Abstract
Wolverines (Gulo gulo) occupy most of the globe’s Arctic tundra. Given the rapidly warming climate and expanding human activity in this biome, understanding wolverine ecology, and therefore the species’ vulnerability to such changes, is increasingly important for developing research priorities and effective management strategies. Here, we review and synthesize knowledge of wolverines in the Arctic using both Western science sources and available Indigenous Knowledge (IK) to improve our understanding of wolverine ecology in the Arctic and better predict the species’ susceptibility to change. To accomplish this, we update the pan-Arctic distribution map of wolverines to account for recent observations and then discuss resulting inference and uncertainties. We use these patterns to contextualize and discuss potential underlying drivers of distribution and population dynamics, drawing upon knowledge of food habits, habitat associations, and harvest, as well as studies of wolverine ecology elsewhere. We then identify four broad areas to prioritize conservation and research efforts: (1) Monitoring trends in population abundance, demographics, and distribution and the drivers thereof, (2) Evaluating and predicting wolverines’ responses to ongoing climate change, particularly the consequences of reduced snow and sea ice, and shifts in prey availability, (3) Understanding wolverines’ response to human development, including the possible impact of wintertime over-snow travel and seismic testing to reproductive denning, as well as vulnerability to hunting and trapping associated with increased human access, and (4) Ensuring that current and future harvest are sustainable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wolverines (Gulo gulo) occur broadly across the Arctic, known by names including qavvik (Iñupiaq), qavvigaarjuk (Inuit/Inuktitut; Laugrand 2017), qafčik (Yup'ik; Bobaljik 1996), kuekuatsheu (Innu, Armitage 1992), иӈгнeй (Nenets; Li 2021), қeper (Chukchi, Weinstein 2018), ńitlә (Tundra Yukaghir; Nikolaeva 2008), and pocoмaxa (Russian). The region is often considered a stronghold for wolverines due to its relatively restricted human footprint and cold climate (Fisher et al. 2022), and the species occupies an important sociocultural role among the region’s Indigenous Peoples (Armitage 1992; Cardinal 2004; Benson 2014). Despite this, many aspects of the species’ ecology in this biome have received little attention by Western science. The paucity of available information has precluded developing formalized research priorities and evaluating potential conservation concerns. Addressing this gap is particularly important as the Arctic changes rapidly in response to anthropogenic disturbance.
The circumpolar Arctic, which we regard here as the area north of latitudinal treeline (Walker et al. 2005), is a dynamic landscape undergoing rapid change (Hinzman et al. 2005). Anthropogenic warming in recent decades has degraded sea ice, snow, and permafrost at a rapid pace, with cascading consequences for wildlife (Post et al. 2013; Berteaux et al. 2017; Huntington et al. 2020). For example, sea ice loss precludes metapopulation mixing among terrestrial island-dwelling species, such as wolves (Canis lupus; Carmichael et al. 2008) and Arctic foxes (Alopex lagopus; Geffen et al. 2007), while enabling range expansions of temperate marine mammals (Kovacs et al. 2011). Thawing permafrost is depleting waterfowl breeding habitat (Perreault et al. 2017), and shifting snowmelt phenology is altering the availability of insects to breeding migratory birds (Saalfeld et al. 2019). Species typically associated with boreal forest, including moose (Alces alces), beavers (Castor canadensis), and snowshoe hares (Lepus americanus) are shifting northward into the treeless Arctic following shrubification (Jung et al. 2016; Tape et al. 2016a, b, 2018), while the ranges of specialist Arctic species such as Lapland longspurs (Calcarius lapponicus) are contracting (Boelman et al. 2015). Disease transmission, mediated by the abiotic and biotic environments, is changing as well (Ruscio et al. 2015; Keatts et al. 2021). Accompanying and driving these changes are an expanding human footprint, largely associated with resource extraction and facilitated by advancements in over-snow travel technology (Landa et al. 2000; Kumpula et al. 2012; Raynolds et al. 2014). Such human activity can produce both positive and negative impacts to individual wildlife species (e.g., through food subsidies, habitat loss, or increased exposure to hunters; Elmhagen et al. 2017; Johnson et al. 2020).
Many aspects of wolverine ecology, including the species’ response to disturbance, are receiving increasing attention in boreal and montane environments, whereas research on this species in the Arctic lags behind. In a recent review of two decades of wolverine research across the species’ global distribution (Fisher et al. 2022), only 10 of 156 articles focused primarily on the Arctic. Although many fundamental aspects of the species’ ecology are undoubtedly similar across biomes, important differences likely exist that necessitate focused research in this region for effective management and conservation. For example, although documented wolverine reproductive dens in the montane contiguous USA occur exclusively in deep, persistent snowdrifts (Copeland et al. 2010) suggesting likely habitat loss with climate change (McKelvey et al. 2011), this habitat requirement is apparently less strict in boreal forest (Webb et al. 2016; Aronsson and Persson 2017). In the Arctic, fundamental facets of the species’ ecology may differ in accordance with the unique characteristics of this landscape, including distinct prey species, forms of structural habitat, and controls over metapopulation dynamics such as sea ice loss for island populations.
To address the lack of formalized research priorities and potential conservation concerns for wolverines in this region, we synthesize existing knowledge, including both Western research and Indigenous Knowledge (IK), and discuss possible implications of anthropogenic change for wolverines in the Arctic. We contextualize our review by updating the pan-Arctic distribution map of wolverines and discussing possible underlying drivers of Arctic wolverine population dynamics.
Methods
We sought to review all available published literature addressing wolverines in the Arctic. To accomplish this, we employed a snowballing procedure (Wohlin 2014), whereby we searched “wolverine* AND Arctic AND ‘Gulo gulo’” in Google Scholar, sorted in descending order of relevance and used the top 20 most relevant studies addressing wolverines north of latitudinal treeline as our start set. We then worked forward and backward, examining papers both cited by and citing each focal paper until we found no new studies. Additionally, to identify studies excluded using English search terms and to locate gray literature (e.g., reports from management agencies) not indexed by Google Scholar or commonly referenced in the peer-reviewed literature, we contacted regional experts across the circumpolar north. We note that, although this approach yielded some studies not identified during the snowballing procedure, our review likely remains biased toward English studies and peer-reviewed literature. All IK we discuss is derived from published reports, which we note limits the contribution of this body of knowledge to this review.
To quantify research and monitoring efforts regarding wolverines in the Arctic, we classified each source listed in References according to topic and whether or not it was published in the peer-reviewed literature. We excluded supporting sources (i.e., those not dealing directly with wolverines in the Arctic) and those used solely to translate “wolverine” into Indigenous languages (e.g., dictionaries). To quantify how this body of literature has grown and changed over time, we then summarized studies according to date published and topical area.
To update the distribution map of wolverines in the Arctic, we relied on reports arising (1) during our literature review, (2) from targeted searches for specific geographical areas, and (3) from communication with regional experts. We note that defining the Arctic by latitudinal treeline excludes nearly all of the Scandinavian Peninsula; this region is therefore not included in the present review (Fig. 2). We analyzed the updated distribution map in a Geographical Information System to calculate the portion of wolverines’ global distribution in the Arctic and the portion of the species’ Arctic distribution that occurs on islands, which we use to contextualize our discussion of island metapopulation dynamics. For non-Arctic areas of wolverines’ global distribution, we used the distribution reported by Copeland et al. (2010) since this is the most recent effort to collate expert opinion of global wolverine distribution of which we are aware. To visualize the discrepancies between currently reported Arctic distribution and our update, we use the range reported by the International Union for Conservation of Nature (Abramov 2016), since this is typically considered the authority on species’ distributions and it broadly parallels Copeland et al. (2010) for the Arctic.
With the exception of northwestern Alaska and northern Labrador (Schmelzer 2006; Poley et al. 2018), as well as snow track surveys near mines in the Northwest Territories (NWT; Golder Associates Ltd. 2017; De Beers 2018), we are not aware of any systematic effort to survey distribution of wolverines across the Arctic. Therefore, evaluating wolverine presence relies mostly on opportunistic reports or harvest (i.e., hunting or trapping) by local residents, land users, and researchers, as well as a few systematic studies focusing on other aspects of wolverine biology (e.g., remote camera grids to measure population density; Mulders et al. 2007; Awan and Boulanger 2016; Awan et al. 2020). We erred conservatively in designating presence, since wolverines occur naturally at low densities and many remote regions of the Arctic are visited only sporadically by people. Specifically, we designated areas to be within the species’ distribution if reproduction had been documented or if the region was consistently considered occupied by wolverines among local land users or regional experts. We defined an intermediate level (“occasionally observed”) for areas with at least one verifiable report but insufficient observations to support the presence of a reproductive population. For islands, we designated wolverine occupancy on a per-island basis and did not attempt to determine within-island variation (except Greenland). Data and citations for distribution on Arctic islands are presented in Fig. 2 and Online Resource 1. This map, while reflective of the current state of knowledge, is subject to biases associated with obtaining presence records for low-density, wide-ranging species in a vast region with relatively few people. Specifically, we suspect that wolverines may occur in some regions where no records exist and that some regions currently reported to support wolverines may no longer do so.
Bibliometrics
We identified 59 peer-reviewed studies and 32 reports, books, or theses dealing directly with wolverines in the Arctic (Table 1, Fig. 1). Of these, the category “harvest” was most represented (n = 18 across publication types), followed by “parasitism, disease, and toxicology” (n = 15), and occupancy/population density (n = 13, Table 1). Studies published in the peer-reviewed literature generally became more abundant in the late 1990s with a recent peak in 2021. Impacts of human development to wolverines have received particularly little attention (n = 3 across publication types), as have diet (n = 7) and sociocultural role/Indigenous knowledge (n = 6).
Sociocultural role
The few available published surveys regarding the sociocultural role of wolverines among Arctic Indigenous communities unsurprisingly affirm that Arctic Indigenous Peoples demonstrate a refined and complex appreciation of wolverine biology (Nelson 1983; Cardinal 2004; Braund 2010). Indeed, conversations of IK holders in the Canadian High Arctic include much of the information contained in the present review, as well as aspects of wolverine biology not yet documented by Western science (Cardinal 2004). There have, however, been few targeted efforts to collate and publish this knowledge, and much of the information discussed here therefore spans both the Arctic and subarctic. As such, there are both scientific and ethical imperatives for the involvement of Arctic Indigenous Peoples in producing management objectives, biological knowledge, and conservation priorities for wolverines in this region.
Indigenous hunters and trappers in northern Canada predominantly characterize the wolverine as smart and tough, adept at raiding traplines and food caches, and enduring harsh conditions (Laugrand 2017; Bonamy et al. 2020). Among the Inuit, the animal’s consumption of human food and resources position it as an adversary and have made it the subject of hatred (Laugrand 2017), whereas for the Innu of Ungava the wolverine is central to creation stories and occupies an archetypal trickster role in mythology (Armitage 1992). In Arctic Russia, Indigenous Peoples including the Nenets report occasional interactions with wolverines as predators of semi-domestic reindeer (Rangifer tarandus) and raiders of supplies (Forbes 1999; Terekhina et al. 2021). Although they report rarely encountering wolverines, Gwich’in hunters and trappers in boreal and Arctic Canada frequently cross and follow tracks, revealing that wolverines travel impressive distances and disregard topography (Benson 2014). Wolverines appear frequently in legend and oral history among the Gwich’in, often characterized as tricksters or by their intelligence (Benson 2014).
Wolverines are hunted and trapped across the Arctic, predominantly for their fur or to mitigate predation of reindeer. Wolverine fur is valued for its resistance to accumulating frost and is therefore desirable for the ruffs of parka hoods (Hardy 1948). In contrast to more southerly regions, where wolverines are typically trapped, wolverines in the Arctic are commonly hunted and taken opportunistically (Fig. 3; Cardinal 2004; Braund 2010). The prevalence of hunting for wolverines increased with the arrival of the snowmachine; prior to this, take of wolverines in the Arctic was likely considerably lower. Total annual reported take based on carcass collections in Nunavut for the years 2009–2012 ranged from 61 to 124, with most animals taken on the mainland in the Kivalliq and Kitikmeot regions and far fewer in the more northerly Qikiqtaaluk region (Awan and Szor 2012). Reported harvest varies spatially across the Arctic and is broadly concentrated near communities (Braund 2010; Awan and Szor 2012), reflecting the importance of access in determining harvest patterns. In Alaska, reported wolverine harvest for Game Management Units 18, 22, 23, and 26, roughly corresponding to the Arctic portion of the state, is strongly male biased and annual totals ranged from 70 (1994) to 177 (2016) for the period 1987–2018 (Fig. 3; Alaska Department of Fish and Game 1986, 1988a, 1988b, 2013, 1990a; 1990b, 1991, 1993, 1998, 2001, 2007, personal communication). However, these values are widely assumed to underestimate true harvest, since most furs in Arctic communities are used or sold locally and therefore not reported (possibly as many as 90% of the total harvest; Magoun 1985; Cardinal 2004), despite reporting mandates in some regions (e.g., Alaska; Alaska Department of Fish and Game 2021).
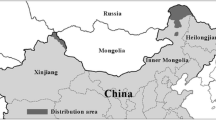
Wolverine (Gulo gulo) distribution in the Arctic. For islands, distribution was assigned to whole islands; no effort was made to distinguish distribution patterns within islands (except Greenland). “Occasional” indicates at least one verifiable record but insufficient observations to support a reproductive population. Latitudinal treeline indicates the southern boundary of the region considered in this review, and the southern boundary of wolverine distribution is derived from wolverine range reported by Copeland et al. (2010). We compared our updated range with that reported by the International Union for Conservation of Nature (IUCN; Abramov 2016); diagonal hatching indicates either that (i) IUCN considers the region occupied, whereas our review found no or only occasional reports or (ii) IUCN considers the region unoccupied, whereas our review found reports of occupation. References for wolverine presence records are in Online Resource 1
Annual wolverine (Gulo gulo) harvest in Arctic Alaska between 1987 and 2018 from fur sealing records. Black lines depict boundaries of Game Management Units 18, 22, 23, and 26, which align roughly with latitudinal treeline. Reported harvest underestimates true harvest, possibly by as much at 90% (Magoun 1985; Cardinal 2004; Lee 2016)
Distribution
We estimated that 25% of wolverines’ global distribution by area occurs in the Arctic and of this 28% is on islands (i.e., 7% of global distribution is on Arctic islands; Fig. 2). In the mainland Arctic regions of North America and Eurasia, wolverines occur everywhere except northern Labrador and Québec (i.e., the Ungava Peninsula). In this region, the wolverine population was severely reduced or extirpated as a result of hunting/trapping and/or caribou (Rangifer tarandus) declines in the early/mid 1900s (Banfield and Tener 1958; Schmelzer 2006; Committee on the Status of Endangered Wildlife in Canada 2014). The species then reappeared in the region’s harvest records between 1963 and 1979 (Moisan 1996; Committee on the Status of Endangered Wildlife in Canada 2014), but since then only two occurrences of wolverines have been confirmed (both trapped in 2019; Guillaume Szor, personal communication), although other unconfirmed observations (i.e., lacking physical evidence or photographs) have been reported since 1979 in both Québec and Labrador (Moisan 1996; Committee on the Status of Endangered Wildlife in Canada 2014). In the Low Arctic (defined as Bioclimate Subzones D and E; Walker et al. 2005) of Alaska, occupancy is non-uniform and associated with terrain ruggedness and soil drainage (Poley et al. 2018).
On High Arctic islands of the Canadian Archipelago and Russia, the species’ distribution is sporadic. Most islands of the Canadian Arctic are considered occupied by wolverines, although the species’ presence on many islands is supported by only a few published reports, and there are few or no reports on the westernmost Queen Elizabeth Islands (van Zyll de Jong 1975; Committee on the Status of Endangered Wildlife in Canada 2014). Wolverines are reported on Ellesmere Island, but there are few historical reports of the species in Greenland, despite being separated from Ellesmere Island only by the 25–35 km seasonally frozen Kennedy Channel. Of Greenland reports, none are verified with photographs, although the abundance of track observations by skilled naturalists in the Thule region during the 1930s and 1940s provides strong support for at least occasional forays by wolverines to the island (Muus et al. 1981). Additionally, although wolverines have been documented on Baffin Island and the northern islands of Hudson Bay (Southampton, Coats, and Mansel), their presence has not been reported for any islands of the more northerly Foxe Basin (Manning 1943; van Zyll de Jong 1975; Mallory et al. 2001; Committee on the Status of Endangered Wildlife in Canada 2014). Occasional sightings are reported on several of the High Arctic Russian islands, but only Wrangel Island has a verified reproductive population apparently resulting from recent colonization (Vekhov 1999; Kolodeznikov 2013; Starova et al. 2014).
The mechanism underlying the sporadic distribution of wolverines across Arctic islands is currently unknown, although availability of prey and carrion is a likely driver (van Zyll de Jong 1975; Cardinal 2004; Committee on the Status of Endangered Wildlife in Canada 2014). There is generally high accordance between the distribution of wolverines’ primary prey species (caribou and muskoxen, Ovibos moschatus) and wolverines. Caribou and muskoxen are sparse or absent on many of the Canadian islands lacking wolverines (Ellef Ringnes, King Christen, Cornwall, Meighen, and Lougheed Islands; Jenkins et al. 2011). Reindeer are similarly unreported on most islands of the Russian High Arctic that lack wolverines, particularly Franz Joseph Land and Severnaya Zemlya (Mizin et al. 2018), whereas muskoxen are absent from all except Wrangel Island, where they were introduced in 1975 followed by the colonization of wolverines in subsequent decades (Starova et al. 2014; Cuyler et al. 2020). Conversely, caribou are present on all islands throughout the Arctic where wolverines are reported, although in regions with lower caribou densities muskoxen comprise a higher portion of the wolverine’s diet (Awan and Szor 2012).
In turn, these patterns of herbivore presence are likely linked to availability of suitable forage. The absence of muskoxen and caribou on High Arctic islands correlates well with Arctic Bioclimate Subzone A, the region of the Arctic characterized by the lowest net primary productivity, lowest mean summer temperatures, and lowest diversity of vascular plants (Walker et al. 2005). Most of the islands lacking wolverines are either predominantly glaciated or belong to subzones A or B. Collectively, these patterns suggest a northern limit to the bioclimatic conditions suitable for wolverines (Copeland et al. 2010), defined by net primary productivity and resulting carrion/prey availability. This hypothesis warrants formal evaluation using species distribution models (e.g., MaxEnt; Elith et al. 2011) and evaluation against possible competing explanations (e.g., availability of suitable denning habitat or cumulative abundance of prey biomass, including small mammals).
There are three interesting exceptions to the above patterns. Svalbard, northern Greenland, and the Ungava Peninsula all contain ungulates, sufficient in Greenland and Ungava to sustain populations of wolves at least periodically (Parker and Luttich 1986; Bergerud et al. 2008; Marquard-Petersen 2009). In Ungava, concurrent crashes of caribou, wolves, and wolverines in the early/mid 1900s, followed by the reappearance of wolverines in the trapping record between 1963 and 1979 associated with an increase in caribou, support the role of caribou in influencing wolverine distribution (Banfield and Tener 1958; Bergerud et al. 2008). Nonetheless, caribou rebounded to over a million individuals in Québec by the 1990s, accompanied by an increase in wolf populations (Courtois et al. 2003), but wolverines apparently did not capitalize on this surge of prey and carrion (Fortin et al. 2005). Ungava caribou populations subsequently experienced dramatic declines between 2000 and 2020 (Gagnon et al. 2019), which may be a contributing factor to wolverines’ continued failure to recolonize the region (Fortin et al. 2005). An additional possible contributing factor is the absence of Arctic ground squirrels (Urocitellus parryii), which have been identified as an important food source in some instances where/when ungulates are less prevalent (Magoun 1987). This persistent absence of wolverines on the Ungava Peninsula presents a ripe opportunity to study factors controlling wolverine recolonization dynamics and the species’ distribution. Similarly, the absence of wolverines in northern Greenland despite the presence of ungulates and carrion-providing wolves, as well as its relative proximity to Ellesmere Island, presents a puzzle that deserves further attention. The absence of wolverines in Svalbard despite the presence of Svalbard Reindeer is also intriguing, perhaps explained simply by its greater distance from potential mainland source populations.
Population structure
Genetic structure of wolverines in the Arctic has been studied in the Low Arctic region of North America. There, populations generally exhibit little nuclear genetic structure (Kyle and Strobeck 2001, 2002), but higher mitochondrial genetic structure (Wilson et al. 2000; Chappell et al. 2004; Tomasik and Cook 2005; Zigouris et al. 2013; Krejsa et al. 2021). This dynamic likely reflects the species’ male-biased dispersal (Vangen et al. 2001; Aronsson and Persson 2018), resulting in lower gene flow among maternally inherited markers. However, the spatial scale of such structuring remains uncertain; Wilson et al. (2000) detected mitochondrial genetic structuring among populations separated by < 100 km in Nunavut, whereas Dalerum et al. (2007) did not find genetic support for male-biased dispersal in the Western Brooks Range of Alaska. Mitochondrial markers also indicate that wolverines in Nunavut may be genetically distinct from those in Alaska and NWT (Tomasik and Cook 2005; Zigouris et al. 2013). Wolverines in Russia are broadly distinct from wolverines in Arctic North America, although specific location information about Russian samples (i.e., Arctic versus non-Arctic, Beringian versus non-Beringian) is generally not reported (Kyle and Strobeck 2002; Zigouris et al. 2013; Rico et al. 2015). We are aware of no study to evaluate genetic structure of wolverines within Russia.
Although 7% of global wolverine distribution occurs on Arctic islands, we are aware of no study to evaluate metapopulation dynamics (i.e., connectivity or genetic structure) among island-dwelling populations or the potential role of sea ice or its loss as a barrier to gene flow. Wolverines are occasionally observed traveling across sea ice (up to seven kilometers from land in some instances; Glass, unpublished data, Starova et al. 2014), and use of sea ice for dispersal is implied from the species’ presence on Arctic islands (e.g., St. Lawrence and Wrangel Islands, 70 km and 140 km from potential mainland source populations, respectively), but dispersal rates to, from, and between islands are unknown.
Population density
Broadly, IK holders report higher wolverine densities in the Low Arctic, decreasing across a northward gradient, with the lowest densities on islands of the High Arctic in Canada (Cardinal 2004). IK holders and genetic evidence suggest an increase in wolverine populations across much of Arctic Canada during recent decades (Tomasik and Cook 2005; Committee on the Status of Endangered Wildlife in Canada 2014), possibly rebounding from historical carnivore poisoning programs (Cardinal 2004). The only quantitative population density estimates come from the North American Low Arctic, and these estimates are inconsistent. In the Low Arctic regions of Napaktulik and Aberdeen Lakes, Nunavut, and Toolik Lake, Alaska, spatial capture–recapture analyses of bait station visits yielded population densities of approximately 2–4 individuals/1000 km2 (Awan and Boulanger 2016; Awan et al. 2020, Glass unpublished data), whereas estimates based on VHF telemetry (Utukok River, Alaska) and closed population models of bait station visits (Daring Lake, NWT) produced estimates of approximately 17–21 individuals/1000 km2 (Magoun 1985; Mulders et al. 2007). The habitat in these regions is superficially similar; all are within roughly 200 km of the latitudinal treeline, fall within the migration paths of substantial caribou herds, and have similar assemblages of potential prey species. Although some of the difference is likely attributable to bias associated with the analytical approaches (Mowat et al. 2020), this may not explain the entire discrepancy, since the territory sizes of radio-collared individuals studied contemporarily are consistent with the densities, i.e., much smaller in the Utukok region than the Toolik region (Magoun 1985, Glass unpublished data). The disparate estimates preclude a generalizable assessment of population density in Low Arctic tundra and highlight the need for additional studies, ideally accounting for spatial and temporal fluctuations in carrion availability, alternative food sources, and wolverine mortality, which can affect density and territorial dynamics of at least reproductive female wolverines (Aronsson and Persson 2018).
Diet and food habits
Most studies of wolverine diet in the Arctic have taken place near latitudinal treeline and suggest that wolverines rely heavily on ungulates for food, consistent with conspecifics in boreal and montane areas. Caribou and reindeer (hereafter “caribou”) account for the majority of ungulates’ contribution to wolverine diet in the Arctic, although muskoxen and moose are also well represented in some regions and individuals. Specifically, muskoxen may be particularly important in regions of the High Arctic where or when caribou are scarce (Awan and Szor 2012), while moose occur more frequently in the diet of wolverines near treeline and in regions with tall shrubs (Dorendorf et al. 2018). Wolverine consumption of ungulates is generally highest during winter, a time when alternate food sources are scarce and ungulates are vulnerable to predation and better preserved as carcasses (Magoun 1987). The species’ reliance on caribou extends even to regions without overwintering caribou, where wolverines consume bones, hide, and remains cached or otherwise left from seasons of caribou presence (Magoun 1987). Although wolverines have been observed to hunt and kill caribou on tundra (Magoun et al. 2018), most ungulates are likely consumed as carrion (Magoun 1987). This point is supported by interannual trends in wintertime consumption of caribou, which is influenced by caribou mortality rate and not by annual fluctuations in caribou abundance (Dalerum et al. 2009). Since overwinter food availability is important to wolverine reproduction (Persson 2005), these findings collectively suggest that the abundance of caribou, muskoxen, and moose carcasses in the Arctic is an important factor in regulating wolverine populations.
In addition to ungulates, wolverines supplement wintertime diet with a variety of species, including Arctic ground squirrels, Arvicoline rodents, ptarmigan (Lagopus spp.), beavers, snowshoe hares, seals (Phoca spp.), and cached geese and goose eggs (Makridin 1964; Magoun 1987; Lee and Niptanatiak 1993; Dorendorf et al. 2018; L’Herault 2018). Of these, Arctic ground squirrels and Arvicoline rodents have been found to occur in wolverine diet at frequencies comparable to ungulates in certain conditions (Magoun 1987; Dorendorf et al. 2018). Arctic ground squirrels consumed during winter are mostly killed and cached the preceding summer, rather than excavated during hibernation from burrows, although at least one instance of the latter has been observed (Magoun 1987). In coastal areas, wolverines consume marine mammals, particularly seals, in accordance with their proximity to the sea (Rausch and Pearson 1972; L’Herault 2018; Robards, unpublished data), and wolverines’ occasional forays onto sea ice are presumably associated with hunting or scavenging seals at least in some cases, as evidenced by spatiotemporal overlap with seal pupping (Glass, unpublished data, Starova et al. 2014).
During spring and summer, wolverines transition to a heavier reliance on hunting, particularly Arctic ground squirrels and Arvicoline rodents, and scavenge or hunt vulnerable caribou and muskoxen calves (Magoun 1987; Starova et al. 2014). As snow melts, wolverines on tussock tundra hunt Arvicolines that are apparently forced to the tussock tops as the inter-tussock space floods with snowmelt unable to permeate the still-frozen ground (Dorendorf et al. 2018). Wolverines have also been observed removing and caching eggs from waterfowl, shorebird, and raptor nests (Samelius et al. 2002; Hoover and Dickson 2007; Chris Latty and Devin Johnson personal communication), and hunting adult geese (Chen spp.; Samelius et al. 2002) and eiders (Somateria mollissima; Hoover and Dickson 2007). The presence of mud-encased waterfowl carcasses and eggshells in wintertime caches made by wolverines or foxes suggests that these food sources can help wolverines survive the relatively food-scarce winter (Magoun 1987, Glass unpublished data).
Parasitism, disease, and toxicology
Most studies of Arctic wolverine health have focused on parasitism. The species’ large territories, generalist diet, and position in regional food webs likely predispose wolverines to high overall parasitism rates, which generally exceed 80% in the Arctic and make the species a good candidate as a sentinel of landscape-level parasite spread (Watson et al. 2020; Sharma et al. 2021). Historical or longitudinal data of wolverine parasitism remain sparse for evaluating such trends, but information is improving. Documented parasites include several taxa of both helminths and protozoa, with > 80% taxa-specific prevalence of Trichinella spp., Taenia twitchelli, Sarcocystis spp., and Baylisascaris devosi and 40–60% prevalence of the protozoan Toxoplasma gondii in some, but not all, regions of the North American Arctic (Rausch 1959; Addison and Boles 1978; Reichard et al. 2008b, a; Dubey et al. 2010; Sharma et al. 2019; Watson et al. 2020). Wolverine is the preferred definitive host for T. twitchelli, which likely uses porcupine (Erethizon dorsatum) as its intermediate host and therefore may be limited to the Low Arctic (Rausch 1959). The recently discovered Trichinella chanchalensis has only been documented in wolverines of Arctic and boreal Canada, although previous methods may not have been able to distinguish it from T. nativa in other species and regions (Sharma et al. 2020). We are not aware of any ectoparasite reports among wolverines in the Arctic, although ear canker mite (Otodectes cynotis) was reported in a wolverine in northern boreal forest of Alaska (Wilson and Zarnke 1985).
Scavenging may increase wolverines’ susceptibility to viral and bacterial diseases, which can be transferred from other scavengers or arise from the scavenged animal’s necrobiome (Dalerum et al. 2005; Watson 2020). The few studies to catalogue potentially pathogenic viruses and bacteria in Arctic wolverines, however, generally indicate low incidence of both. Among 64 wolverines harvested in Alaska’s Brooks Range during 1998–2002, four had antibodies for canine distemper virus, one had antibodies for canine parvovirus type 2, and none had antibodies for canine adenovirus (Dalerum et al. 2005). A single apparently lethal rabies infection has been documented in Arctic Alaska, the only case of rabies in wolverine of which we are aware (Alaska Department of Fish and Game 2012). In Nunavut, pathogenic bacteria accounted for only 0.7% of wolverine gut microbiota, possibly attributable to low exposure mediated by caching behavior, defenses provided by the gut microbiome, or high stomach acidity (Inman et al. 2012; Watson 2020).
Wolverines’ relatively high trophic level and at least occasional consumption of marine mammals may also increase the species’ risk of accumulating toxic levels of anthropogenic contaminants. Total mercury concentrations in wolverines harvested near coastal Arctic Canadian communities, however, were well below expected toxicity level (Hoekstra et al. 2003a), although mercury toxicity has not been directly evaluated for this species. Polychlorinated biphenyl concentrations among the same sample of wolverines were higher than other terrestrial mammals, possibly due to consumption of marine mammals, but still below the expected level of reproductive impairment (Hoekstra et al. 2003b).
The implications of these parasites, viruses, bacteria, and toxins for wolverine and human health remain unclear. The single rabies case is the only Arctic wolverine mortality directly attributable to disease of which we are aware, and sublethal effects of this and other potential pathogens and toxins remain poorly understood. Most parasites found in wolverines use the species as a definitive host, which likely reduces health consequences (Watson et al. 2020), and although several bacterial taxa known to cause disease in other species are found in wolverine, bacterial disease induction in wolverines is so far undocumented (Watson 2020). Toxoplasmosis and Trichinellosis in humans, typically arising from undercooked parasitized meat consumption, are increasing concerns in Arctic communities relying on subsistence hunting (Keatts et al. 2021). Wolverines, which are rarely consumed, are unlikely vectors for these diseases, but handling and skinning animals has been identified as a risk factor in certain contexts and therefore care is warranted when handling live or dead wolverines (McDonald et al. 1990; Keatts et al. 2021; Sharma et al. 2021). Keatts et al. (2021) note the potential for coronavirus transmission between humans and wolverines.
Habitat associations and den-site selection
Arctic Canadian IK holders report that wolverine presence is predominantly determined by food availability, but that wolverines also associate broadly with rugged and rocky habitats, as well as higher elevations (Cardinal 2004). This trend aligns with Western science studies from the winter and spring in northern Alaska tundra, which have found that wolverine habitat selection is driven by terrain ruggedness, soil drainage, streams/rivers, and snow properties (Poley et al. 2018; Glass et al. 2021b). At the occupancy level of selection (i.e., the placement of home ranges), wolverines select more rugged terrain and areas with better drained soils, possibly showing a preference for Arctic ground squirrel habitat (Poley et al. 2018). In Arctic Alaska, this results in higher occupancy in the mountains and foothills of the Brooks Range than the more northerly and less rugged coastal plain (Poley et al. 2018). Within home ranges in Low Arctic Alaska during winter and spring, wolverines select strongly for streams and rivers, where they are observed hunting snowshoe hare and ptarmigan dwelling among the tall shrubs, as well as rugged terrain and deep, dense snow (Glass et al. 2021b).
Snow is used by wolverines across the species’ global range for reproductive den structures and food caching (Copeland et al. 2010; Inman et al. 2012) and its importance may be particularly high in the Arctic where alternative structural habitat is limited (Magoun et al. 2017). Elsewhere in the species’ global distribution, wolverines access subnivean structure including upturned rootwards, abandoned beaver lodges, and boulder complexes for reproductive dens (Magoun and Copeland 1998; Dawson et al. 2010; Scrafford and Boyce 2015; Jokinen et al. 2019). In Arctic Alaska and Russia, wolverines excavate reproductive dens in snow, and in many cases these dens are excavated solely in snowdrifts (Glass et al. In Press; Serebryakov 1983; Magoun and Copeland 1998), although IK holders also report the use of rocks and boulders for dens (Cardinal 2004). Reproductive dens are commonly found in snowdrifts that form in small drainages and along the cut banks associated with lake edges (Glass et al. In Press). Excavations of such dens reveal complex networks of tunnels and chambers, with total tunnel lengths approaching 55 m (Magoun 1985). Wolverine reproductive dens and resting sites have also been documented in subterranean ice caves formed by eroding permafrost (Glass et al. 2021a).
In addition to reproductive dens, wolverines excavate burrows in snow for resting sites and to cache food (Glass et al. In press; Lee and Niptanatiak 1993, 1996). Approximately half of wintertime wolverine resting sites are in burrows, and wolverines rest in these burrows when it is cold and there is little solar radiation, transitioning to the snow surface during periods of higher air temperature (Glass et al. 2021c).
Response to industrial development
The Arctic contains large mineral and petroleum deposits, the extraction of which requires constructing and maintaining transportation networks, buildings, waste processing facilities, and other supporting infrastructure. Russia produces the majority of Arctic petroleum and mineral resources, with petroleum production concentrated in the Yamal and Gydan Peninsulas and gold, iron, apatite, copper, nickel, and cobalt mines distributed across mainland Arctic Russia (Haley et al. 2011; Peters et al. 2011; Mathonniere 2019). In the North American Arctic, petroleum production is concentrated in the Prudhoe Bay region of Alaska’s North Slope, whereas mineral extraction includes several active diamond and gold mines in Arctic Canada, an iron mine on northern Baffin Island, and a zinc mine in Alaska’s Brooks Range (Haley et al. 2011). Trends in extractive infrastructure and activity can be difficult to document and predict, particularly in Arctic Russia where inconsistent reporting standards and methodologies have precluded longitudinal evaluation (Haley et al. 2011). Nonetheless, continued expansion of these industries is indicated by the initiation and support of resource development for economic opportunities by a suite of local communities, as well as Arctic states’ policies signaling continued expansion (Laruelle 2020) and expanding footprints and human presence in most regions with adequate data (Raynolds et al. 2014; Kröger 2019).
Current and projected industrialization highlight the importance of understanding the relationship between wolverines and human infrastructure in an Arctic tundra environment. However, the only systematic attempts to do so, by evaluating habitat selection and movement responses, have been unsuccessful due to low sample sizes or insufficient overlap between monitored wolverines and disturbance features (Johnson et al. 2005; Glass et al. 2021b). Incidental observations suggest possible mechanisms underlying the species’ response, such as apparent short-term attraction to mines in Arctic Canada (Golder Associates Ltd. 2017; De Beers Canada Inc. 2018), lethal control following attraction to grease traps (Gebauer et al. 2014), and possible home range delineation along a highway corridor by six wolverines in Arctic Alaska (Glass, unpublished data). These observations highlight the need for further study of population-level impacts of industrial development to wolverines.
Research and Conservation Priorities
The Arctic is changing rapidly. For wolverines, these changes introduce novel prey species and pathogens, may reduce connectivity among island populations, alter habitat availability, and increase interactions with humans through new infrastructure, transportation corridors, and hunting/trapping opportunities. Some of these changes, such as increased moose abundance, may benefit wolverines, while others will likely incur negative consequences. Although recent studies have dramatically improved our understanding of wolverines in the Arctic, the individual findings of each represent brief periods and typically small spatial scales. As change accelerates, so too does the need to gather longitudinal information about population processes and drivers thereof (Fisher et al. 2022). These research and monitoring efforts should be developed by, or in collaboration with, local Indigenous communities to improve their efficacy, applicability, and equity (Brook et al. 2009). Here, we identify several research priorities focusing on understanding wolverines’ susceptibility and response to ongoing change in the Arctic (summarized in Table 2).
Effective population monitoring
Conservation-driven management of wolverines in the Arctic requires an accurate understanding of spatiotemporal trends and drivers of occupancy, population abundance, and demographics. Since obtaining such information can be logistically challenging, we advocate partnering with existing wildlife monitoring efforts (e.g., aerial surveys for caribou and muskoxen) and/or local hunters and trappers to design and implement these studies. For example, existing aerial surveys can be adapted to include wolverine track observations if observers are skilled at identifying wolverine tracks, snow conditions are amenable, and survey protocol such as height above ground and aircraft speed are compatible, potentially yielding occupancy and population density estimates (Golden et al. 2007; Poley et al. 2018). Alternatively, baited camera sites or hair snares operated by local hunters and trappers can simultaneously strengthen collaborative relationships and gather longitudinal data regarding reproduction, population density, and occupancy, as has been successful elsewhere (Webb et al. 2016). In concert with these efforts, we recommend developing community-based reporting initiatives to more accurately document harvest, including demographic information, such as sex and age, as are currently implemented in Arctic Canada (Lee 1994, 2016; Awan and Szor 2012; Kukka and Jung 2016). Such information can supplement population abundance estimates, enabling study of harvest influences on population dynamics (Mowat et al. 2020).
Improved understanding of population dynamics will also facilitate a mechanistic study of the limits of wolverine distribution in the Arctic, including the potential role of bioclimatic conditions and ungulate abundance. Parsing the drivers underlying distribution will aid in predicting and understanding the species’ response to climate warming, including the potential to expand into currently unoccupied regions, as bioclimatic conditions change.
Understanding and forecasting impacts of climate change
We expect that impacts of climate change to wolverines in the Arctic will primarily be mediated by reduced snow and sea ice availability, changing prey species assemblage, and shifts in disease dynamics. We advocate prioritizing study of these changes’ mechanistic links to wolverine fitness and population dynamics.
Net primary productivity is broadly increasing in the Arctic, and this trend extends to the regions currently unoccupied by wolverines (Yu et al. 2017), suggesting future conditions more favorable for wolverine prey species and the possibility of wolverines’ northward range expansion. Arctic shrubification is driving a northward range expansion of moose, beaver, and snowshoe hare, all species identified by modern studies to be consumed by wolverines in Arctic Alaska despite their arrival to the region only decades ago (Tape et al. 2016a, b, 2018). The availability of these species to wolverines will likely continue to increase under climate change (Zhou et al. 2020). Conversely, wintertime ground-icing events, which are increasingly common and severe with climate change, are dampening the peaks of Arvicoline population cycles and driving mass mortality events in caribou and muskoxen (Aars and Ims 2002; Kohler and Aanes 2004; Ims et al. 2008; Jenkins et al. 2011). Earlier springs and later autumns are altering the migration phenology of species including caribou and geese (Lameris et al. 2018; Mallory et al. 2020), and sea ice degradation is reducing wolverines’ access to marine resources (Post et al. 2013). Although wolverines have a broad diet, the unprecedented magnitude and synchrony of these environmental changes have the potential to impact wolverine fitness, particularly where ungulates such as caribou are negatively impacted (Mallory and Boyce 2018). Cumulatively, these changes highlight the need for continued study of wolverines’ diet throughout the Arctic, with a focus on mechanistic links between diet composition and wolverine fitness.
Disease vectors are strongly influenced by environmental conditions, and climate change may facilitate range expansion of temperate vectors into the Arctic and modify transmission pathways of existing Arctic vectors (Bradley et al. 2005; Keatts et al. 2021). For instance, warmer and longer summers can increase developmental rates of some parasites and may ultimately result in higher prevalence and individual parasite load (Bradley et al. 2005). Information regarding pathogen and parasite disease potential to wolverines in the Arctic is insufficient to predict the possible impacts of such changes to the species. We therefore recommend continued monitoring of wolverine health using community-based initiatives in collaboration with hunters and trappers (Oakley et al. 2016; Sharma et al. 2019; Watson et al. 2020) and study of physiological response to potential disease vectors. The connection between human and animal health in the Arctic and subarctic is increasingly recognized under the “One Health” framework (Ruscio et al. 2015), yielding successful longitudinal monitoring efforts of wildlife health across diverse taxa (e.g., caribou, muskoxen, and ringed seal (Phoca hispida); Brook et al. 2009; Tomaselli et al. 2018; Harwood et al. 2020); we advocate development of similar long-term efforts for wolverines.
Wolverines’ occupancy of Arctic islands may make the species susceptible to reduced inter-island dispersal opportunities resulting from sea ice decline (Post et al. 2013). The abrupt loss of sea ice that began in the mid twentieth century is likely reducing gene flow among island populations of this vagile species, a dynamic supported by genetics studies in arctic foxes, wolves, and caribou (Geffen et al. 2007; Carmichael et al. 2008; Jenkins et al. 2016). This rapid change likely increases the susceptibility of wolverines and their prey to stochastic environmental events and genetic drift. Islands that have been historically occupied by wolverines may therefore become unsustainable as sea ice reductions disconnect them from other populations of wolverines and their prey species. We suggest community-based campaigns using tissues from harvested animals or bait stations to monitor gene flow among island populations and possible impacts of changing sea ice conditions, building upon existing efforts that already include southern portions of the Canadian Archipelago (Awan and Szor 2012; Lee 2016).
Finally, spring snowmelt is advancing rapidly in the Arctic, with the duration of the snow-covered period contracting by as much as nearly 9 days per decade in some regions (Callaghan et al. 2011). This advancing melt is likely exposing vulnerable wolverine kits to predators and inclement weather at a younger age, but the capacity of wolverines to respond by shifting reproductive phenology or structural requirements for den sites is unknown. Moreover, advancing snowmelt and warmer air temperatures may reduce the efficacy of springtime food caches upon which wolverines rely to fuel lactation (Inman et al. 2012). The cumulative effects of these snow-mediated changes have received considerable attention in contiguous USA (e.g., McKelvey et al. 2011) and warrant further research in the Arctic.
Understanding and forecasting impacts of industrial development
We suggest prioritizing research regarding development impacts into three possible disturbance pathways. First, wintertime over-snow travel, which is common in the Arctic to reduce tundra compaction, could negatively impact wolverines occupying subnivean reproductive dens and resting burrows, either through compaction or auditory/visual disturbance. Exploratory surveys for mineral or petroleum deposits, which typically deploy high-energy seismic pulses into tundra, can have footprints covering 10% of the landscape (Raynolds et al. 2020). Mitigating the impacts of over-snow travel and/or associated seismic pulses has received considerable attention for polar bears (Ursus maritimus) and ringed seals, which have similar reproductive phenology and denning requirements (Kelly et al. 2010; Wilson and Durner 2020; Owen et al. 2021). Observations of wolverine den abandonment following interaction with humans indicate that this species may be susceptible to these disturbances (Glass et al. In press). However, population-level response of wolverines to den-site disturbance has so far not been evaluated, and we are not aware of any existing measures to mitigate impacts of over-snow travel.
Second, increased ice-breaking ship traffic may reduce connectivity among island populations of wolverines in the Arctic, exacerbating impacts of climate-induced sea ice decline. Such shipping traffic inhibits caribou migration (Dumond et al. 2013), and springtime ice breakers may be of particular concern for wolverines given the temporal overlap with juvenile dispersal (Vangen et al. 2001). We recommend study of wolverine dispersal dynamics across sea ice to better understand and mitigate any impacts from this form of disturbance.
Finally, industrial development may increase mortality by facilitating harvest in new regions or prompting lethal control at development sites. Roads associated with mines and oilfields provide hunting and trapping access to regions where harvest is otherwise more difficult and development-related capital flowing into Arctic communities increases harvest efficiency through modernization of equipment, particularly snowmachines (Gebauer et al. 2014; Fauchald et al. 2017). We suggest community-based monitoring initiatives to address these potential concerns and ensure sustainability of landscape-scale harvest. Mortality arising from lethal control at mine sites or oilfields can be proactively mitigated using appropriate deterrence procedures consistent with those used for other species, such as bear and fox (e.g., secure waste storage and processing containers).
Ensuring harvest sustainability
The absence of information regarding population trends, demographics, or vital rates for most Arctic regions, coupled with unreported harvest due to use of fur within and among communities, complicates efforts to evaluate sustainability of current harvest levels. Most regions report higher harvest of males than females (Awan and Szor 2012; Alaska Department of Fish and Game 2013; Lee 2016) consistent with expectations for a species exhibiting male-biased dispersal. This provides some support for the sustainability of current harvest levels, since persistence of species with low reproductive output requires low mortality of adult females (Kukka et al. 2017). The precise relationship between demographics of hunted/trapped wolverines and harvest sustainability remains unknown, however (Kukka et al. 2017), and additional information is required to evaluate impacts of harvest to wolverine populations in the Arctic. We recommend coupled monitoring of wolverine population parameters and harvest rates, as discussed in “Effective population monitoring,” to address this knowledge gap, with the ultimate goal of harvest management policies that are based in a firm understanding of wolverine population dynamics.
As technological and industrial development continue to facilitate harvest farther from population centers, it may become increasingly important to proactively establish refugia. Harvest is currently concentrated near population centers, and in some cases harvest rates are likely unsustainable at a local level but enabled by immigration from surrounding de facto refugia as is seen in subarctic regions (Kukka et al. 2022). Construction of road networks and development of more efficient and faster over-snow vehicles may enable access to these regions and necessitate codifying refugia (Lee 2016).
Conclusion
The Arctic is often considered a stronghold for wolverines, given the species’ prevalence and the region’s low human population density and cold climate (Fisher et al. 2022). Nonetheless, much remains unknown about wolverines in this region, including the potential impacts of modern anthropogenic change to the species. Climate change in particular offers numerous potential pathways to negatively impact the species, as does the expansion of extraction-based industries and, in many regions, underestimated harvest. Ensuring long-term persistence of wolverines in the Arctic requires considerable additional research and monitoring to resolve mechanisms driving abundance and demographics, and mitigation of growing threats as the Arctic continues to change.
Data availability
This article did not involve previously unpublished data.
References
Alaska Department of Fish and Game (2021) 2021–2022 Alaska Trapping Regulations. Juneau
Aars J, Ims RA (2002) Intrinsic and climatic determinants of population demography: the winter dynamics of tundra voles. Ecology 83:3449–3456. https://doi.org/10.1890/0012-9658(2002)083[3449:IACDOP]2.0.CO;2
Abramov AV (2016) Gulo gulo. IUCN Red List Threat Species e.T9561A45198537. https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T9561A45198537.en
Addison EM, Boles B (1978) Helminth parasites of wolverine, Gulo gulo, from the District of Mackenzie, Northwest Territories. Can J Zool 56:2241–2242. https://doi.org/10.1139/z78-304
Alaska Department of Fish and Game (1988a) Annual Report of Survey-Inventory Activities - Furbearers (February 1988a). Juneau
Alaska Department of Fish and Game (1988b) Annual Report of Survey-Inventory Activities - Furbearers (December 1988b). Juneau
Alaska Department of Fish and Game (1990a) Annual Report of Survey-Inventory Activities 1 July 1987 - 30 June 1988 - Furbearers. Juneau
Alaska Department of Fish and Game (1990b) Annual Report of Survey-Inventory Activities 1 July 1988 - 30 June 1989 - Furbearers. Juneau
Alaska Department of Fish and Game (1993) Survey-Inventory Management Report - 1 July 1989 - 30 June 1991 - Furbearers. Juneau
Alaska Department of Fish and Game (2001) Furbearer Management Report of Survey-Inventory Activities - 1 July 1997–30 June 2000. Juneau
Alaska Department of Fish and Game (2007) Furbearer Management Report of Survey-Inventory Activities 1 July 2003 - 30 June 2006. Juneau
Alaska Department of Fish and Game (2013) Furbearer Management Report of Survey-Inventory Activities, 1 July 2009 – 30 June 2012. Juneau
Alaska Department of Fish and Game (1986) Annual Report of Survey-Inventory Activities: Part XIV. Furbearers. Juneau
Alaska Department of Fish and Game (2012) Rabies in Alaska: Rabid Wolverine Found on North Slope. In: Alaska Fish & Wildllife News. http://www.adfg.alaska.gov/index.cfm?adfg=wildlifenews.view_article&articles_id=582. Accessed 6 Oct 2021
Alaska Department of Fish and Game (1991) Annual Performance Report of Survey-Inventory Activities - 1 July 1989 - 30 June 1990 - Furbearers. Juneau
Alaska Department of Fish and Game (1998) Furbearer Management Report of Survey-Inventory Activities - 1 July 1994 - 30 June 1997. Juneau
Armitage P (1992) Religious ideology among the innu of eastern quebec and labrador. Religiologiques 6:63–110
Aronsson M, Persson J (2017) Mismatch between goals and the scale of actions constrains adaptive carnivore management: the case of the wolverine in Sweden. Anim Conserv 20:261–269. https://doi.org/10.1111/acv.12310
Aronsson M, Persson J (2018) Female breeding dispersal in wolverines, a solitary carnivore with high territorial fidelity. Eur J Wildl Res 64:1–10. https://doi.org/10.1007/s10344-018-1164-3
Awan M, Boulanger J (2016) Estimates of wolverine density from mark ‐ recapture DNA sampling, Aberdeen Lake, Kivalliq Region, Nunavut, 2013–14. Department of Environment, Government of Nunavut, Iqaluit
Awan M, Szor G (2012) Wolverine (Gulo gulo) carcass collection and harvest monitoring in Nunavut, Summary Report. Department of Environment, Government of Nunavut, Iqaluit
Awan M, Efford M, Boulanger J (2020) Estimates of wolverine density from mark-recapture DNA sampling, Napaktulik Lake, Kitikmeot Region, Nunavut 2018–2019, Final Report. Department of Environment, Government of Nunavut, Iqaluit
Banfield AWF, Tener JS (1958) A preliminary study of Ungava caribou. J Mammal 39:560–573. https://doi.org/10.2307/1376795
Benson K (2014) Gwich’in Traditional Knowledge: Nèhtrùh (Wolverine). Gwich’in Social and Cultural Institute, Inuvik
Bergerud AT, Luttich SN, Camps L (2008) The return of caribou to Ungava. McGill-Queen’s University Press, Ithaca
Berteaux D, Gauthier G, Domine F et al (2017) Effects of changing permafrost and snow conditions on tundra wildlife: critical places and times. Arct Sci 3:65–90. https://doi.org/10.1139/as-2016-0023
Bobaljik JD (1996) Assimilation in the Inuit languages and the place of the uvular nasal. Int J Am Linguist 62:323–350. https://doi.org/10.1086/466303
Boelman NT, Gough L, Wingfield J et al (2015) Greater shrub dominance alters breeding habitat and food resources for migratory songbirds in Alaskan arctic tundra. Glob Chang Biol 21:1508–1520. https://doi.org/10.1111/gcb.12761
Bonamy M, Herrmann TM, Harbicht AB (2020) ‘I think it is the toughest animal in the North’: human-wolverine interactions among hunters and trappers in the Canadian Northwest territories. Polar Geogr 43:1–24. https://doi.org/10.1080/1088937X.2019.1685020
Bradley M, Kutz SJ, Jenkins E, O’Hara TM (2005) The potential impact of climate change on infectious diseases of Arctic fauna. Int J Circumpolar Health 64:468–477. https://doi.org/10.3402/ijch.v64i5.18028
Braund SR (2010) Subsistence mapping of Nuiqsut, Kaktovik, and Barrow. Stephen R. Braund & Associates, Anchorage
Brook RK, Kutz SJ, Veitch AM et al (2009) Fostering community-based wildlife health monitoring and research in the Canadian North. EcoHealth 6:266–278. https://doi.org/10.1007/s10393-009-0256-7
Callaghan TV, Johansson M, Brown RD et al (2011) The changing face of Arctic snow cover: a synthesis of observed and projected changes. Ambio 40:17–31. https://doi.org/10.1007/s13280-011-0212-y
Cardinal N (2004) Aboriginal traditional knowledge COSEWIC status report on wolverine (Gulo gulo, Qavvik). Committee on the Status of Endangered Wildlife in Canada, Ottowa
Carmichael LE, Krizan J, Nagy JA et al (2008) Northwest passages: conservation genetics of Arctic Island wolves. Conserv Genet 9:879–892. https://doi.org/10.1007/s10592-007-9413-0
Chappell DE, Van Den Bussche RA, Krizan J, Patterson B (2004) Contrasting levels of genetic differentiation among populations of wolverines (Gulo gulo) from northern Canada revealed by nuclear and mitochondrial loci. Conserv Genet 5:759–767. https://doi.org/10.1007/s10592-004-1976-4
Committee on the Status of Endangered Wildlife in Canada (2014) COSEWIC Assessment and Status Report Wolverine (Gulo gulo) in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottowa
Copeland JP, McKelvey KS, Aubry KB et al (2010) The bioclimatic envelope of the wolverine (Gulo gulo): Do climatic constraints limit its geographic distribution? Can J Zool 88:233–246. https://doi.org/10.1139/Z09-136
Courtois R, Ouellet JP, Gingras A et al (2003) Historical changes and current distribution of caribou, Rangifer tarandus, in Québec. Can Field-Naturalist 117:399–414. https://doi.org/10.22621/cfn.v117i3.742
Cuyler C, Rowell J, Adamczewski J et al (2020) Muskox status, recent variation, and uncertain future. Ambio 49:805–819. https://doi.org/10.1007/s13280-019-01205-x
Dalerum F, Shults B, Kunkel K (2005) A serologic survey for antibodies to three canine viruses in wolverines (Gulo gulo) from the Brooks Range, Alaska. J Wildl Dis 41:792–795. https://doi.org/10.7589/0090-3558-41.4.792
Dalerum F, Loxterman J, Shults B et al (2007) Sex-specific dispersal patterns of wolverines: insights from microsatellite markers. J Mammal 88:793–800. https://doi.org/10.1644/05-MAMM-A-427R1.1
Dalerum F, Kunkel K, Angerbjörn A, Shults BS (2009) Diet of wolverines (Gulo gulo) in the western Brooks range, Alaska. Polar Res 28:246–253. https://doi.org/10.1111/j.1751-8369.2008.00090.x
Dawson FN, Magoun AJ, Bowman J, Ray JC (2010) Wolverine, Gulo gulo, home range size and denning habitat in lowland boreal forest in Ontario. Can Field-Naturalist 124:139–144. https://doi.org/10.22621/cfn.v124i2.1052
De Beers Canada Inc. (2018) 2017 Annual Wildlife Effects Monitoring Program, Snap Lake Mine. De Beers Canada, Inc., Toronto
Dorendorf RR, Sivy KJ, Robards MD et al (2018) Spring food habits of wolverine (Gulo gulo) in the Colville River watershed, Alaska. Can Field-Naturalist 132:268–278
Dubey JP, Reichard MV, Torretti L et al (2010) Two new species of Sarcocystis (Apicomplexa: Sarcocystidae) infecting the wolverine (Gulo gulo) from Nunavut, Canada. J Parasitol 96:972–976. https://doi.org/10.1645/GE-2412.1
Dumond M, Sather S, Harmer R (2013) Observation of Arctic island barren-ground caribou (Rangifer tarandus groenlandicus) migratory movement delay due to human induced sea-ice breaking. Rangifer 33:115–121. https://doi.org/10.7557/2.33.2.2533
Elith J, Phillips SJ, Hastie T et al (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57. https://doi.org/10.1111/j.1472-4642.2010.00725.x
Elmhagen B, Berteaux D, Burgess RM et al (2017) Homage to Hersteinsson and Macdonald: climate warming and resource subsidies cause red fox range expansion and Arctic fox decline. Polar Res 36:1–15. https://doi.org/10.1080/17518369.2017.1319109
Fauchald P, Hausner VH, Schmidt JI, Clark DA (2017) Transitions of social-ecological subsistence systems in the Arctic. Int J Commons 11:275–329. https://doi.org/10.18352/ijc.698
Fisher JT, Murray S, Barrueto M et al (2022) Wolverines (Gulo gulo) in a changing landscape and warming climate : a decadal synthesis of global conservation ecology research. Glob Ecol Conserv 34:1–17. https://doi.org/10.1016/j.gecco.2022.e02019
Forbes BC (1999) Reindeer herding and petroleum development on Poluostrov Yamal: sustainable or mutually incompatible uses? Polar Rec (gr Brit) 35:317–322. https://doi.org/10.1017/S0032247400015667
Fortin C, Banci V, Brazil J et al (2005) National Recovery Plan for the Wolverine (Gulo gulo) [Eastern Population]. Recovery of Nationally Endangered Wildlife, Ottowa
Gagnon M, Yannic G, Perrier C, Côté SD (2019) No evidence of inbreeding depression in fast declining herds of migratory caribou. J Evol Biol 32:1368–1381. https://doi.org/10.1111/jeb.13533
Gebauer M, Crampton A, Shaw J, Laing I (2014) Meadowbank mine 2013 wildlife monitoring summary report. Nunavut Environmental Consulting Ltd., Baker Lake
Geffen E, Waidyaratne S, Dalén L et al (2007) Sea ice occurrence predicts genetic isolation in the Arctic fox. Mol Ecol 16:4241–4255. https://doi.org/10.1111/j.1365-294X.2007.03507.x
Glass TW, Breed GA, Iwahana G et al (2021a) Permafrost ice caves: an unrecognized microhabitat for Arctic wildlife. Ecology 102:1–4. https://doi.org/10.1002/ecy.3276
Glass TW, Breed GA, Liston GE et al (2021b) Spatiotemporally variable snow properties drive habitat use of an Arctic mesopredator. Oecologia 195:887–899. https://doi.org/10.1007/s00442-021-04890-2
Glass TW, Breed GA, Robards MD et al (2021c) Trade-off between predation risk and behavioural thermoregulation drives resting behaviour in a cold-adapted mesocarnivore. Anim Behav 175:163–174. https://doi.org/10.1016/j.anbehav.2021.02.017
Glass TW, Breed GA, Laird CR, et al (2022) Terrain features and architecture of wolverine (Gulo gulo) resting burrows and reproductive dens on Arctic tundra. Arctic 75:291–299. https://doi.org/10.14430/arctic75576
Golden HN, Henry JD, Becker EF et al (2007) Estimating wolverine Gulo gulo population size using quadrat sampling of tracks in snow. Wildlife Biol 13:52–61. https://doi.org/10.2981/0909-6396(2007)13[52:ewggps]2.0.co;2
Golder Associates Ltd (2017) Analysis of environmental effects from the diavik diamond mine on wildlife in the lac de gras region. Golder Associates Ltd., Vancouver
Haley S, Klick M, Szymoniak N, Crow A (2011) Observing trends and assessing data for Arctic mining. Polar Geogr 34:37–61. https://doi.org/10.1080/1088937X.2011.584449
Hardy TMP (1948) Wolverine fur frosting. J Wildl Manage 12:331–332
Harwood LA, Smith TG, Alikamik J et al (2020) Long-term, harvest-based monitoring of ringed seal body condition and reproduction in Canada’s Western Arctic: an update through 2019. Arctic 73:206–220. https://doi.org/10.14430/arctic70428
Hinzman LD, Bettez ND, Bolton WR et al (2005) Evidence and implications of recent climate change in Northern Alaska and other Arctic regions. Clim Change 72:251–298. https://doi.org/10.1007/s10584-005-5352-2
Hoekstra PF, Braune BM, Elkin B et al (2003a) Concentrations of selected essential and non-essential elements in arctic fox (Alopex lagopus) and wolverines (Gulo gulo) from the Canadian Arctic. Sci Total Environ 309:81–92. https://doi.org/10.1016/S0048-9697(02)00684-8
Hoekstra PF, Braune BM, Wong CS et al (2003b) Profile of persistent chlorinated contaminants, including selected chiral compounds, in wolverine (Gulo gulo) livers from the Canadian Arctic. Chemosphere 53:551–560. https://doi.org/10.1016/S0045-6535(03)00514-9
Hoover AK, Dickson DL (2007) Nesting Ecology and Survival of the Pacific Common Eider (Somateria mollissima v-nigra) in Central Arctic Canada. Canadian Wildlife Service, Edmonton
Huntington HP, Danielson SL, Wiese FK et al (2020) Evidence suggests potential transformation of the Pacific Arctic ecosystem is underway. Nat Clim Chang 10:342–348. https://doi.org/10.1038/s41558-020-0695-2
Ims RA, Henden JA, Killengreen ST (2008) Collapsing population cycles. Trends Ecol Evol 23:79–86. https://doi.org/10.1016/j.tree.2007.10.010
Inman RM, Magoun AJ, Persson J, Mattisson J (2012) The wolverine’s niche: linking reproductive chronology, caching, competition, and climate. J Mammal 93:634–644. https://doi.org/10.1644/11-MAMM-A-319.1
Jenkins DA, Lecomte N, Schaefer JA et al (2016) Loss of connectivity among island- dwelling Peary caribou following sea ice decline. Biol Lett 12:3–7. https://doi.org/10.1098/rsbl.2016.0235
Jenkins DA, Campbell M, Hope G, et al (2011) Recent trends in abundance of Peary caribou (Rangifer tarandus pearyi) and muskoxen (Ovibos moschatus) in the Canadian Arctic Archipelago, Nunavut. Department of Environment, Government of Nunavut, Pond Inlet
Johnson CJ, Boyce MS, Case RL et al (2005) Cumulative effects of human developments on Arctic wildlife. Wildl Monogr 160:1–36. https://doi.org/10.2193/0084-0173(2005)160[1:CEOHDO]2.0.CO;2
Johnson HE, Golden TS, Adams LG et al (2020) Caribou use of habitat near energy development in Arctic Alaska. J Wildl Manage 84:401–412. https://doi.org/10.1002/jwmg.21809
Jokinen ME, Webb SM, Manzer DL, Anderson RB (2019) Characteristics of Wolverine (Gulo gulo) dens in the lowland boreal forest of north-central Alberta. Can Field-Naturalist 133:1–15. https://doi.org/10.22621/cfn.v133i1.2083
Jung TS, Frandsen J, Gordon DC, Mossop DH (2016) Colonization of the Beaufort coastal plain by beaver (Castor canadensis): a response to shrubification of the tundra? Can Field-Naturalist 130:332–335. https://doi.org/10.22621/cfn.v130i4.1927
Keatts LO, Robards M, Olson SH et al (2021) Implications of Zoonoses from hunting and use of wildlife in North American Arctic and boreal biomes: pandemic potential, monitoring, and mitigation. Front Public Heal 9:1–27. https://doi.org/10.3389/fpubh.2021.627654
Kelly BP, Bengtson JL, Boveng PL, et al (2010) Status Review of the Ringed Seal (Phoca hispida). U.S. Department of Commerce, Springfield
Kohler J, Aanes R (2004) Effect of winter snow and ground-icing on a svalbard population: results of a simple snowpack model. Arctic Antarct Alp Res 36:333–341. https://doi.org/10.1657/1523-0430(2004)036[0333:EOWSAG]2.0.CO;2
Kolodeznikov VE (2013) Фayнa птиц и млeкoпитaющиx нoвocибиpcкиx ocтpoвoв (Bird and Mammal Fauna in New Siberian Islands). Bull North-Eastern Fed Univ 10:43–49
Kovacs KM, Lydersen C, Overland JE, Moore SE (2011) Impacts of changing sea-ice conditions on Arctic marine mammals. Mar Biodivers 41:181–194. https://doi.org/10.1007/s12526-010-0061-0
Krejsa DM, Talbot SL, Sage GK et al (2021) Dynamic landscapes in northwestern North America structured populations of wolverines (Gulo gulo). J Mammal 102:891–908. https://doi.org/10.1093/jmammal/gyab045
Kröger M (2019) The Global Land Rush and the Arctic. In: Heininen L, Finger M (eds) The globalarctic handbook. Springer, Cham, pp 27–43
Kukka PM, Jung TS, Robitaille JF, Schmiegelow FKA (2017) Temporal variation in the population characteristics of harvested wolverine (Gulo gulo) in northwestern Canada. Wildl Res 44:497–503. https://doi.org/10.1071/WR17063
Kukka PM, Jung TS, Schmiegelow FKA (2022) Spatiotemporal patterns of wolverine (Gulo gulo) harvest: the potential role of refugia in a quota-free system. Eur J Wildl Res. https://doi.org/10.1007/s10344-022-01566-x
Kukka PM, Jung TS (2016) Wolverine carcass collection project: 2013–2014 Annual report. Yukon Department of Environment, Whitehorse
Kumpula T, Forbes BC, Stammler F, Meschtyb N (2012) Dynamics of a coupled system: multi-resolution remote sensing in assessing social-ecological responses during 25 years of gas field development in Arctic Russia. Remote Sens 4:1046–1068. https://doi.org/10.3390/rs4041046
Kyle CJ, Strobeck C (2001) Genetic structure of North American wolverine (Gulo gulo) populations. Mol Ecol 10:337–347. https://doi.org/10.1046/j.1365-294X.2001.01222.x
Kyle CJ, Strobeck C (2002) Connectivity of peripheral and core populations of North American wolverines. J Mammal 83:1141–1150. https://doi.org/10.1644/1545-1542(2002)083%3c1141:COPACP%3e2.0.CO;2
L’Herault V (2018) Niche alimentaire et écologie du loup et du carcajou dans l’arctique canadien: Des analyses isotopiques au savoir Iniut (Dietary niche and ecology of wolves and wolverines in the Canadian Arctic: From isotopic analyses to Inuit knowledge). Dissertation, Université du Québec à Montréal
Lameris TK, van der Jeugd HP, Eichhorn G et al (2018) Arctic geese tune migration to a warming climate but still suffer from a phenological mismatch. Curr Biol 28:2467–2473. https://doi.org/10.1016/j.cub.2018.05.077
Landa A, Lindén M, Kokjola I (2000) Action Plan for the conservation of Wolverines (Gulo gulo) in Europe. Council of European Publishing, Strasbourg
Laruelle M (2020) Russia’s Arctic Policy: A Power Strategy and Its Limits. Institut Français des Relations Internationales, Paris
Laugrand F (2017) La perception du carcajou/glouton par les Inuit du Nord canadien: Du passé au présent (How Inuit in the Canadian North perceive the wolverine: from past to present). Études Inuit 41:243–263. https://doi.org/10.7202/1061440ar
Lee J, Niptanatiak A (1993) Ecology of the wolverine on the central arctic barrens. Department of Renewable Resources, Government of Northwest Territories, Yellowknife
Lee J, Niptanatiak A (1996) Observation of repeated use of a wolverine, Gulo gulo, den on the tundra of the Northwest Territories. Can Field-Naturalist 110:349–350
Lee J (1994) Wolverine harvest and carcass collection Coppermine, Bay Chimo and Bathurst Inlet, 1992/1993. Department of Renewable Resources, Government of Northwest Territories, Yellowknife
Lee J (2016) Database Description, Data Summary and Analysis of the Wolverine Harvest from Kugluktuk, Umingmaktok and Bathurst Inlet, Northwest Territories. Environment and Natural Resources, Government of Northwest Territories, Yellowknife
Li PI (2021) The graphic analysis of the first syllable vowel system in Nenets dictionaries from A M. Sjögren’s Archive. Ural Stud 1:47–60. https://doi.org/10.37892/2500-2902-2021-40-1-47-60
Magoun AJ (1987) Summer and winter diets of wolverines, Gulo gulo, in Arctic Alaska. Can Field-Naturalist 101:392–397
Magoun AJ, Copeland JP (1998) Characteristics of wolverine reproductive den sites. J Wildl Manage 62:1313–1320. https://doi.org/10.2307/3801996
Magoun AJ, Robards MD, Packila ML, Glass TW (2017) Detecting snow at the den-site scale in wolverine denning habitat. Wildl Soc Bull 41:381–387. https://doi.org/10.1002/wsb.765
Magoun AJ, Laird CR, Keech MA et al (2018) Predation on Caribou (Rangifer tarandus) by Wolverines (Gulo gulo) after long pursuits. Can Field-Naturalist 132:382–385. https://doi.org/10.22621/cfn.v132i4.2050
Magoun AJ (1985) Population characteristics, ecology, and management of wolverines in Northwestern Alaska. Dissertation, University of Alaska Fairbanks
Makridin VP (1964) O pacпpocтpaнeнии и биoлoгии pocoмaxи нa кpaйнeм ceвepe (On the distribution and biology of wolverine in the far north). Zool Zhurnal 43:1688–1692
Mallory CD, Boyce MS (2018) Observed and predicted effects of climate change on Arctic caribou and reindeer. Environ Rev 26:13–25. https://doi.org/10.1139/er-2017-0032
Mallory CD, Williamson SN, Campbell MW, Boyce MS (2020) Response of barren-ground caribou to advancing spring phenology. Oecologia 192:837–852. https://doi.org/10.1007/s00442-020-04604-0
Mallory ML, Akearok J, Fontaine AJ (2001) Community knowledge on the distribution and abundance of species at risk in southern Baffin Island, Nunavut, Canada. Canadian Wildlife Service, Iqaluit
Manning TH (1943) Notes on the mammals of South and Central West Baffin Island. J Mammal 24:47–59
Marquard-Petersen U (2009) Abundance, social organization, and population trend of the arctic wolf in north and east greenland during 1978–1998. Can J Zool 87:895–901. https://doi.org/10.1139/Z09-078
Mathonniere J (2019) The growth of Russian crude oil production. Independent Commodity Intelligence Services, London
McDonald JC, Gyrokos TW, Alberton B et al (1990) An outbreak of congenital toxoplasmosis in pregnant women in northern Quebec. J Infect Dis 161:769–774. https://doi.org/10.1093/infdis/161.4.769
McKelvey KS, Copeland JP, Schwartz MK et al (2011) Climate change predicted to shift wolverine distributions, connectivity, and dispersal corridors. Ecol Appl 21:2882–2897. https://doi.org/10.1890/10-2206.1
Mizin IA, Sipko TP, Davydov AV, Gruzdev AR (2018) The wild reindeer (Rangifer tarandus: Cervidae, Mammalia) on the Arctic islands of Russia: a review. Nat Conserv Res 3:1–14. https://doi.org/10.24189/ncr.2018.040
Moisan M (1996) Rappot sur la situation du carcajou (Gulo gulo) au Québec. Ministére de l’Environnement et de la Faune, Québec
Mowat G, Clevenger AP, Kortello AD et al (2020) The sustainability of wolverine trapping mortality in Southern Canada. J Wildl Manage 84:213–226. https://doi.org/10.1002/jwmg.21787
Mulders R, Boulanger J, Paetkau D (2007) Estimation of population size for wolverines Gulo gulo at daring lake, Northwest territories, using DNA based mark-recapture methods. Wildlife Biol 13:38–51. https://doi.org/10.2981/0909-6396(2007)13[38:EOPSFW]2.0.CO;2
Muus B, Salomonsen F, Vibe C (1981) Jærv. Grønlands Fauna: fisk. Fugle. Pattedyr. Gyldendal, Copenhagen, pp 390–391
Nelson RK (1983) Make prayers to the raven: a koyukon view of the Northern forest. The University of Chicago Press, Chicago
Nikolaeva I (2008) Chuvan and Omok languages? In: Houtzagers P, Kalsbeek J, Schaeken J (eds) Evidence and counter- evidence: essays in honour of frederik kortlandt. Rodopi, New York, pp 313–336
Oakley MP, Jung TS, Kukka PM, Robitaille JF (2016) Prevalence of renal calculi in wolverine (Gulo gulo) from northwestern Canada. Mamm Biol 81:189–193. https://doi.org/10.1016/j.mambio.2015.11.004
Owen MA, Pagano AM, Wisdom SS et al (2021) Estimating the audibility of industrial noise to denning polar bears. J Wildl Manage 85:384–396. https://doi.org/10.1002/jwmg.21977
Parker GR, Luttich S (1986) Characteristics of the Wolf (Canis lupus labradorius Goldman) in Northern Quebec and Labrador. Arctic 39:145–149. https://doi.org/10.14430/arctic2062
Perreault N, Lévesque E, Fortier D et al (2017) Remote sensing evaluation of High Arctic wetland depletion following permafrost disturbance by thermo-erosion gullying processes. Arct Sci 3:237–253. https://doi.org/10.1139/as-2016-0047
Persson J (2005) Female wolverine (Gulo gulo) reproduction: reproductive costs and winter food availability. Can J Zool 83:1453–1459. https://doi.org/10.1139/z05-143
Peters GP, Nilssen TB, Lindholt L et al (2011) Future emissions from shipping and petroleum activities in the Arctic. Atmos Chem Phys 11:5305–5320. https://doi.org/10.5194/acp-11-5305-2011
Poley LG, Magoun AJ, Robards MD, Klimstra RL (2018) Distribution and occupancy of wolverines on tundra, northwestern Alaska. J Wildl Manage 85:991–1002. https://doi.org/10.1002/jwmg.21439
Post E, Bhatt US, Bitz CM et al (2013) Ecological consequences of sea-ice decline. Science 341:519–524. https://doi.org/10.1126/science.1235225
Rausch RA (1959) Studies of the Helminth Fauna of Alaska. XXXVI. Parasites of the Wolverine with observations of the biology of Taneia twitchelli. J Parasitol 45:465–484
Rausch RA, Pearson AM (1972) Notes on the Wolverine in Alaska and the Yukon territory. J Wildl Manage 36:249–268. https://doi.org/10.2307/3799057
Raynolds MK, Walker DA, Ambrosius KJ et al (2014) Cumulative geoecological effects of 62 years of infrastructure and climate change in ice-rich permafrost landscapes, Prudhoe Bay Oilfield, Alaska. Glob Chang Biol 20:1211–1224. https://doi.org/10.1111/gcb.12500
Raynolds MK, Jorgenson JC, Jorgenson MT et al (2020) Landscape impacts of 3D-seismic surveys in the Arctic National Wildlife Refuge, Alaska. Ecol Appl 30:1–20. https://doi.org/10.1002/eap.2143
Reichard MV, Torretti L, Garvon JM, Dubey JP (2008a) Prevalence of antibodies to Toxoplasma gondii in wolverines from Nunavut, Canada. J Parasitol 94:764–765. https://doi.org/10.1645/GE-1497R.1
Reichard MV, Torretti L, Snider TA et al (2008b) Trichinella T6 and Trichinella nativa in Wolverines (Gulo gulo) from Nunavut, Canada. Parasitol Res 103:657–661. https://doi.org/10.1007/s00436-008-1028-y
Rico Y, Morris-Pocock J, Zigouris J et al (2015) Lack of spatial immunogenetic structure among wolverine (Gulo gulo) populations suggestive of broad scale balancing selection. PLoS ONE 10:1–21. https://doi.org/10.1371/journal.pone.0140170
Ruscio BA, Brubaker M, Glasser J et al (2015) One health—a strategy for resilience in a changing arctic. Int J Circumpolar Health 74:1–10. https://doi.org/10.3402/ijch.v74.27913
Saalfeld ST, McEwen DC, Kesler DC et al (2019) Phenological mismatch in Arctic-breeding shorebirds: Impact of snowmelt and unpredictable weather conditions on food availability and chick growth. Ecol Evol 9:6693–6707. https://doi.org/10.1002/ece3.5248
Samelius G, Alisauskas RT, Larivière S et al (2002) Foraging behaviours of wolverines at a large arctic goose colony. Arctic 55:148–150. https://doi.org/10.14430/arctic699
Schmelzer I (2006) Occurrence and Distribution of Wolverines in Northern Labrador: An Aerial Survey to Clarify Status and Focus Recovery. Department of Environment and Conservation, Government of Newfoundland and Labrador, Corner Brook
Scrafford M, Boyce M (2015) Effects of industrial development on wolverine (Gulo gulo) ecology in the boreal forest of northern Alberta - Progress Report 2014/2015. University of Alberta
Serebryakov VF (1983) Лoгoвa pocoмaxи в бoльшeзeмeльcкoи тyндpe (Wolverine dens in Bolshezemelsky tundra). Zool Zhurnal 953–955
Sharma R, Parker S, Elkin B et al (2019) Risk factors and prevalence of antibodies for Toxoplasma gondii in diaphragmatic fluid in wolverines (Gulo gulo) from the Northwest Territories, Canada. Food Waterborne Parasitol 12:1–8. https://doi.org/10.1016/j.fawpar.2019.e00056
Sharma R, Thompson PC, Hoberg EP et al (2020) Hiding in plain sight: discovery and phylogeography of a cryptic species of Trichinella (Nematoda: Trichinellidae) in wolverine (Gulo gulo). Int J Parasitol 50:277–287. https://doi.org/10.1016/j.ijpara.2020.01.003
Sharma R, Harms NJ, Kukka PM et al (2021) High prevalence, intensity, and genetic diversity of Trichinella spp. in wolverine (Gulo gulo) from Yukon. Canada Parasit Vectors 14:1–9. https://doi.org/10.1186/s13071-021-04636-2
Starova O, Kozhechkin V, Kazmin V (2014) Boлк и pocoмaxa o. Bpaнгeлa (Wolf and wolverine on Wrangel Island). Oxoтa и oxoтничьe xoзяйcтвo (Hunting Hunt Econ 10:12–14
Tape KD, Christie K, Carroll G, O’Donnell JA (2016a) Novel wildlife in the Arctic: the influence of changing riparian ecosystems and shrub habitat expansion on snowshoe hares. Glob Chang Biol 22:208–219. https://doi.org/10.1111/gcb.13058
Tape KD, Gustine DD, Ruess RW, Adams LG (2016b) Range expansion of moose in Arctic Alaska linked to warming and increased shrub habitat. PLoS ONE 11:1–12. https://doi.org/10.1371/journal.pone.0152636
Tape KD, Grosse G, Jones BM et al (2018) Tundra be dammed: Beaver colonization of the Arctic. Glob Chang Biol. https://doi.org/10.1111/gcb.14332
Terekhina A, Volkovitskiy A, Sokolova N et al (2021) The context of an emerging predation problem: nenets reindeer herders and Arctic foxes in Yamal. Eur J Wildl Res 67:1–13. https://doi.org/10.1007/s10344-021-01497-z
Tomaselli M, Kutz S, Gerlach C, Checkley S (2018) Local knowledge to enhance wildlife population health surveillance: conserving muskoxen and caribou in the Canadian Arctic. Biol Conserv 217:337–348. https://doi.org/10.1016/j.biocon.2017.11.010
Tomasik E, Cook JA (2005) Mitochondrial phylogeography and conservation genetics of wolverine (Gulo gulo) of northwestern North America. J Mammal 86:386–396. https://doi.org/10.1644/BER-121.1
van Zyll de Jong CG (1975) The Distribution and Abundance of the Wolverine (Gulo gulo) in Canada. Can Field-Naturalist 89:431–437
Vangen KM, Persson J, Landa A et al (2001) Characteristics of dispersal in wolverines. Can J Zool 79:1641–1649. https://doi.org/10.1139/cjz-79-9-1641
Vekhov NV (1999) Ecological consequences of hunting, trapping, and fishing on the Novaya Zemlya archipelago. Polar Geogr 23:309–319. https://doi.org/10.1080/10889379909377682
Walker DA, Raynolds MK, Daniëls FJA et al (2005) The circumpolar arctic vegetation map. J Veg Sci 16:267–282
Watson SE, Hailer F, Lecomte N et al (2020) Parasites of an Arctic scavenger; the wolverine (Gulo gulo). Int J Parasitol Parasites Wildl 13:178–185. https://doi.org/10.1016/j.ijppaw.2020.10.004
Watson SE (2020) Arctic health: Investigating the gut microbiota and parasite diversity of Arctic species. Dissertation, Cardiff University
Webb SM, Anderson RB, Manzer DL et al (2016) Distribution of female wolverines relative to snow cover, Alberta, Canada. J Wildl Manage 80:1461–1470. https://doi.org/10.1002/jwmg.21137
Weinstein C (2018) Chukchi-French-English-Russian dictionary. Anadyr. https://unesdoc.unesco.org/ark:/48223/pf0000367748
Wilson RR, Durner GM (2020) Seismic Survey Design and Effects on Maternal Polar Bear Dens. J Wildl Manage 84:201–212. https://doi.org/10.1002/jwmg.21800
Wilson N, Zarnke RL (1985) Occurrence of the ear canker mite, Otodectes cynotis (Hering), on the wolverine, Gulo gulo (L.). J Wildl Dis 21:180. https://doi.org/10.7589/0090-3558-21.2.180
Wilson GM, Van Den Bussche RA, Kennedy PK et al (2000) Genetic variability of wolverines (Gulo gulo) from the Northwest Territories, Canada: conservation implications. J Mammal 81:186–196. https://doi.org/10.1644/1545-1542(2000)081%3c0186:GVOWGG%3e2.0.CO;2
Wohlin C (2014) Guidelines for snowballing in systematic literature studies and a replication in software engineering. In: ACM International Conference Proceeding Series. Association for Computing Machinery
Yu Q, Epstein H, Engstrom R, Walker D (2017) Circumpolar arctic tundra biomass and productivity dynamics in response to projected climate change and herbivory. Glob Chang Biol 23:3895–3907. https://doi.org/10.1111/gcb.13632
Zhou J, Tape KD, Prugh L et al (2020) Enhanced shrub growth in the Arctic increases habitat connectivity for browsing herbivores. Glob Chang Biol 26:3809–3820. https://doi.org/10.1111/gcb.15104
Zigouris J, Schaefer JA, Fortin C, Kyle CJ (2013) Phylogeography and post-glacial recolonization in wolverines (Gulo gulo) from across their circumpolar distribution. PLoS ONE 8:1–13. https://doi.org/10.1371/journal.pone.0083837
Acknowledgements
We are grateful to M. Awan, E. Bragina, D. Johnson, C. Latty, U. Marquard-Petersen, M. Meldgaard, J. Ray, M. Scrafford, E. Stacy, R. Stimmelmayr, G. Szor, and F. Ugarte for reviewing early versions of this manuscript or providing regional expertise. T.S. Jung and an anonymous reviewer generously provided feedback that strengthened this work.
Funding
A National Science Foundation Graduate Research Fellowship under Grant No. 1650114 provided salary support to T. Glass while writing this manuscript and Wilburforce Foundation supported M. Robards’ contributions.
Author information
Authors and Affiliations
Contributions
TG reviewed literature and drafted the original manuscript; AM, KK, and MR edited the original draft and contributed additional text.
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no competing interests relevant to the content of this article.
Research involving human and animals rights
This article did not involve research with live animals.
Consent to participate
This article did not involve research with human subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Glass, T.W., Magoun, A.J., Robards, M.D. et al. Wolverines (Gulo gulo) in the Arctic: Revisiting distribution and identifying research and conservation priorities amid rapid environmental change. Polar Biol 45, 1465–1482 (2022). https://doi.org/10.1007/s00300-022-03079-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03079-4