantic cod into and out of the fjord, as well as migration to different water layers is possible. As shown in Ingvaldsen (2017) adult Atlantic cod can be expected generally in between 150- and 600-m water depth on Svalbard. It is reported for 2013 that the subsurface (Payne and Roesler, 2019) and bottom waters (Fransson et al. 2016) of Kongsfjorden were colder in 2013 (∼ 2–4 °C) than in 2014 (∼ 3.5–6 °C). The temperature in the shallow water zone remained rather constant (4.3–5.4 °C). It is known that adult Atlantic cod prefers higher temperatures (Nakken and Raknes 1987) than juveniles. The cold temperatures in the deeper water layers could have led to a partial avoidance by adults. This might have resulted in the special mix of different age classes in the shallow water zone that could only be observed in 2013 (see Fig. 2, 3).
The observation might only be true for central Kongsfjorden and its hydrography. Mark (2013a, b) reports for Forlandsundet, at the mouth of Kongsfjorden, vertical separation between different age classes with adult Atlantic cod at the bottom (170–220 m) and smaller specimens in the shallower waters (0–50 m). Such vertical separation is also reported for Atlantic cod along the Norwegian coastline, known as the Norwegian Coastal Cod (NCC). Juvenile specimens of this stock, which have a non-migratory lifestyle, are known to settle in shallow waters of coastal areas and fjords (Løken et al. 1994). After 2 years, these specimens start a vertical separation, and adult specimens can be found in deeper waters of up to 500 m (Bakketeig and Bakketeig 2018). In NCC a connection between increasing genetic differentiation and geographic distance has been shown by Dahle et al. (2018).
Origin of specimens
In Kongsfjorden, hydrographic conditions and sea ice show an inter-annual variability. Advection of seawater from the WSC leads to generally increasing water temperatures and is influencing food availability (Cottier et al. 2007; Hop et al. 2002, 2019; Hegseth et al. 2019). This might open an ecological window of opportunity for the Atlantic cod to establish a permanent non-migratory population. Iversen (1934) reported Atlantic cod in the spawning stage at Isfjorden bank and around Bear Island during the last Arctic warm period from 1920 to 1940. He also reported age class 0+ specimens at Grønfjorden on Svalbard and mentioned that sporadic spawning seemed to occur close to Isfjorden and in the Bear Island area. However, he stressed that the largest number of Atlantic cod in Svalbard waters was likely associated with the spawning grounds off the coast of Norway. Andrade et al. (2020) suggest that a NEAC population has established itself in Isfjorden and Kongsfjorden and that specimens in Isfjorden show limited local movement which is typical for NCC. NCC and NEAC both spawn at some adjacent locations along the Norwegian coast, and eggs and larvae can be subject to the same processes of transport and dispersal (Brander 2005). Therefore, eggs and larvae in Kongsfjorden could be transported via the Norway Coastal Current and WSC toward Svalbard and Kongsfjorden. Recent studies by Spotowitz et al. (2022) show that specimens of NEAC and NCC can be found in fjords on Svalbard. These NCC specimens were found in age class 0+ and adult form and showed genetic differences to NCC on the Norwegian coast. This indicates a separation into a Svalbard CC population.
Outlook
It is likely that the shallow water zone of Kongsfjorden can provide a nursery and foraging habitat for Atlantic cod, enabling growth rates comparable to conspecifics in the Barents Sea, as described in Brander (2005). The origin of Atlantic cod in Kongsfjorden is less clear than in the past because a mix of NEAC, NCC, and potential local forms need to be considered. For a better understanding of the current state and further development of the Atlantic cod population in Kongsfjorden, regular monitoring seems worthwhile. To differentiate between populations of Atlantic cod a mix of genetic and otolith analyses as applied in Spotowitz et al. (2022) seems to be reasonable. Automated underwater observatories with hydrographic sensors and camera systems can additionally provide valuable contributions. They allow year-round monitoring of local fish populations and may allow assessing the influence of the hydrographic regime on abundances. The sampling in shallow water and deep-water zones should be coordinated for a holistic assessment of the local fish communities, as it must be understood that the data of this study are strictly limited to 0–12 m water depth. Recent research has shown that the northward expansion of Atlantic cod might also affect the Greenland shelf (Strand et al. 2017). A reasonable effort in monitoring should be conducted to clarify the origins of these specimens as it might provide valuable insights into the change in Arctic fish communities.
Data availability
Not applicable.
Code availability
Not applicable.
References
Ailloud LE, Hoenig JM (2019) A general theory of age-length keys: combining the forward and inverse keys to estimate age composition from incomplete data. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsz072
Andrade H, van der Sleen P, Black BA, Godiksen JA, Locke VWL, Carroll ML, Ambrose WG Jr, Geffen A (2020) Ontogenetic movements of cod in Arctic fjords and the Barents Sea as revealed by otolith microchemistry. Polar Biol 43:409–421. https://doi.org/10.1007/s00300-020-02642-1
Bakketeig GH, Bakketeig IE (2018) Ressursoversikten, Fisken og havet 6–2018. Havforskningsinstituttet, Bergen.
Bartsch I, Paar M, Fredriksen S, Schwanitz M, Daniel C, Hop H, Wiencke C (2016) Changes in kelp forest biomass and depth distribution in Kongsfjorden, Svalbard, between 1996–1998 and 2012–2014 reflect Arctic warming. Polar Biol 39:2021–2036. https://doi.org/10.1007/s00300-015-1870-1
Baschek B, Schroeder F, Brix H, Riethmüller R, Badewien T, Breitbach G, Brügge B, Colijn F, Doerffer R, Eschenbach C, Friedrich J, Fischer P, Garthe S, Horstmann J, Krasemann H, Metfies K, Merckelbach L, Ohle N, Petersen W, Pröfrock D, Röttgers R, Schlüter M, Schulz J, Schulz-Stellenfleth J, Stanev E, Winter C, Wirtz K, Wollschläger J, Zielinski O, Ziemer F (2017) The Coastal Observing System for Northern and Arctic Seas (COSYNA). Ocean Sci 13:379–410. https://doi.org/10.5194/os-13-379-2017
Berge J, Cottier F, Last KS, Varpe Ø, Leu E, Søreide J, Eiane K, Falk-Petersen S, Willis K, Nygård H, Vogedes D, Griffiths C, Johnsen G, Lorentzen D, Brierley AS (2009) Diel vertical migration of Arctic zooplankton during the polar night. Biol Lett 5:69–72. https://doi.org/10.1098/rsbl.2008.0484
Berge J, Daase M, Renaud PE, Ambrose WG, Darnis G, Last KS, Leu E, Cohen JH, Johnsen G, Moline MA, Cottier F, Varpe Ø, Shunatova N, Bałazy P, Morata N, Massabuau JC, Falk-Petersen S, Kosobokova K, Hoppe CJM, Węsławski JM, Kukliński P, Legezyńska J, Nikishina D, Cusa M, Kędra M, Włodarska-Kowalczuk M, Vogedes D, Camus L, Tran D, Michaud E, Gabrielsen TM, Granovitch A, Gonchar A, Krapp R, Callesen TA (2015a) Unexpected Levels of Biological Activity during the Polar Night Offer New Perspectives on a Warming Arctic. Curr Biol 25:2555–2561. https://doi.org/10.1016/j.cub.2015.08.024
Berge J, Heggland K, Lønne OJ, Cottier F, Hop H, Gabrielsen GW, Nottestad L, Misund OA (2015b) First record of Atlantic Mackerel (Scomber scombrus) from the Svalbard Archipelago, Norway, with possible explanation for the extension of its distribution. Arctic 68(1), 54–61. https://doi.org/10.14430/arctic4455
Beszczynska-Möller A, Fahrbach E, Schauer U, Hansen E (2012) Variability in Atlantic water temperature and transport at the entrance to the Arctic Ocean, 1997–2010. ICES J Mar Sci 69(5):852–863. https://doi.org/10.1093/icesjms/fss056
Brand M, Fischer P (2016) Species composition and abundance of the shallow water fish community of Kongsfjorden. Svalbard Polar Biol 39(11):2155–2167. https://doi.org/10.1007/s00300-016-2022-y
Brander K (2005) Spawning and life history information for North Atlantic cod stocks. ICES Coop Res Rep 274:152
Christiansen JS, Mecklenburg CW, Karamushko OV (2014) Arctic marine fishes and their fisheries in light of global change. Glob Change Biol 20:352–359. https://doi.org/10.1111/gcb.12395
Cottier FR, Nilsen F, Inall ME, Gerland S, Tverberg V, Svendsen H (2007) Wintertime warming of an Arctic shelf in response to large-scale atmospheric circulation. Geophys Res Lett 34:L10607. https://doi.org/10.1029/2007GL029948
Dahle G, Quintela M, Johansen T, Westgaard JI, Besnier F, Aglen A, Jørstad KE, Glover KA (2018) Analysis of coastal cod (Gadus morhua L.) sampled on spawning sites reveals a genetic gradient throughout Norway’s coastline. BMC Genet 19:42. https://doi.org/10.1186/s12863-018-0625-8
Driscoll WC (1996) Robustness of the ANOVA and Tukey-Kramer Statistical Tests. Compute Ind Eng 31(1–2):265–268. https://doi.org/10.1016/0360-8352(96)00127-1
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6(3):241–252. https://doi.org/10.2307/1266041
Fischer P, Schwanitz M, Loth R, Posner U, Brand M, Schröder F (2017) First year of practical experiences of the new Arctic AWIPEV-COSYNA cabled Underwater Observatory in Kongsfjorden. Spitsbergen Ocean Sci J 13(2):259–272. https://doi.org/10.5194/os-13-259-2017
Fischer P, Schwanitz M, Brand M, Posner U, Brix H, Baschek B (2018a) Hydrographical time series data of the littoral zone of Kongsfjorden, Svalbard 2012. Alfred Wegener Institute - Biological Institute Helgoland, Pangaea. https://doi.org/10.1594/PANGAEA.896828
Fischer P, Schwanitz M, Brand M, Posner U, Brix H, Baschek B (2018b) Hydrographical time series data of the littoral zone of Kongsfjorden, Svalbard 2013. Alfred Wegener Institute - Biological Institute Helgoland, Pangaea. https://doi.org/10.1594/PANGAEA.896822
Fischer P, Schwanitz M, Brand M, Posner U, Brix H, Baschek B (2018c) Hydrographical time series data of the littoral zone of Kongsfjorden, Svalbard 2014. Alfred Wegener Institute - Biological Institute Helgoland, Pangaea. https://doi.org/10.1594/PANGAEA.896821
Fossheim M, Primicerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV (2015) Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat Clim Change 5:673–678. https://doi.org/10.1038/nclimate2647
Fransson A, Chierici M, Hop H, Findlay HS, Kristiansen S, Wold A (2016) Late winter-to-summer change in ocean acidification state in Kongsfjorden, with implications for calcifying organisms. Polar Biol 39:1841–1857. https://doi.org/10.1007/s00300-016-1955-5
Godø OR (1984a) Migration, mingling and homing of North-East Arctic cod from two separate spawning grounds. In: Godø OR, Tilseth S (eds) Proceedings of the Soviet-Norwegian Symposium on Reproduction and Recruitment of Arctic Cod. Institute of Marine Research, Bergen, pp 289–302.
Godø OR (1984b) Cod (Gadus morhua L.) off Møre - composition and migration. In: Dahl E, Danielssen DS, Moksness E, Solemdal P (eds) The Propagation of Cod (Gadus morhua L.) Flødevigen Rapportserie 1, pp 591–608.
Gotceitas V, Fraser S, Brown JA (1995) Habitat use by juvenile Atlantic cod (Gadus morhua) in the presence of an actively foraging and non-foraging predator. Mar Biol 123:421–430
Hayward PJ, Ryland JS (2009) Handbook of the Marine Fauna of North-West Europe. Oxford University Press, Oxford
Hegseth EN, Assmy P, Wiktor JM, Wiktor J, Kristiansen S, Leu E, Tverberg V, Gabrielsen TM, Skogseth R, Cottier F (2019) Phytoplankton Seasonal Dynamics in Kongsfjorden, Svalbard and the Adjacent Shelf. In: Hop H, Wiencke C (ed) The Ecosystem of Kongsfjorden, Svalbard. Adv Pol Ecol 2. https://doi.org/10.1007/978-3-319-46425-1_6
Hop H, Pearson T, Hegseth EN, Kovacs KM, Wiencke C, Kwasniewski S, Eiane K, Mehlum F, Gulliksen B, Włodarska-Kowalczuk M, Lydersen C, Węsławski JM, Cochrane SC, Gabrielsen GW, Leakey RJG, Lonne OJ, Zajaczkowski M, Falk-Petersen S, Kendall M, Wängberg SA, Bischof K, Voronkov AY, Kovaltchouk NA, Wiktor J, Poltermann M, Prisco G, Papucci C, Gerland S (2002) The marine ecosystem of Kongsfjorden, Svalbard. Polar Res 21:167–208. https://doi.org/10.1111/j.1751-8369.2002.tb00073.x
Hop H, Cottier F, Berge J (2019) Autonomous Marine Observatories in Kongsfjorden, Svalbard. In: Hop H, Wiencke C (ed) The Ecosystem of Kongsfjorden, Svalbard. Adv Pol Ecol 2. https://doi.org/10.1007/978-3-319-46425-1_13
IPCC (2014) Climate Change 2014: Synthesis Report. In: Core Writing Team, Pachauri RK, Meyer LA (eds) Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva. pp 151
Ingvaldsen RB, Gjøsæter H, Ona E, Michalsen K (2017) Atlantic cod (Gadus morhua) feeding over deep water in the high Arctic. Polar Biol 40:2105–2111. https://doi.org/10.1007/s00300-017-2115-2
Iversen T (1934) Some observations on cod in Northern Waters. In: Report on Norwegian Fishery and Marine Investigations Vol. 4 Fiskeridirektoratets Skrifter, Bergen
Klekowski RZ, We̜sławski JM (1990) Atlas of the marine fauna of Southern Spitsbergen Vol.1, Ossolineum, Wroclaw
Kruskal WH, Wallis AW (1952) Use of Ranks in One-Criterion variance analysis. J Am Stat Assoc 47:583–621. https://doi.org/10.2307/2280779
Løken S, Pedersen T, Berg E (1994) Vertebrae numbers as an indicator for the recruitment mechanism of coastal cod of northern Norway. ICES Mar Sci Symp 198:510–519
Mark FC (2013a) Cruise Report RV Heincke HE408. https://doi.org/10.1594/PANGAEA.829679
Mark FC (2013b) Physical oceanography during HEINCKE cruise HE408. Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven,. https://doi.org/10.1594/PANGAEA.824703
Misund OA, Heggland K, Skoseth R, Falck E, Gjøsæter H, Sundet J, Watne J, Lønne OJ (2016) Norwegian fisheries in the Svalbard zone since 1980 Regulations, profitability and warming waters affect landings. Polar Sci 10(3):312–322
Nakken O, Raknes A (1987) The Distribution and Growth of Northeast Arctic Cod in Relation to Bottom Temperatures in the Barents Sea, 1978–1984. Fish Res 5:243–252. https://doi.org/10.1016/0165-7836(87)90044-0
Neat F, Righton D (2007) Warm water occupancy by North Sea cod. Proc R Soc B 274:789–798. https://doi.org/10.1098/rspb.2006.0212
Norderhaug KM, Christie H, Fosså JH, Fredriksen S (2005) Fish-macrofauna interactions in a kelp (Laminaria hyperborea) forest. J Mar Biol Assoc UK 85:1279–1286
Ogle DH (2016) Introductory Fisheries Analysis with R. CRC Press, Boca Raton
Ottersen G, Michalsen K, Nakken O (1998) Ambient temperature and distribution of North-east Arctic cod. ICES J Mar Sci 55:67–85. https://doi.org/10.1006/jmsc.1998.0414
Ottersen G, Bogstad B, Yaragina NA, Stige LC, Vikebø FB, Dalpadado P (2014) A review of early history dynamics of Barents Sea cod (Gadus morhua). ICES J Mar Sci 71:2064–2087. https://doi.org/10.1093/icesjms/fsu037
Payne CM, Roesler CS (2019) Characterizing the influence of Atlantic water intrusion on water mass formation and phytoplankton distribution in Kongsfjorden, Svalbard. Cont Shelf Res 191. https://doi.org/10.1016/j.csr.2019.104005
Pohlert T (2021) PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended. R package version 1.9.3 http://CRAN.R-project.org/package=PMCMRplus
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Renaud PE, Løkken TS, Jørgensen LL, Berge J, Johnson BJ (2015) Macroalgal detritus and food-web subsidies along an Arctic fjord depth-gradient. Front Mar Sci 2:31. https://doi.org/10.3389/fmars.2015.00031
Righton DA, Andersen KH, Neat F et al (2010) Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima. Mar Ecol Prog Ser 420:1–13. https://doi.org/10.3354/meps08889
Seitz RD, Wennhage H, Bergström U, Lipcius RN, Ysebaert T (2014) Ecological value of coastal habitats for commercially and ecologically important species. ICES J Mar Sci 71:648–665. https://doi.org/10.1093/icesjms/fst152
Snoeijs-Leijonmalm P, Flores H, Sakinan S, Hildebrandt N, Svenson A, Castellani G, Vane K, Mark F, Heuzé C, Tippenhauer S, Niehoff B, Hjelm J, Sundberg JH, Schaafsma FL, Engelmann R (2022) Unexpected fish and squid in the central Arctic deep scattering layer. Sci Adv. https://doi.org/10.1126/sciadv.abj7536
Spotowitz L, Johansen T, Hansen A, Berg E, Stransky C, Fischer P (2022) New evidence for the establishment of coastal cod Gadus morhua in Svalbard fjords. Mar Ecol Prog Ser 696:119–133. https://doi.org/10.3354/meps14126
Stevenson DK, Campana SE (1992) Otolith microstructure examination and analysis. Can Spec Publ Fish Aquat Sci. https://doi.org/10.13140/RG.2.2.22258.61127
Strand KO, Sundby S, Albretsen J, Vikebø FB (2017) The Northeast Greenland Shelf as a Potential Habitat for the Northeast Arctic Cod. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00304
Sundby S (2000) Recruitment of Atlantic cod stocks in relation to temperature and advection of copepod populations. Sarsia 85:277–298
Sundby S, Nakken O (2008) Spatial shifts in spawning habitats of Arcto-Norwegian cod related to multidecadal climate oscillations and climate change. ICES J Mar Sci 65:953–962
Suthers IM, Sundby S (1993) Dispersal and growth of pelagic juvenile Arcto-Norwegian cod (Gadus morhua), inferred from otolith microstructure and water temperature. ICES J Mar Sci 50:261–270
Tukey J (1949) Comparing individual means in the analysis of variance. Biometrics 5:99–114. https://doi.org/10.2307/3001913
Woodhead AD (1959) Variations in the activity of the thyroid gland of the cod, Gadus callarias L., in relation to its migrations in the Barents Sea, II. The ‘Dummy of run’ of the immature fish. J Mar Biol Ass UK 38:417–422
Acknowledgements
This work was performed at the Ny-Ålesund Research Station, Svalbard, Norway. We thank the staff of the AWIPEV Arctic Research Base for their support during our fieldwork. We appreciate the helpful comments of our reviewers in the preparation of this manuscript. Some data were collected within the framework of the AWIPEV underwater observatory and were financed and developed by the projects COSYNA, ACROSS, and MOSES.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MB performed the whole sampling campaign described in the proposed manuscript. The initial explorative age reading of otoliths were conducted by LS with support from MB. ELL performed a complete age reading of all otoliths. JMW performed the analysis of stomach content samples. The analysis of the dataset was performed by MB. All listed authors participated equally in the writing process, whereas PF is the PI of the project and JB as well as FM supported the discussion fundamentally so that without them it would not be possible to submit the proposed manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the submitted work was carried out in the absence of any personal, professional, or financial relationships that could potentially be construed as a conflict of interest.
Ethical approval
The local regulations related to fauna harvest on Svalbard exclude saltwater fish, except for salmonids. Additionally, an application for the research project submitted to the local governor (Sysselmesteren) was approved by his authority in accordance with local legislation. All work was performed in accordance with the act on animal welfare and by accepted research methods. We were also in contact with NARA (Norwegian Animal Research Authority), and they confirmed that for our sampling no additional approval had to be applied for.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brand, M., Spotowitz, L., Mark, F.C. et al. Age class composition and growth of Atlantic cod (Gadus morhua) in the shallow water zone of Kongsfjorden, Svalbard. Polar Biol 46, 53–65 (2023). https://doi.org/10.1007/s00300-022-03098-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03098-1