Abstract
Changes in habitat availability and prey abundance are predicted to adversely influence survival and reproduction of wildlife in the Southern Ocean. Some populations of southern right whale (SRW; Eubalaena australis) are showing dramatic changes in habitat use. Surveys were undertaken in the austral winters of 2020 and 2021 at the key nursery and socialising ground for New Zealand SRWs: Port Ross, Auckland Islands, with 548 encounters and 599 skin biopsy samples collected. Data from these two surveys spanned peak periods of use and were used to test the hypothesis there have been shifts in the phenology, demographic composition and behaviour of SRWs using the Auckland Islands over the past three decades. The behavioural phenology and demographic composition of SRW resembles that observed in the 1990s. In contrast, the proportion of groups containing cow-calf pairs increased from 20% in the 1998 survey to 50% in 2020/21. These changes are consistent with a growing population undergoing strong recruitment, not limited by food resources. Continued use of Port Ross by all SRW demographic classes confirms this as key habitat for SRW in New Zealand waters, and we support increased enforcement of existing management measures to reduce whale-vessel interactions in this remote subantarctic archipelago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctic and subantarctic ecosystems are facing a variety of complex human-mediated pressures including climate change, invasive species, and resource exploitation (Chown and Brooks 2019). The cumulative impact of such anthropogenic pressures will vary by region and species (Bestley et al. 2020). Expected changes include alterations in population dynamics, behaviour, phenology and habitat use (Trathan et al. 2007; Hindell et al. 2020).
Long-lived predators, such as marine mammals, can serve as indicators of the overall ecosystem structure and function due to the comparable ease of monitoring their distribution and abundance, compared with lower trophic levels (Weimerskirch et al. 1993; Hindell et al. 2003, 2020; Taylor et al. 2007b). The population dynamics of baleen whale species, including right whales (e.g., Meyer-Gutbrod et al. 2015; Record et al. 2019b), humpback whales (e.g., Fleming et al. 2016), and fin whales (e.g., Ramp et al. 2015; Jory et al. 2021) have been found to respond to environmental change, making them valuable sentinels of ecosystem health in polar regions. Furthermore, recovering populations of large whales may provide important ecosystem services such as nutrient recycling and carbon storage (Nicol et al. 2010; Roman and McCarthy 2010; Ratnarajah et al. 2014, 2017, 2018).
Here we focus on the southern right whale (Eubalaena australis, hereafter SRW), a Southern Ocean predator for which changes in reproductive success, foraging ecology and habitat use have been observed in recent years (Harcourt et al. 2019; Carroll et al. 2020). SRW are migratory baleen whales, moving between coastal winter nursery/calving grounds (hereafter calving) and socializing grounds (i.e., areas where social and/or sexual activity is observed, such as surface active groups) (Kraus and Hatch 2001), and offshore summer feeding grounds (International Whaling Commission (IWC) 1986). The species typically shows fidelity to natal wintering grounds, with both sexes returning to the same region to breed, and with females calving at approximately 3 year intervals (Burnell 2001; Rowntree et al. 2001; Carroll et al. 2013, 2016; Charlton 2017; Davidson et al. 2018).
In the southwest Atlantic, research shows that there is a strong association between environmental conditions and food availability at high latitude feeding grounds and reproductive success and population recovery at wintering grounds (Leaper et al. 2006; Seyboth et al. 2016). In the southeast Atlantic, there have been recent changes in population dynamics, such that fewer unaccompanied adults have been observed returning to the South African wintering grounds (Vermeulen et al. 2020) concurrent with an increase in the calving interval from 3 years to 4 or 5 years (Brandão et al. 2018). Stable isotope analyses have revealed a change in foraging grounds coincident with this shift in population dynamics. For example, in South Africa the whales are now feeding further north and on a wider variety of prey types than the previously suspected key prey, Antarctic krill (van den Berg et al. 2021). A similar increase in calving interval, from 3 to 4 years on average, is estimated to have occurred since 2015 in the Head of the Bight calving ground, Australia (Charlton et al. 2022).
SRWs have also shown noticeable shifts in wintering habitat use over time. This has been seen on a small scale, with the highest concentrations of whales on the wintering grounds at Península Valdés, Argentina, shifting from the outer coast of the Península to two adjacent Gulfs (Rowntree et al. 2001). Increasing density of SRWs has also been documented to result in shifts in distribution of different demographic classes, with cow-calf pairs preferentially inhabiting shallow coastal waters over socializing adults (Crespo et al. 2019). Changes in habitat use are also occurring over larger scales. For example, the Falkland Islands/Islas Malvinas have become an important migratory habitat for SRW over the last few winters (Weir and Stanworth 2020).
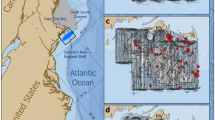
In this study, we focus on New Zealand SRWs (Tohorā nō Aotearoa in the Māori language) that winter in Port Ross, Auckland Islands Maungahuka (hereafter Auckland Islands, Fig. 1). Port Ross is a long, shallow harbour (~ 13 km long and up to ~ 2 km wide; typically < 30 m deep) shaped by glaciers over repeated glaciation cycles with varied substrate types (Tidey and Hulbe 2018; Rainsley et al. 2019). SRWs in this region have been subject to long-term genetic monitoring with surveys during the austral winters of 1995–1998 and 2006–2009 (Patenaude and Baker 2001; Carroll et al. 2013). This research program has shown that the New Zealand SRW population is a single breeding stock that uses wintering habitat around the Auckland Islands (Patenaude and Baker 2001; Carroll et al. 2013), Campbell Island (Stewart and Todd 2001; Torres et al. 2017), and mainland New Zealand (Carroll et al. 2014b). Earlier modelling estimated that the New Zealand SRW stock had an abundance of approximately 2300 whales for the period of 1995–2009, a time interval during which the population was growing at 7% per annum (95% CI: 5, 9%) (Carroll et al. 2013). The Port Ross region of the Auckland Islands (Fig. 1) is the key calving and socialising ground for the New Zealand SRW population (Patenaude and Baker 2001), and is demographically and genetically distinct from populations that use other wintering grounds around the Southern Hemisphere (Patenaude et al. 2007; Carroll et al. 2019).
This recovery from whaling, from as few as 40 whales in 1920 as a result of historical and illegal Soviet whaling in the 1960s (Tormosov et al. 1998; Smith et al. 2012; Carroll et al. 2014a), has prompted a revision and improvement in New Zealand threat status of SRW from Nationally Endangered to At Risk—Recovering in 2019 (Carroll et al. 2013; Jackson et al. 2016; Baker et al. 2019). We undertook surveys at the Auckland Islands in the austral winters of 2020 and 2021 to continue the genetic monitoring work and to further investigate recovery and foraging ecology. Information was collected to test the hypothesis that there have been shifts in the phenology, demographic composition and behaviour of SRWs using the Auckland Islands over the past three decades. Here we summarise group composition and behaviour of whales per week over the 2020 and 2021 survey periods and compare our results to similar data collected in 1998, the only previous field survey that comprehensively surveyed the full wintering period (Patenaude and Baker 2001; Patenaude 2002). We also provide an overall summary of the field effort and data collection of the 2020 and 2021 field seasons, and compare it more broadly to surveys conducted during 2008 and the 1990s (Patenaude and Baker 2001; Patenaude 2002; Childerhouse and Dunshea 2008; Childerhouse et al. 2009).
Materials and methods
Field methods
Field work dates
Fieldwork was conducted in Port Ross, Auckland Islands (50° 32′ S, 166° 15′ E: Fig. 1), with the field season spanning 1–18 August 2020 (living aboard the SV Evohe) and from 27 June to 27 July 2021 (MV Strannik: 27 June–12 July; SV Evohe: 12 July–27 July 2021). Below we detail methodology for vessel-based surveys to provide 1-day counts, a trial drone-based survey approach, and small boat surveys to collect encounter and photo-identification (photo-ID) data, and skin biopsy samples, activities which were conducted when weather permitted.
Vessel-based surveys to provide one-day counts
A non-systematic survey of Port Ross was conducted from the top deck of the MV Strannik (3.6 m above sea level) on 10 July 2021, following methods broadly similar to that first used by Patenaude (2002) in Port Ross in 1995. The vessel sailed through Port Ross at 5–10 knots, with one experienced observer on the port side and another on the starboard side of the vessel searching for whales. As the whale passed abeam, a waypoint was made on a handheld GPS, and the group size and composition and an estimated distance to the whales was recorded. A whale was considered to be a calf if it was less than half the length of an accompanying adult. A sighting was considered to include only one individual unless additional whales were counted at the surface within one and a half whale body lengths (Patenaude 2002). While surveying, whale behaviour was not specifically recorded. The vessel’s track and SRW groups were plotted using QGIS (version 3.4) by offsetting each group sighting waypoint on the vessel’s track to the side they were recorded on (port or starboard) by their estimated distance from the vessel. Error in sightings distance was not considered, but the scale of plotting means that the dots represents a space ~ 150 m in diameter, likely soaking up some variance in these estimates. Furthermore, we plotted results from a survey following the same methodology conducted in 2008 on the SV Evohe (~ 3 m above sea level; Childerhouse and Dunshea 2008) to allow a qualitative comparison across time.
Drone-based survey
Drone-based surveys potentially offer a more efficient way to survey Port Ross. Accordingly, Port Ross was divided into 18 sections (Online Resource Fig. S1) that were flown with a DJI Mavic Pro2 in 2020. Using the inbuilt Hasselblad camera and Ground Station Pro mapping software, photos were taken at a height of 100 m with a 20% overlap between successive images. Images at this height resulted in a GSD (ground sample distance) of 2.54 cm px−1, thus the dimensions of each photo were approximately 139 m (w) × 93 m (h), taken approximately 45 m ± 5 m apart (variance due to wind error). In 2021, the same method was used for an expanded grid survey in Sandy Bay. No attempt was made to correct for detection probability of whales, as this initial trial was to determine feasibility of drone surveys in a region that is characterised by frequent high winds and rain.
Small boat surveys to collect encounter and photo-identification, and biopsy samples
Small, rigid-hulled inflatable boats (RHIB; 4–5 m) were used to collect photo-ID images and skin biopsy samples, following methods used in previous surveys, including the 1998 field season (Patenaude et al. 1998; Carroll et al. 2013). Briefly, skin biopsy samples were collected using small, stainless steel biopsy darts deployed from a crossbow or a modified veterinary capture device (Lambertsen 1987; Krützen et al. 2002). Satellite tagging of 17 whales was also conducted during this field work, but results will be presented in a future paper. During each encounter, defined as a close (i.e., < 25 m) approach during which a biopsy sample was taken, the number of whales in the group, group composition (e.g., adult, cow-calf pair) and pre-approach behaviour were recorded.
Behaviour definitions were adapted from Patenaude & Baker (2001):
-
(1)
Log: whale is resting at the surface, only moving slightly to breath.
-
(2)
Travel: directional forward movement that resulted in change of location.
-
(3)
Social: two or more non-calves interacting at the surface.
-
(4)
Milling: movement that is not directional in nature.
We also recorded all whales seen with unusual pigmentation or suspected anthropogenic scarring during encounters, and photographs of these features were taken where possible. Other observations were also recorded where time allowed, including the presence of other vessels in Port Ross and interactions between the whales and other species.
Analysis methods
Difference in habitat use by groups with and without cow-calf pairs within 2020 and 2021 field seasons
We investigated whether habitat use patterns within Port Ross differed between groups with and without cow-calf pairs using the non-parametric kernel utilisation density approach (Worton 1989). First, we created a 50 × 50 m spatial grid that covered the study area using QGIS (version 3.4). Kernel density estimates of whale space use were computed from encounter locations across the combined field seasons of 2020 and 2021. Following Rayment et al. (2012) we used a search radius of 1,000 m in order to confine the kernel density estimates to Port Ross. This ensures we do not predict density outside the area where data were collected. Kernel density output (cell size 10 m) was then matched to the 50 × 50 m grid by averaging raster cell values within a given grid cell using the 'zonal statistics' tool in QGIS. Whale density was adjusted for search effort by dividing each grid cell’s whale density value by the total distance (in meters) spent travelling inside each cell of the predetermined 50 × 50 m grid. This index of whale density per effort was normalised within each reproductive group (groups with and without cow-calf pairs) to range between zero and one, to allow for easy visual comparison of high-use areas.
We then investigated whether there was spatial clumping in the encounter data by examining the occurrence of groups with and without calves along the survey tracks of research boats. Specifically, we calculated the difference in mean distances between animals in each encounter of the same type (e.g., groups with calves to groups with calves) and encounters of different type (e.g., groups with calves to groups without calves). We compared these mean values to simulated distributions, which were taken by sampling the inter-group distances regardless of group type, to see if the mean distances to groups of the same type were smaller compared with the overall dataset. This was implemented in the statistical programming language ‘R’ (version 4.0 R Core Team 2021).
Changes in demographic composition and behaviour of groups within 2020 and 2021 field seasons
The field season was divided into approximately weekly blocks spanning the wintering period; early- to mid-wintering season: late June to late July for the 2021 season; and late wintering season: early to late August for the 2020 season. The group composition, pre-approach behaviour, and presence of cow-calf pairs was plotted for each week over the winter period. The location of each group encountered was also plotted for each week block.
Changes in demographic composition and behaviour of groups across decades
Combined, the 2020 and 2021 surveys span approximately the same wintering period as the 1998 survey: late June to late August (Patenaude and Baker 2001). This allowed for an assessment of the change in group composition and behaviour across the wintering period between decades. This was not possible previously as other surveys started in late July and lasted 2–3 weeks (Patenaude 2002; Carroll et al. 2013).
The 1998 survey data used the same definitions for log (rest), travel, and socialising but had additional categories: approached boat (whale altered course to actively investigate research vessel) which we subsumed into travel; play (above surface activity not apparently linked to social activity) which we did not regularly record but would have been captured by the behavioural category milling. We qualitatively compared the change in group behaviour and demographic composition across the wintering period for the 1998 and combined 2020 and 2021 datasets. We quantitatively compared the proportion of encounters with and without cow-calf pairs and proportions of socialising and non-socialising behaviours between the two datasets using a χ2 test in R. Quantitative weekly comparisons were not possible as the 1998 data are only available on an aggregated rather than on a per week basis.
Results
Field work summary
Over the two field seasons, there were 548 encounters with SRWs during which 599 skin biopsy samples were collected (Table 1). On average, encounters were 5.5 min long (standard error, SE, ± 0.3 min, n = 548), although the distribution has a substantial leftward skew with a mode of 1 min. The longest two encounters (40 and 52 min) were with large social groups (minimum 6 adult whales). The duration of the longest encounter was due to the concurrent collection of photogrammetry and matched biopsy, which was achieved from 3 adult whales.
Vessel-based Port Ross surveys
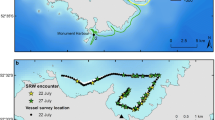
The non-systematic survey on 10 July 2021 counted 157 whales in 86 groups, of which 35 were cow-calf pairs (Fig. 2a). The 2008 survey was conducted on 29 July, and counted 213 whales, including 57 cow-calf pairs (Fig. 2b).
Southern right whale (Eubalaena australis) sightings during vessel-based surveys in Port Ross. a Map of survey track of the MV Strannik on 10 July 2021, with location and composition of southern right whale groups, b map of approximate survey track of the SV Evohe on 29 July 2008, with location and composition of southern right whale groups sighted. b Has been modified from Childerhouse & Dunshea (2008)
Drone-based surveys
In 2020, we undertook drone-based surveys of six grids (Online Resource Fig. S1). Review of the images showed that between 0 and 27 whales were available for image collection per grid (Online Resource Table S1).
Gull harassment
During the 2020 expedition, we noted observations of occasional red bill gull (tarāpunga in the Māori language, Larus novaehollandiae) harassment of SRW at Port Ross. There was no mention of such harassment in the previous surveys (Patenaude and Baker 2001; Patenaude 2002; Childerhouse et al. 2009). In 2021, we observed more of these interactions than in 2020 (Fig. 3). On multiple, separate, occasions per day of field effort we observed gulls landing primarily on calves but also on adult SRWs, walk on their backs and peck at either the skin and/or on the callosities populated with cyamid whale lice (Cyamus spp.). This was also observed as a group activity, with up to four gulls harassing a single calf. Adult whales were observed strongly reacting to the gulls landing on their back, by raising their head and tail in what is termed the galleon pose (Marón et al. 2015; Azizeh et al. 2021). However, no lesions or sores were observed on SRW, in contrast to reports for the Argentinean SRW population (Marón et al. 2015).
Fishing vessels observed in Port Ross and Carnley Harbour
Two different commercial fishing vessels were observed visiting and anchoring in Port Ross during the 2021 field season. The vessels stayed several days each. One vessel transited Port Ross in the dark at approximately 5 kn. The Regional Coastal Plan: Kermadec and Subantarctic Islands (2017: https://www.doc.govt.nz/about-us/our-role/managing-conservation/coastal-management/regional-coastal-plan-kermadec-and-subantarctic-islands/) states “A bow watch for whales is kept on vessels entering, transiting and departing Port Ross between 1 April and 31 October inclusive”; vessels transiting in the dark are unlikely to have such a watch, and if they do, it is likely to be ineffective at sighting whales. Five other fishing vessels were observed anchored in Carnley Harbour in 2021.
Human impacts
In the 2021 field season, five whales were observed with scarring or injuries that are consistent with anthropogenic impacts. No similar injuries were observed in 2020. Photographs were obtained for three (Fig. 4) of these whales. Images were reviewed by Dr Michael Moore, a marine mammal pathologist from the Woods Hole Oceanographic Institution, who provided an assessment of the injury and the potential cause (Table 2). One whale was observed with a fresh injury (i.e., it did not show sign of healing or repair) the morning after a fishing vessel was observed transiting Port Ross in the dark (Fig. 4b).
Southern right whales (Eubalaena australis) with scarring or fresh wounds. a Minor healed scars along the dorsal ridge of the peduncle, b abrasion caudal to and involving a post-blowhole callosity and c multiple linear healed incisions, bisected by a linear furrow perpendicular to the incisions. Photographs taken in Port Ross, Auckland Islands in July 2021
Similar habitat use patterns by groups with and without cow-calf pairs
Kernel density analysis of data collected over the whole wintering period showed that areas of high density were very similar for groups with and without calves (Fig. 5, Online Resource Figs. S2 and S3). That is, there was no evidence of spatial clumping of groups with or without calves. This was confirmed with the results of the permutation test, which did not find a significant difference in distance to groups of the same or different type (1000 permutations, p > 0.05).
Search effort adjusted southern right whale (Eubalaena australis) density (2020 and 2021 seasons combined). a Groups including cow-calf pairs and b groups with only adult individuals. The kernel density estimtates are calculated in 50 × 50 m grid cells. The colour-scale is directly comparable between a and b; darkest red colour indicates most preferred areas, and darkest blue colour indicates least preferred areas for each group type
Changes in demographic composition and behaviour of groups within 2020 and 2021 field seasons
Encounter data were plotted by week along the wintering period, spanning 7 weeks: 4 weeks from late June to late July in 2021 and 3 weeks from early to late August in 2020 (Figs. 6, 7). Earlier in the season, there is a trend towards higher mean and maximum group size, with two SRWs becoming the mean and mode of the group size later in the season (Table 3, Fig. 6). This decline in group size happens concurrently to the proportion of cow-calf pairs increasing (Table 3). The proportion of encounters with whales engaged in socialising also changes over the course of the season (Fig. 7). The proportion of groups where socialising is the pre-approach behaviour increases until weeks 3 and 4 (mid-late July) and then decreases again (Fig. 7).
When group composition and behaviour were plotted by location over the week blocks (Online Resource Fig. S2), there is no obvious distinction between where groups with and without calves were encountered. Indeed, shallow, sheltered areas like Terror and Erebus Cove that are typically considered key areas appear to be areas highly used by adult-only groups earlier in the winter season. The exception is Laurie Harbour, which seems to have comparably fewer cow-calf pairs than adult-only groups across the season.
Comparison of encounter data from 2020/21 and 1998
The proportion of encounters of the 2020 and 2021 and 1998 field survey by group size, composition and behaviour are shown in Fig. 8 and Online Resource Table S2. Socialising, which had the same definition across both survey periods, occurred in very similar proportions across survey periods in groups both with and without cow-calf pairs. Logging had the same definition and very similar incidence across survey periods for groups without cow-calf pairs. The incidence of logging in the 1998 surveys has similar incidence to logging plus milling in the 2020/21 surveys for groups with cow-calf pairs. This likely reflects that if the cow was resting but calf moving, it was classified as logging in 1998, but this behaviour would have been likely to be classified as milling in 2020/21.
Summary of southern right whale (Eubalaena australis) pre-approach behaviour by group size and category for the 1998 field season, and the combined 2020 and 2021 field seasons. Shown are the percentage of groups observed with each behaviour. The 1998 data are from Patenaude and Baker (2001)
Differences were observed in the proportion of groups with cow-calf pairs observed in the overall 2020/21 vs 1998 dataset. The 1998 survey had a significantly smaller proportion of groups with cow-calf pairs (93 with cow-calf pairs; 275 without) compared with the 2020/21 field season (273 with and 275 without, χ2 test: χ1 statistic = 54.27, p < 0.001). There was also a significantly smaller proportion of social groups in the 2020/21 dataset (121 social vs 427 non-social) compared with 1998 (128 vs 179, respectively, χ2 test: χ1 statistic = 35.73, p < 0.001).
Discussion
Here we show that Port Ross, Auckland Islands, is increasingly important as the key calving and socialising ground for SRWs in New Zealand waters. Earlier recognition of this region contributed to the Auckland Islands being listed as a no-take marine reserve, marine mammal sanctuary (established in 1993), and part of the New Zealand Subantarctic Islands IUCN Important Marine Mammal Area (IMMA, established in 2020, https://www.marinemammalhabitat.org/imma-eatlas/) (Patenaude et al. 1998; Carroll et al. 2013). The only other New Zealand subantarctic region where SRWs are frequently seen, Campbell Island, appears to be an important socialising area, but no cow-calf pairs have been reported in this region this century (Stewart and Todd 2001; Torres et al. 2017).
Many key SRW winter habitats are considered primarily socialising grounds (e.g., Uruguay; Costa et al., 2007; or Falkland Islands/Las Malvinas; Weir & Stanworth, 2020), or calving grounds (e.g., Warrnambool, Victoria, Australia: Watson et al. 2021). Other wintering grounds have SRWs distributed along an expanse of coastline, with (e.g., Australia; Carroll et al. 2015; Charlton et al. 2019; Watson et al. 2021; Kemper et al. 2022) or without (e.g., South Africa; Best 2000) spatial segregation of demographic classes. The Auckland Islands has all demographic classes present together in ~ 24 km2, documented here and in previous surveys (Patenaude and Baker 2001; Carroll et al. 2011), making it unique. This indicates that data collected at the Auckland Islands is still representative of the whole New Zealand population, consistent with earlier work showing individual matches between the mainland and the subantarctic islands (Carroll et al. 2014a, b).
Limited variability in phenology across three decades
Growing populations of baleen whales can change habitat use and distribution patterns (e.g., SRW and humpbacks whales; Rowntree et al. 2001; Dulau et al. 2017; Weir and Stanworth 2020). Here we have found that the phenology of the whales using the Auckland Islands remained fairly constant across three decades. Overall, the 2020 and 2021 surveys showed that the change in demographic composition of SRWs over the winter season broadly followed the trend described in the 1998 surveys (Patenaude and Baker 2001; Patenaude 2002). Cow-calf pairs increased over the 1998 winter season, and peak abundance of unaccompanied adults and cow-calf pairs was in mid-July and mid-August, respectively (Patenaude 2002). This is qualitatively similar to what we documented in 2020 and 2021, with the proportion of groups socialising being highest in mid-July, and an increase in the proportion of cow-calf pairs over the season, peaking during the week of 12–18 August 2020. Pre-approach behaviour of the groups, categorised by group size and presence/absence of cow-calf pairs, was very similar across the survey periods. Using data collected in 1998, Fewster & Patenaude (2009) estimated residency for cows and non-cows in the Auckland Islands was 31 and 37 days, respectively. Port Ross provides key resting areas for groups with and without cow-calf pairs, and is still an important region for socialising whales in 2020 and 2021.
The consistency of timing across decades is similar to that observed in some other SRW wintering grounds, where aerial surveys have been undertaken at the same time each year for decades to count and photo-ID cow-calf pairs (Bannister 2011; Charlton et al. 2019). The mid-August timing of peak cow-calf abundance is similar to that of the Head of Bight winter calving grounds in Australia, which have shown consistent timing in peak of abundance across two decades (Charlton et al. 2019). By contrast, in the South Atlantic, the timing of peak abundance is slightly different to that in the Indo-Pacific and has shown changes in recent years. In Argentina, the peak abundance is now a month earlier, late August to mid-September (Crespo et al. 2019) compared with mid-September to mid-October from 1971 to 1990 (Rowntree et al. 2001), while in Brazil it is mid-September to mid-October (Groch et al. 2005). The South African population may have shown a similar earlier shift in peak abundance as observed in Argentina (Vermeulen et al. 2018). This phenological change has coincided with a dramatic decrease in the proportion of unaccompanied adults observed along the South African coast (Vermeulen et al. 2020).
In the absence of annual aerial survey data comparable to that from other SRW wintering grounds (e.g., Charlton et al. 2019; Crespo et al. 2019), we report the results of non-systematic vessel-based one-day counts conducted in 2008 and 2021. These vessel-based surveys have limitations in the Auckland Islands, as logistical challenges and bad weather meant that our surveys were conducted at different times in the winter season, under different weather conditions with different numbers of observers. The counts could be considered underestimates due to unobserved whales not being counted, or biased high as whales could move and be counted twice. The results of the surveys can only be considered as minimum abundance of whales using Port Ross on a given day and broadly compared in a qualitative manner to each other. Additionally, the 2021 boat-based survey also omitted surveying north of Enderby Islands due to poor weather conditions, meaning a substantial proportion of whales would not have been available for counting (> 20% of all groups were found in this region in both 2008 and 1998 surveys). This missed region could potentially contribute to the comparatively low total in 2021, but this could also be due to the survey being earlier in the season.
This work contributes to the growing body of knowledge on changes in population dynamics, behaviour, phenology and habitat use in baleen whales. Some species show changes in these parameters linked to an environmental change that has occurred at a previously undocumented pace. For example, Ramp et al. (2015) found that fin and humpback whales adapted seasonal movements to a shift in productivity in one of their prime feeding grounds in the North Atlantic. In the Gulf of St. Lawrence, climate driven reductions in prey size and energy content appear to be impacting reproduction of North Atlantic right whales (Gavrilchuk et al. 2021), while in the Gulf of Maine, warming ocean conditions are predicted to alter the distribution of North Atlantic right whales’ prey, which will most likely drive the whales to forage in new areas (Pendleton et al. 2009; Runge et al. 2015; Record et al. 2019a). The consistent phenology of New Zealand SRW across time is in line with the finding that this population has the highest body condition scores of any studied SRW population (Christiansen et al. 2020), indicating their summer foraging grounds at this time are still productive enough to support this growing population.
Indeed, we suggest that both the consistent phenology and increase in the proportion of cow-calf pairs reflect population growth and recruitment. The New Zealand SRW population was growing at an estimated rate of 7% (95% CI 5–9%) between 1995 and 2009, so it is now not only larger but due to recruitment it is also expected to have a higher proportion of reproductive females than in the 1990s (Taylor et al. 2007a). This could be driving the distinct change in the relative proportion of groups with cow-calf pairs, an increase from 20% in 1998 to 50% in 2020/21. Other factors that could influence these findings include emigration and inter-annual variation in the number of breeding females. We believe that a substantial amount of emigration is unlikely given the New Zealand wintering ground is demographically closed (Carroll et al. 2012) and genetically distinct (Carroll et al. 2019) to other wintering grounds. Additionally, inter-annual variation in the number of breeding females drives large changes in the abundance of whales on wintering grounds in South Africa (Vermeulen et al. 2020) and Australia (Charlton et al. 2019). However, this would be expected to influence the sex ratio, as in the absence of breeding females we would expect a male bias in whales on the wintering ground (as seen in humpback whale wintering grounds; (e.g., Constantine et al. 2012). Instead, we found an increase in the proportion of females in the 2006–2009 surveys (1.5:1.0 females vs males) compared with the 1995–1998 datasets (1.0:1.1 females vs males) (Carroll et al. 2013) (2020/21 data not yet available). Ideally, we would compare not just the proportion of females but population abundance and growth rate, as these are stronger indications of recruitment and reproductive rate. However, these analyses were ongoing and not available at the time of manuscript preparation.
We were surprised to find no difference in the distribution of groups with and without calves based on kernel density analysis. In Argentina, SRW cow-calf pairs preferentially occupy coastal regions, and solitary adults and social groups are moving further out into deeper areas over time (Crespo et al. 2019). In Australia, spatial segregation between cow-calf pairs and unaccompanied adults has also been observed in Encounter Bay (Kemper et al 2022) and Head of Bight (Burnell 2001; Charlton et al. 2019). At Head of Bight, SRW are typically found along the coast within 2 km of shore (Charlton et al. 2019, 2020), whereas Port Ross is a shallow harbour typically sheltered from the prevailing wind and at most 2 km wide (Tidey and Hulbe 2018); perhaps the more sheltered waters in the latter allows the cow-calf pairs to use a wider distribution. Previous kernel density analyses of SRW in the Auckland Islands showed similar results to those reported here, with higher density along the southern coast of Enderby Island, as well as the middle and southwest of Port Ross (Rayment et al. 2012). Species-habitat modelling of SRW in Port Ross further suggested that cow-calf pairs preferred nearshore habitats that were sheltered from prevailing swell and wind (Rayment et al. 2015). We suggest that the differences between the kernel density and species-habitat modelling could be linked to differences in methodologies but also behavioural state of groups or age of calves included in the analyses. For example, we suggest that future work should investigate whether cow-calf pairs observed in social groups with other whales (~ 35% of observed groups with calves in this study) use different habitats from cow-calf pairs that are solitary and resting (~ 50%). At Head of Bight the spatial distribution of cow-calf pairs changes from clustered in shallow, sandy habitat to becoming more widely distributed along deeper water as the winter progresses (Charlton et al. 2019). Given the trends seen in other populations, we suggest that as the proportion of cow-calf pairs increases in line with the population growth in Port Ross, SRWs of other demographic classes may move to socialising areas around Campbell Island or the mainland (Carroll et al. 2014b; Torres et al. 2017). Although we were able to directly assess this hypothesis, our study and previous studies are limited in their ability to assess habitat use across time and space in the New Zealand subantarctic, specifically, the inclusion of survey data and habitat modelling of SRW in waters around Campbell Island would be a critical next step.
Observations of gull-southern right whale interactions
During the 2020 expedition, opportunistic observations were made of red bill gulls in close association with the SRWs for the first time, and the incidence appeared more frequently in the 2021 expedition. The gulls appeared to be following the whales and diving for material at the surface of the water in the whales’ wake, landing on the whales, and in some circumstances pecking at the whales’ backs or heads. We observed the gulls primarily targeting calves, eating sloughed whale skin and cyamids from the water’s surface or while directly standing on the whale itself. The gulls elicited a strong startle-type reaction from adult whales on more than one occasion and some used the galleon position at times to evade the gulls as described in Península Valdés, Argentina (Marón et al. 2015). We also recorded one observation of multiple gulls following, landing on and pecking the back of a SRW calf. Gull feeding on whales has been documented at Península Valdés, Argentina leading to large sores on the back of SRW calves (Marón et al. 2015; Sironi et al. 2018). However, no lesions consistent with skin damage from gulls like that reported in Península Valdés were observed, in fact, no lesions were observed in any SRW sighted or photographed over the 2 years of field effort. This could be because red bill gulls are considerably smaller (37 cm) than the black backed or kelp gulls (Larus dominicanus, 60 cm (Moon 2011)) that predate SRW in Argentina, and may be removing already flaking skin or cyamids, so do not cause obvious physical damage to the SRW. We suggest that the primary target could be young calves, whose skin flakes heavily after birth and that there is currently evidence for minimal physical impact. In fact, this could be an important food source for the red bill gulls, which are listed as At Risk—Declining under New Zealand legislation (Robertson et al. 2021).
Management implications of findings
The high observed density of whales (~ 5–10 per km2, derived from the vessel-based surveys) means that the New Zealand SRW wintering grounds is particularly vulnerable to disturbance or disaster (e.g., oil spill or epizootic diseases). In 2021, we documented five whales with likely anthropogenic scarring, injuries or wounds. Expert opinion is that the injuries likely arose from entanglements or ship strikes, the two factors that represent the greatest threats to right whale populations globally (Harcourt et al. 2019). Of particular concern was our finding of a whale with a recent wound that had occurred either the same day or only a few days before it was photographed as there was no evidence of healing (M. Moore pers. comm.). The wound was observed after the departure of a vessel from Port Ross during the hours of darkness, when these large, dark, whales are very difficult to discern. A similar number of whales with likely anthropogenic scarring (n = 5, N. Patenaude unpublished data) were observed during the 1995–1998 field seasons in 88 days of field effort, compared to 44 days of effort here. Differences in population abundance and age structure make comparison of these figures difficult, but in South Africa more anthropogenic scarring, ship strike and entanglements have been observed in line with population growth (Best et al. 2001).
We cannot say definitively that this and the other whales were injured in Port Ross. However, given the aforementioned residency times (Fewster and Patenaude 2009) and high-use of the Auckland Islands and Campbell Island by SRW in the austral winter, it seems likely that the fresh wound occurred in New Zealand subantarctic waters. The Regional Coastal Plan for the Kermadec and Subantarctic Islands, established in 2017, has recommendations to protect the SRW. One such recommendation of having a bow watch for whales while transiting Port Ross implies it should be done in daylight hours, which was not done by one vessel observed in 2021. Safe anchorages are available outside of Port Ross in nearby areas with a far lower density of SRWs (Patenaude 2002; Rayment et al. 2012). The use of these other areas would reduce potential impacts on the SRWs such as vessel strike and the risk of disasters like oil spills, and also reduce changes in whale behaviour arising from vessel noise and close approaches. Controlled vessel approach experiments have documented that SRW cows with calves are the most sensitive demographic classes to disturbance (Lundquist 2007). Controlled vessel approaches undertaken in Port Ross showed that cow-calf pairs start reacting to boat approaches from more than 1 km away (Barrett 2000). We suggest that monitoring and more vigorous enforcement of the existing rules would help reduce the chances of whale-vessel interactions and other anthropogenic impacts in Port Ross.
Given the significance of gull-SRW interactions to SRWs elsewhere (Rowntree et al. 2013; Marón et al. 2015), further monitoring of these interactions is recommended to assess the potential frequency and impact of this seabird behaviour. The continued harassment by gulls over a winter season has led to behavioural changes in Argentinean SRW including changes in breathing frequency and nursing frequency in calves that could have energetic costs (Azizeh et al. 2021). We did not document any lesions similar to that described in Península Valdés and current impact appears minimal, but behavioural studies at the Auckland Islands would give insight into whether similar behavioural changes are occurring.
Finally, we suggest that future studies should consider larger scale surveys within the Auckland Islands over longer time periods, and ideally, encompass Campbell Island as well. We consider such surveys imperative to determine any shifts in core habitat for the SRWs to better inform future management decisions that protect these whales as the population slowly recovers. Here, we reported on non-systematic boat-based surveys and pilot data from drone-based surveys, but other technological innovations may be useful in future, such as satellite imagery (e.g., Cubaynes et al. 2019). The drone-based survey pilot study was successful at detecting SRWs, and could be used in future to fully survey Port Ross with methodological developments such as estimating detection probabilities (Hodgson et al. 2017). Improving drone technology, and potentially satellite imagery (Cubaynes et al. 2019), could provide important methods for conducting surveys in these remote regions in future. Surveying multiple years for the whole wintering period would also provide important information on year to year variation in factors such as the cohort size of reproductive females. This is an important factor that we were unable to account for in our present study, due to the logistical difficulties in working in such a remote archipelago during the austral winter in a pandemic.
Conclusion
The 2020 and 2021 surveys show that the Port Ross region of the Auckland Islands remains the most important area for SRWs during winter in New Zealand waters, particularly for cow-calf pairs. As the population increases, we find evidence of increasing interactions with human activities, in line with increases in ship strike and fishing gear entanglements seen in other growing SRW populations (Best et al. 2001). Population growth is often associated with changes in habitat use in SRW. Continued monitoring of Port Ross will be necessary to ensure that protections remain relevant to this growing population, which we hope will continue to recover and recolonise New Zealand coastal regions.
Data availability
The underlying encounter data are available as a supplementary excel file to the manuscript.
Code availability
The code used in this paper is available on github: https://github.com/emmcarr/PolarBioPaper/new/main.
References
Azizeh T, Sprogis K, Soley R et al (2021) Acute and chronic behavioral effects of kelp gull micropredation on southern right whale mother-calf pairs off Península Valdés, Argentina. Mar Ecol Prog Ser 668:133–148. https://doi.org/10.3354/meps13716
Baker CS, Boren LJ, Childerhouse SJ et al (2019) Conservation status of New Zealand marine mammals, 2019. N Z Threat Classif Ser 14:18
Bannister JL (2011) Population trend in right whales off southern Australia 1993–2010. Unpubl Rep Present to Int Whal Comm Work South right whales, 13–16 Sept 2011, Buenos Aires, Argentina
Barrett B (2000) Nearshore habitat use by southern right whales (Eubalaena australis) cow/calf pairs in Port Ross Harbour, Auckland Islands. MSc Thesis, University of Auckland
Best P (2000) Coastal distribution, movements and site fidelity of right whales Eubalaena australis off South Africa, 1969–1998. S Afr J Mar Sci 22:43–55. https://doi.org/10.2989/025776100784125618
Best P, Peddemors V, Cockcroft VG, Rice N (2001) Mortalities of right whales and related anthropogenic factors in South African waters, 1963–1998. J Cetacean Resour Manag 2:171–176
Bestley S, Ropert-Coudert Y, Bengtson Nash S et al (2020) Marine ecosystem assessment for the Southern Ocean: birds and marine mammals in a changing climate. Front Ecol Evol. https://doi.org/10.3389/fevo.2020.566936
Brandão A, Vermeulen E, Ross-gillespie A, et al (2018) Updated application of a photo-identification based assessment model to southern right whales in South African waters, focussing on inferences to be drawn from a series of appreciably lower counts of calving females over 2015 to 2017. Unpublished report SC/67B/SH2 presented to the Scientific Committee of the International Whaling Commission, Cambridge, UK. https://doi.org/10.13140/RG.2.2.14456.88325
Burnell SR (2001) Aspects of the reproductive biology, movements and site fidelity of right whales off Australia. J Cetacean Res Manag Spec Issue 2:89–102
Carroll E, Patenaude NJ, Childerhouse SJ et al (2011) Abundance of the New Zealand subantarctic southern right whale population estimated from photo-identification and genotype mark-recapture. Mar Biol. https://doi.org/10.1007/s00227-011-1757-9
Carroll E, Childerhouse SJ, Christie M et al (2012) Paternity assignment and demographic closure in the New Zealand southern right whale. Mol Ecol. https://doi.org/10.1111/j.1365-294X.2012.05676.x
Carroll EL, Childerhouse SJ, Fewster R et al (2013) Accounting for female reproductive cycles in a superpopulation capture—recapture framework. Ecol Appl 23:1677–1690. https://doi.org/10.1890/12-1657.1
Carroll E, Jackson JA, Paton D, Smith TD (2014a) Two intense decades of 19th century whaling precipitated rapid decline of right whales around New Zealand and East Australia. PLoS ONE. https://doi.org/10.1371/journal.pone.0093789
Carroll E, Rayment W, Alexander AMAM et al (2014b) Reestablishment of former wintering grounds by New Zealand southern right whales. Mar Mamm Sci 30:206–220. https://doi.org/10.1111/mms.12031
Carroll E, Baker CS, Watson M et al (2015) Cultural traditions across a migratory network shape the genetic structure of southern right whales around Australia and New Zealand. Sci Rep 5:16182
Carroll EL, Fewster RM, Childerhouse SJ et al (2016) First direct evidence for natal wintering ground fidelity and estimate of juvenile survival in the New Zealand southern right whale Eubalaena australis. PLoS ONE 11:e0146590. https://doi.org/10.1371/journal.pone.0146590
Carroll E, Alderman R, Bannister JL et al (2019) Incorporating non-equilibrium dynamics into demographic history inferences of a migratory marine species. Heredity 122:53–68. https://doi.org/10.1038/s41437-018-0077-y
Carroll E, Charlton C, Vermeulen E, et al (2020) Roadmap to success for the International Whaling Commission - Southern Ocean Research Parternship (IWC-SORP) Theme 6—the right sentinel for climate change: linking southern right whale foraging ecology to demographics, health and climate. Report SC/68B/
Charlton CM (2017) Population demographics of southern right whales (Eubalaena australis) in Southern Australia. PhD Thesis. Curtin University
Charlton C, Ward R, McCauley RD et al (2019) Southern right whale (Eubalaena australis), seasonal abundance and distribution at Head of Bight, South Australia. Aquat Conserv Mar Freshw Ecosyst 29:576–588. https://doi.org/10.1002/aqc.3032
Charlton C, Marsh O, Shannessy BO, et al (2020) Long term southern right whale research at Head of Bight, South Australia 1991–2020: annual field report 2020. Unpublished paper SC/67C/SH/11 presented to the Scientific Committee of the International Whaling Commission. Available at www.iwc.int. Accessed July 2022
Charlton C, McCauley RD, Brownell RL et al (2022) Southern right whale (Eubalaena australis) population demographics at major calving ground Head of Bight, South Australia, 1991–2016. Aquat Conserv Mar Freshw Ecosyst 32:671–686. https://doi.org/10.1002/aqc.3771
Childerhouse SJ, Dunshea G (2008) Preliminary trip report for Southern right whale research trip to the Auckland Islands July/August 2008. Unpublished report to the New Zealand Department of Conservation Te Papa Atawhai
Childerhouse SJ, Carroll E, Rayment W (2009) Preliminary trip report for southern right whale research trip to the Auckland Islands July/August 2009. Unpublished report to the New Zealand Department of Conservation
Chown SL, Brooks CM (2019) The state and future of Antarctic environments in a global context. Annu Rev Environ Resour 44:1–30. https://doi.org/10.1146/annurev-environ-101718-033236
Christiansen F, Dawson S, Durban J et al (2020) Population comparison of right whale body condition reveals poor state of the North Atlantic right whale. Mar Ecol Prog Ser 640:1–16. https://doi.org/10.3354/meps13299
Constantine R, Jackson JA, Steel DJ et al (2012) Abundance of humpback whales in Oceania using photo-identification and microsatellite genotyping. Mar Ecol Prog Ser 453:249–261. https://doi.org/10.3354/meps09613
Costa P, Piedra M, Franco P, Paez E (2007) Distribution and habitat use patterns of southern right whales, Eubalaena australis, off Uruguay. J Cetacean Res Manag 9:45–51
Crespo EA, Pedraza SN, Dans SL et al (2019) The southwestern Atlantic southern right whale, Eubalaena australis, population is growing but at a decelerated rate. Mar Mamm Sci 35:93–107. https://doi.org/10.1111/mms.12526
Cubaynes HC, Fretwell PT, Bamford C et al (2019) Whales from space: four mysticete species described using new VHR satellite imagery. Mar Mamm Sci 35:466–491. https://doi.org/10.1111/mms.12544
Davidson AR, Rayment W, Dawson SM et al (2018) Estimated calving interval for the New Zealand southern right whale (Eubalaena australis). N Z J Mar Freshw Res 52:372–382. https://doi.org/10.1080/00288330.2017.1397034
Dulau V, Pinet P, Geyer Y et al (2017) Continuous movement behavior of humpback whales during the breeding season in the southwest Indian Ocean: on the road again! Mov Ecol 5:1–17. https://doi.org/10.1186/s40462-017-0101-5
Fewster R, Patenaude NJ (2009) Cubic splines for estimating the distribution of residence time using individual resighting data. Environ Ecol Stat 3:393–415
Fleming AH, Clark CT, Calambokidis J, Barlow J (2016) Humpback whale diets respond to variance in ocean climate and ecosystem conditions in the California Current. Glob Change Biol 22:1214–1224. https://doi.org/10.1111/gcb.13171
Gavrilchuk K, Lesage V, Fortune SME et al (2021) Foraging habitat of North Atlantic right whales has declined in the Gulf of St. Lawrence, Canada, and may be insufficient for successful reproduction. Endanger Species Res 44:113–136. https://doi.org/10.3354/ESR01097
Groch K, Palazzo J, Flores P et al (2005) Recent rapid increase in the right whale (Eubalaena australis) population off southern Brazil. Lat Am J Aquat Mamm 4:41–47
Harcourt R, van der Hoop J, Kraus S, Carroll E (2019) Future directions in Eubalaena spp.: comparative research to inform conservation. Front Mar Sci 5:530. https://doi.org/10.3389/fmars.2018.00530
Hindell M, Bradshaw C, Harcourt R, Guinet C (2003) Ecosystem monitoring: are seals a potential tool for monitoring change in marine systems? In: Gales N, Hindell M, Kirkwood R (eds) Marine mammals. Fisheries, tourism and management issues. CSIRO Publishing, Melbourne, pp 330–343
Hindell MA, Reisinger RR, Ropert-Coudert Y et al (2020) Tracking of marine predators to protect Southern Ocean ecosystems. Nature 580:87–92. https://doi.org/10.1038/s41586-020-2126-y
Hodgson A, Peel D, Kelly N (2017) Unmanned aerial vehicles for surveying marine fauna: assessing detection probability. Ecol Appl 27:1253–1267. https://doi.org/10.1002/eap.1519
IWC (1986) Right whales: past and present status. Rep Int Whal Comm Spec Issue 10:146–152
Jackson JA, Carroll E, Smith TD et al (2016) An integrated approach to historical population assessment of the great whales: case of the New Zealand southern right whale. R Soc Open Sci 3:150669
Jory C, Lesage V, Leclerc A et al (2021) Individual and population dietary specialization decline in fin whales during a period of ecosystem shift. Sci Rep 11:17181. https://doi.org/10.1038/s41598-021-96283-x
Kemper CM, Steele-Collins E, Al-Humaidhi A et al (2022) Encounter Bay, South Australia, an important aggregation and nursery area for the southern right whale, Eubalaena australis (Balaenidae: Cetacea). Trans R Soc South Aust. https://doi.org/10.1080/03721426.2021.2018759.10.1080/03721426.2021.2018759
Kraus SD, Hatch LT (2001) Mating strategies in the North Atlantic right whale (Eubalaena glacialis). J Cetacean Res Manag Spec Issue 2:237–244
Krützen M, Barré L, Möller L et al (2002) A biopsy system for small cetaceans; darting success and wound healing in Tursiops spp. Mar Mamm Sci 18:863–878
Lambertsen R (1987) A biopsy system for large whales and its use for cytogenetics. J Mamm 68:443–445
Leaper R, Cooke J, Trathan P et al (2006) Global climate drives southern right whale (Eubalaena australis) population dynamics. Biol Lett 2:289–292
Lundquist DJ (2007) Behavior and movement of southern right whales: effects of boats and swimmers behavior and movement of southern right whales: effects of boats and swimmers. MSc Thesis, Texas A&M University
Marón CF, Beltramino L, Di Martino M et al (2015) Increased wounding of southern right whale (Eubalaena australis) calves by kelp gulls (Larus dominicanus) at Península Valdés, Argentina. PLoS ONE 10:e0139291. https://doi.org/10.1371/journal.pone.0139291
Meyer-Gutbrod EL, Greene CH, Sullivan PJ, Pershing AJ (2015) Climate-associated changes in prey availability drive reproductive dynamics of the North Atlantic right whale population. Mar Ecol Prog Ser 535:243–258. https://doi.org/10.3354/meps11372
Moon G (2011) A photographic guide to birds of New Zealand. New Holland Publishers, Auckland
Nicol S, Bowie A, Jarman S et al (2010) Southern Ocean iron fertilization by baleen whales and Antarctic krill. Fish Fish 11:203–209
Patenaude NJ (2002) Demographic and genetic status of right whales at the Auckland Islands, New Zealand. PhD thesis. University of Auckland, Auckland, New Zealand
Patenaude NJ, Baker CS (2001) Population status and habitat use of southern right whales in the sub-Antarctic Auckland Islands of New Zealand. J Cetacean Res Manag Spec Issue 10:111–116
Patenaude NJ, Baker CS, Gales N (1998) Observations of southern right whales on New Zealand’s subantarctic wintering grounds. Mar Mamm Sci 14:350–355
Patenaude NJ, Portway V, Schaeff C et al (2007) Mitochondrial DNA diversity and population structure among southern right whales (Eubalaena australis). J Hered 98:147–157
Pendleton DE, Pershing AJ, Brown MW et al (2009) Regional-scale mean copepod concentration indicates relative abundance of North Atlantic right whales. Mar Ecol Prog Ser 378:211–225. https://doi.org/10.3354/meps07832
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed July 2022
Rainsley E, Turney CSM, Golledge NR et al (2019) Pleistocene glacial history of the New Zealand subantarctic islands. Clim past 15:423–448. https://doi.org/10.5194/cp-15-423-2019
Ramp C, Delarue J, Palsbøll PJ et al (2015) Adapting to a warmer ocean—seasonal shift of baleen whale movements over three decades. PLoS ONE 10:e0121374. https://doi.org/10.1371/journal.pone.0121374
Ratnarajah L, Bowie AR, Lannuzel D et al (2014) The biogeochemical role of baleen whales and krill in Southern Ocean nutrient cycling. PLoS ONE 9:1–18. https://doi.org/10.1371/journal.pone.0114067
Ratnarajah L, Lannuzel D, Townsend AT et al (2017) Physical speciation and solubility of iron from baleen whale faecal material. Mar Chem 194:79–88. https://doi.org/10.1016/j.marchem.2017.05.004
Ratnarajah L, Nicol S, Bowie AR (2018) Pelagic iron recycling in the Southern Ocean: exploring the contribution of marine animals. Front Mar Sci 5:1–9. https://doi.org/10.3389/fmars.2018.00109
Rayment W, Davidson A, Dawson S et al (2012) Distribution of southern right whales on the Auckland Islands calving grounds. N Z J Mar Freshw Res 46:431–436
Rayment W, Dawson S, Webster T (2015) Breeding status affects fine-scale habitat selection of southern right whales on their wintering grounds. J Biogeogr 42:463-474. https://doi.org/10.1111/jbi.12443
Record NR, Balch WM, Stamieszkin K (2019a) Century-scale changes in phytoplankton phenology in the Gulf of Maine. PeerJ 7:e6735. https://doi.org/10.7717/peerj.6735
Record NR, Runge JA, Pendleton DE et al (2019b) Rapid climate-driven circulation changes threaten conservation of endangered North Atlantic right whales. Oceanography 32:162–169. https://doi.org/10.5670/oceanog.2019.201
Robertson HA, Baird KA, Elliott GP et al (2021) Conservation status of birds in Aotearoa New Zealand, 2021. N Z Threat Classif Ser 36:43
Roman J, McCarthy J (2010) The whale pump: marine mammals enhance primary productivity in a coastal basin. PLoS ONE 5:e13255
Rowntree VJ, Payne R, Schell D (2001) Changing patterns of habitat use by southern right whales (Eubalaena australis) on their nursery ground at Península Valdés, Argentina, and in their long-range movements. J Cetacean Res Manag Spec Issue 2:133–143
Rowntree VJ, Uhart M, Sironi M et al (2013) Unexplained recurring high mortality of southern right whale Eubalaena australis calves at Península Valdés, Argentina. Mar Ecol Prog Ser 493:275–289. https://doi.org/10.3354/meps10506
Runge JA, Ji R, Thompson CRS et al (2015) Persistence of Calanus finmarchicus in the western Gulf of Maine during recent extreme warming. J Plankton Res 37:221–232. https://doi.org/10.1093/plankt/fbu098
Seyboth E, Groch KR, Dalla Rosa L et al (2016) Southern right whale (Eubalaena australis) reproductive success is influenced by krill (Euphausia superba) density and climate. Sci Rep 6:28205. https://doi.org/10.1038/srep28205
Sironi M, Rowntree VJ, Di Martino M, et al (2018) Southern right whale mortalities at Península Valdés, Argentina: updated information for 2016–2017. Report SC/67B/CMP/06 presented to the Scientific Committee of the International Whaling Commission, Cambridge, UK. Available at: https://iwc.int. Accessed July 2022
Smith TD, Reeves RR, Josephson E, Lund JN (2012) Spatial and seasonal distribution of American whaling and whales in the age of sail. PLoS ONE 7:e34905. https://doi.org/10.1371/journal.pone.0034905
Stewart R, Todd B (2001) A note on observations of southern right whales at Campbell Island, New Zealand. J Cetacean Res Manag Spec Issue 2:117–120
Taylor BL, Chivers SJ, Larese J, Perrin WF (2007a) Generation length and percent mature estimates for IUCN assessments of cetaceans. Adm Rep LJ-07-01 24p
Taylor BL, Martinez M, Gerrodete T et al (2007b) Lessons from monitoring trends in abundance of marine mammals. Mar Mamm Sci 23:157–175
Tidey EJ, Hulbe CL (2018) Bathymetry and glacial geomorphology in the sub-Antarctic Auckland Islands. Antarct Sci 30:357–370. https://doi.org/10.1017/S0954102018000342
Tormosov D, Mikhaliev YA, Best PB et al (1998) Soviet catches of Southern right whales Eubalaena australis 1951–1971. Biol Conserv 86:185–197
Torres LG, Rayment W, Olavarría C et al (2017) Demography and ecology of southern right whales Eubalaena australis wintering at sub-Antarctic Campbell Island, New Zealand. Polar Biol 40:95–106. https://doi.org/10.1007/s00300-016-1926-x
Trathan P, Forcada J, Murphy E (2007) Environmental forcing and Southern Ocean marine predator populations: effects of climate change and variability. Philos Trans R Soc Lond B Biol Sci 362:2351–2365
van den Berg GL, Vermeulen E, Valenzuela LO et al (2021) Decadal shift in foraging strategy of a migratory southern ocean predator. Glob Chang Biol 27:1052–1067. https://doi.org/10.1111/gcb.15465
Vermeulen E, Wilkinson C, Thornton M, et al (2018) Report on the Mammal Research Institute Whale Unit southern right whale survey. Report SC/67B/SH/01 presented to the Scientific Committee of the International Whaling Commission, Cambridge, UK. Available from www.iwc.int. Accessed July 2022
Vermeulen E, Wilkinson C, Van den Berg G (2020) Report of the southern right whale aerial surveys – 2019. Report SC/68B/SH02 submitted to the Scientific Committee of the International Whaling Commission, Cambridge, UK. Available from https://iwc.int/home. Accessed July 2022
Watson M, Stamation K, Charlton C, Bannister J (2021) Calving intervals, longrange movements and site fidelity of southern right whales (Eubalaena australis) in southeastern Australia. J Cetacean Res Manag 22:17–28. https://doi.org/10.47536/JCRM.V22I1.210
Weimerskirch H, Salamolard M, Sarrazin F, Jouventin P (1993) Foraging strategies of wandering albatrosses through the breeding season—a study using satellite telemetry. Auk 110:325–342
Weir CR, Stanworth A (2020) The Falkland Islands (Malvinas) as sub-Antarctic foraging, migratory and wintering habitat for southern right whales. J Mar Biol Assoc UK 100: 153-163. https://doi.org/10.1017/S0025315419001024
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Acknowledgements
We thank the Kaitiaki Roopū o Murihiku for discussions around and support of this project. We thank the research field team including Bill Morris, Arie Spyksma, Ian Skipworth (NZ Geographic) and Richard Robinson (Depth and NZ Geographic), Captain Steve Kafka, Sandra Carrod and crew of the Evohe Jim Dilley, Tori Muir, Johan Domeij, Murray Watson and Jan Hudson; Captain Rodney Russ and the crew of the Strannik: Scott Sinton, Julie Chandelier, Simon Truebridge and Konrad Richter. We thank Mike Double (Australian Antarctic Division), Jennifer Jackson (British Antarctic Survey), Becky Macneil and Lou Duncan (Antarctic New Zealand), Strannik Ocean Voyages, Spindrift Images and the Bluff Yacht Club for logistic and in-kind support. We thank John Peterson, Janice Kevern, Lochie Morton and Sharon Trainor from the New Zealand Department of Conservation – Te Papa Atawhai Southland for biosecurity and logistic support and Simon Wotherspoon for statistical assistance. Thanks to Vanessa Barry for help with reviewing the drone survey footage. Fieldwork was conducted under New Zealand Marine Mammal Protection Act Permit 84845-MAR and Marine Reserve Act Permit 87513-MAR. We thank Robert Brownell and one anonymous reviewer for their constructive comments on the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was funded by generous support from the Royal Society – Te Apārangi Rutherford Discovery Fellowship, Live Ocean, Lou and Iris Fisher Charitable Trust, Joyce Fisher Charitable Trust, Brian Sheth/Sangreal Foundation, University of Auckland Science Faculty Research Development Fund, International Whaling Commission – Southern Ocean Research Partnership, Antarctic and Southern Ocean Coalition, New Zealand Department of Conservation – Te Papa Atawhai and the Cawthron Institute.
Author information
Authors and Affiliations
Contributions
ELC and SC designed the study; ELC, LR, RCole, RC, RH, CM, MO, RR, ROR, ES, ALVDR performed field and analysis; all authors wrote and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest or competing interests to declare.
Ethical approval
Field work was conducted under University of Auckland Animal Ethics approved protocol 002072 to Emma Carroll.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carroll, E.L., Riekkola, L., Andrews-Goff, V. et al. New Zealand southern right whale (Eubalaena australis; Tohorā nō Aotearoa) behavioural phenology, demographic composition, and habitat use in Port Ross, Auckland Islands over three decades: 1998–2021. Polar Biol 45, 1441–1458 (2022). https://doi.org/10.1007/s00300-022-03076-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03076-7