Abstract
Anthropogenic debris, including plastic pollution, is a growing concern in the Arctic and negatively impacts both marine and coastal organisms. The aim of this study was to investigate the potential for using Arctic fox (Vulpes lagopus) faeces as a monitoring tool for plastic pollution in the Arctic environment. Arctic fox faeces were collected in different regions of Iceland and analysed for anthropogenic debris presence larger than 300 µm, and diet composition. In total, 235 faecal samples from 1999, 2017, 2018 and 2020 were analysed. The overall frequency of occurrence of plastic and other anthropogenic material was 5.11% and was found in samples across all regions and years. There were no statistical differences in anthropogenic debris ingested, depending on year or region. There were no obvious differences in diet composition between samples that contained anthropogenic debris and samples without. The suitability of Arctic fox faeces as a method to monitor plastic and anthropogenic debris levels in the Arctic environment remains debatable: Whilst the vast distribution range of the Arctic fox and the non-invasive collection methodology of faecal samples could be utilised as a good monitoring tool, the overall low uptake and unclear source of plastic and anthropogenic debris (marine or terrestrial) makes the interpretation of the data difficult. Nevertheless, debris ingestion by Arctic foxes remains a concern and warrants further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropogenic debris such as plastic is pervasive and present throughout ecosystems and across trophic chains (Rochman and Hoellein 2020). As a versatile material, plastic is in demand in many sectors. This demand leads to an increased accumulation of produced plastic, and the global plastic production reached 368 million tonnes in 2019 (PlasticsEurope 2020).
Research on plastic prevalence in marine species has shown that many species have plastics in their stomachs or faeces (Kühn and Van Franeker 2020). Plastic ingestion is recorded in marine organisms ranging from millimeter-sized zooplankton, up to blue whales (Balaenoptera musculus; Baxter 2009; Yu et al. 2020). Plastics and other man-made substances are ingested directly, either by mistaking these particles for prey or passively ingested together with prey (Ryan 2016). Once ingested, plastic and other anthropogenic debris (henceforth anthropogenic debris) can lead to lethal and sub-lethal consequences such as malnutrition or starvation that can be caused by e.g. the obstruction of the gastro-intestinal tract (Kühn et al. 2015; Bergmann et al. 2022; Tekman et al. 2022). Furthermore, research has shown that harmful chemical additives such as plasticizers, flame retardants, and UV stabilizers can leach from the ingested plastic particles into the organism, although direct health effects are difficult to quantify (Tanaka et al. 2015, 2020; Kühn et al. 2020).
With increasing latitudes and comparatively lower human population density and activity in the Arctic, plastic levels in the environment are expected to decrease (Kühn and Van Franeker 2012; Trevail et al. 2015). However, recent studies show that an increase in plastic levels in the Arctic in the years and decades to come is likely due to the warming of the climate and the poleward expansion of shipping and fishing activities (Trevail et al. 2015; Cózar et al. 2017; Collard and Ask 2021). For example, studies on plastic ingestion in northern fulmars (Fulmarus glacialis) in Arctic environments show a high incidence of plastics even in remote locations (Mallory 2006, 2008; Provencher et al. 2009; Kühn and Van Franeker 2012; Trevail et al. 2015; Baak et al. 2020a, b).
A research bias towards anthropogenic debris in marine organisms has side-lined studies on anthropogenic debris in terrestrial ecosystems and organisms (Rillig 2012; Mai et al. 2018; Puskic et al. 2020; Collard and Ask 2021). Available reports on anthropogenic debris ingestion in Arctic land mammals are scarce (Collard and Ask 2021) and include studies on Arctic wolves (Canis lupus arctos; Marquard-Petersen 1998), Svalbard reindeer (Rangifer tarandus platyrhynchus; Nashoug 2017), the Grant's caribou (Rangifer tarandus granti; Beach et al. 1976) and Arctic foxes (Vulpes lagopus). More specifically, anthropogenic debris ingestion in Arctic foxes was documented in Alaska (Garrott et al. 1983), in Greenland (Kapel 1999), in Svalbard (Prestrud 1992; Hallanger et al. 2022), and in Iceland (Skúladóttir 2019). The curious and opportunistic nature of Arctic foxes may lead to the accidental uptake of anthropogenic litter, in particular when attracted to garbage sites close to human settlements (Kapel 1999; Savory et al. 2014). Still, several knowledge gaps remain in assessing anthropogenic debris exposure of Arctic terrestrial organisms. These include pathways of exposure of anthropogenic debris in terrestrial mammals (e.g. trophic transfer via northern fulmars) as well as the biological effects of anthropogenic debris on the organism.
Currently, no terrestrial indicator species for environmental pollution by anthropogenic debris has been designated for Arctic food webs (Lusher et al. 2022). The Arctic Monitoring and Assessment Programme (AMAP) recommends the northern fulmar as a primary monitoring indicator of marine plastic particles larger than 1 mm (Baak et al. 2020b; AMAP 2021), other species are also discussed including terrestrial species such as the Arctic fox as well as the caribou, the reindeer and the polar bear (Ursus maritimus) to cover other environmental departments (AMAP 2021).
Establishing a terrestrial Arctic organism as a bioindicator for anthropogenic debris pollution can help in identifying spatial as well as temporal trends, polluters entry points into ecosystems and potential trophic transfer between marine and terrestrial organisms. Furthermore, it would effectively comply with the recent request on further plastic ingestion studies in Arctic terrestrial mammals as brought forward by several fora such as AMAP, but also the Protection of the Marine Environment (PAME 2019), and Conservation of the Arctic Flora and Fauna (CAFF). Intergovernmental organizations such as the Helsinki Commission (HELCOM 2021), Nordic Councils (Raubenheimer and Urho 2020), the International Council for the Exploration of the Sea (ICES 2018), and the United Nations Environment Programme (UNEP; GESAMP 2019) all discuss anthropogenic debris in the Arctic as a source of great concern and express a need for further research and monitoring programmes.
The aim of this study was to quantify anthropogenic debris in faecal samples of Arctic foxes in Iceland and to analyse whether Arctic fox faecal analysis might represent a suitable approach to monitor anthropogenic debris in the Arctic environment. Faeces collection was chosen as the preferred methodology due to its simplicity and minimally invasive nature as it limits disturbance of the animals. In order to investigate potential transfer pathways of ingested anthropogenic debris, dietary analysis was performed on the faecal samples.
Methods and materials
Field sample collection
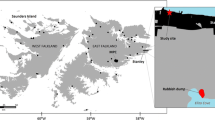
Faecal samples were collected in Iceland in 1999, 2017, 2018 and 2020 (Fig. 1). Faeces from 1999 (n = 80) were collected at known den sites in the nature reserve of Hornstrandir in the Westfjords. Tourism in the uninhabited peninsula is usually restricted to the summer months when access is possible by boat. Hornstrandir visitors were approximated about 3600 visitors in 1999 and about 5000 visitors in 2020 (Kristín Ósk Jónasdóttir, Umhverfisstofnun, pers. comm., 16.05.2022). Visitors of Hornstrandir have increased since 2011 when tour operators started offering tours more frequently and earlier in the season.
In 2020, faecal samples (n = 77) were collected in the Westfjords, more specifically in the regions of Dynjandi waterfall, Ísafjarðarbær and Hornstrandir. The region of the Westfjords is a peninsula in Northwest Iceland with around 7.000 inhabitants (Vestfjarðastofa 2022). The Westfjords and the region of Dynjandi in particular are generally well visited by tourists in the summer. However, during 2020 the effects of the COVID pandemic led to a drop in tourist numbers by 83% in 2020. No cruise ships arrived that year, compared to > 100 in previous years (Ferðamálastofa, 2021).
All faecal samples were collected randomly with some samples collected close to den areas and others further away. But as the area is highly populated, most locations are within breeding territories. During both years (1999 and 2020) collection took place in May, June, and July, when breeding foxes are still rearing cubs. After collection, the samples were stored at – 18 °C before analysis took place.
Faecal precursors were extracted by removing the last part of the large intestine (around 10 cm), close to the anus from dead Arctic foxes received from hunters in 2017 (n = 30) and 2018 (n = 48) in four different regions, namely the Westfjords, the Northwest, the Northeast, and the Southwest of Iceland (Fig. 1). Removing the whole set of intestines is part of the standardized procedure during Arctic fox dissections and was initially not intended for plastic research. Taking only the last part was done to be able to compare the faecal precursors to the scats found on ground. To account for the varying length of the gut that was removed, faecal matter mass was recorded. These samples were collected throughout all seasons in both years.
Laboratory processing of samples
All faecal samples were weighed with a scale of a precision of 0.0001 g. To obtain the total mass of faecal matter from the faecal precursors, it was weighed before and after the removal of the faeces from the intestines. All samples were then placed in glass vials with a solution of water and washing detergent Biotex (3 g/L; Sara Lee H and HB Netherland B.V) for enzymatic digestion of the samples. To remove soft tissue hampering the extraction of hard prey remains and anthropogenic debris, this method has been applied in previous studies analysing plastic ingestion as well as natural diet in marine mammals and seabirds, (e.g. Bravo Rebolledo et al. 2013; Harris et al. 2015). The samples were put in a shaking bath (Julabo SW23) at 40 °C for a minimum of 48 h, and were shaken at 120 revolutions/min. They were then rinsed with fresh tap water over a sieve of 300 μm to remove the Biotex and the dissolved soft organic material. After sieving, the samples were transferred to a Bogorov counting chamber for microscopic analysis.
Identification of dietary items and anthropogenic debris
Faecal samples were analysed using a Discovery V8 stereomicroscope (Zeiss, Germany), and hard prey remains such as bones, feathers, beaks, and non-food natural items such as wood and stones were separated and counted. A minimum number of individual prey (MNI) was estimated for each prey taxa based on, e.g. the number of otoliths or lenses of fish, or the number of similar bone fragments such as jaws. For plant material, such an estimate is more difficult. The MNI of seaweeds was based on the number of different types found in the faecal matter. For terrestrial plants the MNI was based on either the number of different plant types or estimated based on the abundance of plant material, such as berries, which provides a rather rough estimate. Prey remains were categorized as either terrestrial, marine, a mixture of both, or as unknown, identified to the lowest taxonomic level possible. Bird species could not be determined based on the feather remains in the sample. Fish prey remains were identified using otoliths and vertebrae appearance (Härkönen 1986; Camphuysen and Henderson 2017).
Anthropogenic debris particles were extracted from the sample and left to dry for at least 24 h. According to the method of Van Franeker et al. (2011) these particles were then further categorised into industrial pellet, sheet, thread, foam, fragment, other plastic, and other anthropogenic debris (e.g., tar, paper, aluminium foil, etc.). Particles were counted and weighed per category using a scale to an accuracy of 0.0001 g. Their size (length, width, and height with 0.1 mm accuracy) was measured with sliding callipers. By doing this, the particles were classified according to their maximum length in either micro- (< 5 mm), meso- (5–25 mm) and macro particles (> 25 mm; Arthur et al. 2009). The exact colour was defined using the RAL system including 213 colours. These colours were grouped in categories: transparent, black, grey, white, blue, green, red, brown and yellow. In multicoloured pieces, the dominant colour was defined. All items were photographed under the microscope (Zeiss AxioCam MRc with AxioVision 40 V4.8.2.0 software). Fourier-Transform Infrared Spectroscopy (FTIR; Shimadzu IRSpirit) was applied to identify the specific polymer type. Regular background scans were performed to correct for background noise. For each item, 45 scans were performed, and spectrum was measured between 400 and 4000 nm. Results of spectrum overlap are expressed as a match score from 0% (no overlap) to 100% (total overlap). A match score threshold of 80% was accepted in line with recommendation by Kühn et al. (2021). As samples were exposed to the air for an uncertain amount of time prior to collection, this study excluded fibres from the analysis due to the high risk of contamination (e. g. Kühn et al. 2018). All anthropogenic debris in the samples were microscopically checked for similarity with the materials used during processing of the samples in the laboratory such as plastic tape which was used to maintain the labels in place.
Data processing
To indicate the proportion of samples that contained anthropogenic debris particles, the frequency of occurrence (%FO) was calculated. Average number and mass were expressed as population averages, including all samples that did not contain any anthropogenic debris. Differences between average particle numbers, and masses in different years, regions and found when using different sample collection methods (faecal or precursor samples) were assessed using an ANOVA, followed by a non-parametric Tukey HSD test. To test whether anthropogenic debris ingestion could be linked to specific diet components, the difference in diet composition between faecal samples containing anthropogenic debris items or not was tested with ANOSIM (Analysis of Similarities) using untransformed data and a Euclidean distance matrix (Clarke and Warwick 2001). In addition, the differences in diet composition between faecal samples from the four different regions was investigated using ANOSIM. ANOSIM is a multivariate test based on a similarity matrix that is used to uncover differences between groups of samples, including the counts of all dietary items (Clarke and Warwick 2001). In this case, the MNI in the dietary categories benthos, crustaceans, fish, squid, marine plants, mammals, insects, worms, birds, gastropods and terrestrial plants were used. For the performed ANOSIM tests, the R statistic is given, providing a measure for the degree of separation between groups. This value is one when all replicates within a group are more similar than any replicates from different group and groups are, thus, completely separated. The value is zero when similarities between and within groups are, on average, the same. There is, thus, no difference between groups when the R is small (Clarke and Warwick 2001). Statistical tests were performed using the R software version 4.0.2 (R Core Team 2021) and the “vegan” package (Oksanen et al. 2020). Differences were regarded as statistically significant when α < 0.05.
Results
Detected anthropogenic debris
The total frequency of occurrence of anthropogenic debris in all samples was 5.11%, equalling 12 anthropogenic debris particles in 235 samples (Table 1). None of the samples contained more than one anthropogenic debris item. Foxes from all sample groups ingested on average 0.050 items/sample, with an average mass of 0.0048 g/sample. There was no statistical difference between the number of anthropogenic debris (F1, 233 = 0.38, p = 0.54) or the weight (F1, 233 = 0.185, p = 0.67) of anthropogenic debris found in the samples when comparing faecal samples and faeces from precursor samples. Likewise, the number or weight of anthropogenic debris in both faecal and precursor samples did not differ significantly between years (for numbers: F3, 231 = 0.194, p = 0.90; for weight F3, 231 = 0.969, p = 0.41) or regions (for numbers: F3, 231 = 0.949, p = 0.42; for weight F3, 231 = 1.58, p = 0.20).
Anthropogenic debris characteristics
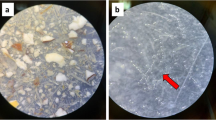
Nine out of twelve items were categorized as plastic. These particles included three plastic sheets, three plastic fragments, as well as three items categorized as ‘other plastic’. Two other items were categorized as ‘paper’ and one as ‘tar’. A photographic overview of all found plastic particles is shown in Fig. 2. Detailed descriptions and pictures of all particles can be found in the Online Resources 1 and 2, respectively.
The maximum length of the litter items ranged from 0.5 to 89.6 mm, with an average size of 18.3 mm and an average weight of 0.093 g per item. The smallest particle measured approximately 0.5 × 0.3 mm. Of the 12 items found, five were categorized as microparticles, four as mesoparticles, and three were larger than 25 mm and were categorized as macroparticles. Colours varied from transparent, to black, grey, white, blue, beige, brown, and yellow.
Of the 12 items found, two particles were identified as cellulose, one of which had the largest dimensions and the appearance of toilet paper. Another particle was identified as tar mixed with cellulose. Two particles were identified as polyethylene and one as polypropylene, two particles as acrylic, one particle as polyester, one as ethylene–vinyl acetate (EVA), and one as acrylonitrile butadiene styrene (ABS). One particle could not be identified with FTIR due to its very small size.
Ecotype and diet variations
Judging from the items in the faecal samples, the diet of Icelandic Arctic foxes was highly variable. Bird remains and terrestrial plant material, such as small leaves and branches as well as berries, dominated the diet in terms of numbers and were found in all regions and years. Both prey types were found in the vast majority of the samples investigated. The proportional contribution of different prey categories to the diet per region are presented in Fig. 3.
Benthic prey items were exclusively found in samples from the Westfjords, including both faeces and precursor samples. They included sea urchin (Echinacea spp.), sea snails or limpets (Lepetellidae), and bivalves. Avian prey items found likely originated both from seabirds and terrestrial birds. Identification of bird species at a lower taxonomic level was achieved for two passerines in total, based on the remains of beaks, feathers and claws, which indicated that these birds might have been a redwing (Turdus iliacus) and a meadow pipit (Anthus pratensis). Squid remains such as eyes and beaks were found in two samples from the Westfjords, once in 1999, and once in 2020. Crustacean prey included small shrimp-like species such as ostracods, amphipods, and decapods. These were found in 32 samples in total, of which 27 in the diet of foxes from the Westfjords from 2020, and five samples from the southwest region from 2017 (3 samples) and 2018 (2 samples). Fish remains were found in 36 samples and belonged to three different orders. The gadifomes (cod-like fish) included whiting (Merlangius merlangus), Norway pout (Trisopterus esmarkii) and northern rockling (Ciliata septentrionalis). Osmeriformes (smelt-like) were represented by capelin (Mallotus villosus) and perciformes (perch-like) included the lesser sandeel (Ammodytes tobianus). The majority of the fish remains (72.2%) were found in faecal samples collected in the Westfjords during both 1999 and 2020. The other 27.8% came from precursor samples collected from the southwest of Iceland. Insects were detected in the Westfjords in 1999 and 2020, in the southwest in 2017 and in 2018 and in the northeast in 2017 and in 2018. Only one individual from 1999 could be identified as belonging to the order of the flying insects (Odonata).
Mammal remains such as skin, fur, teeth and bones were found in 54 samples from all regions and years but predominantly in the northwest and southwest precursor samples. All but two skin and bone fragments found belonged to the order of rodents. Rodent remains from the Westfjords from 1999 and 2020 were most likely wood mouse (Apodemus sylvaticus). The two remaining samples contained skin fragments that could have originated from seals, based on thickness and appearance. Plants represented the largest diet components in terms of numbers in all four regions. This category includes both terrestrial plants (mostly crowberries; Empetrum nigrum) and marine algae. The frequency of plant material found in almost every sample might be an indication that foxes deliberately eat berries, grass, or seaweed. Parasites were detected in three samples from the Westfjords in 1999. The category ‘other’ included stones and was found in all four regions and during all years. A table with an overview of natural prey in Arctic fox faeces and faecal precursors can be found in Online Resource 3. Examples of natural prey remains are depicted in the Online Resource 4.
There was no statistical difference in diet composition between samples from different regions (p = 0.44, R = 0.01). Plastic remains were found in faecal samples together with diet remains of marine and terrestrial origin and bird remains (Fig. 4a). Terrestrial and marine prey remains were found together with plastic in samples from faecal precursors (Fig. 4b). Comparing the abundances of prey items in the different diet categories, no significant difference between samples that included plastic and samples that did not was found (p = 0.15, R = 0.10).
Discussion
This study quantified anthropogenic debris in faecal and faecal precursor samples in Iceland and reports an overall occurrence of anthropogenic debris ingestion in Arctic foxes of 5.11%. It was found that anthropogenic debris is present in Arctic fox faeces from all studied regions. Anthropogenic debris were found in all sampling years (1999, 2017, 2018 and 2020), indicating that anthropogenic debris pollution in the Arctic has been present for several decades.
Garrott et al. (1983) analysed 566 Arctic fox faeces in Prudhoe Bay, Alaska and found anthropogenic debris in 6% of the samples. In that study debris was defined as any man-made substances such as wrapping plastic and paper. The debris found in faecal samples was thought to be wrapping plastic or aluminium foil of the type found in processed food wrappings. On Svalbard, Prestrud (1992) analysed stomach contents from 751 individuals in a study from 1977 to 1989 and found that 5% contained anthropogenic debris which was defined as paper and plastic particles, however methodological procedures and examination details are not given. Both studies do not indicate further details on plastic and anthropogenic debris characteristics.
There are a few other studies with a dedicated anthropogenic litter protocol. A very recent study by Hallanger et al. (2022) report anthropogenic litter in 15% of 20 Arctic fox intestines from Svalbard collected in winter. Another study with a dedicated protocol on Arctic fox plastic ingestion found plastic in 4% of 125 Arctic fox stomachs from Iceland (Skúladóttir 2019). The study reports an average amount of 0.36 plastic particles per sample and an average plastic weight of 0.17 g per sample (Skúladóttir 2019). Of the stomachs found to contain plastic particles, two came from the Westfjords, two from the north, and one from the south of Iceland. The plastic debris found were generally larger than 1 cm (length) with clearly identifiable particles such as a sheep ear tag, and a plastic rope. The similarities in all the aforementioned results might indicate that the collection method (stomachs, precursors of faeces) does not influence the amount of anthropogenic debris detected. Whether combining several methodologies provides greater accuracy in anthropogenic debris detection is yet to be analysed.
The current study of faecal sample analysis revealed that Arctic foxes are able to excrete anthropogenic debris. However particularly large pieces might get stuck in the digestive tract and would therefore not be accounted for in this study.
A variety of polymer types was detected within this study. All identified polymer types were also found in other ingestion studies from the Atlantic Arctic. Carlsson et al. (2021) found several different polymer types in walrus (Odobenus rosmarus) faeces. Both Malinen (2021) and De Vries et al. (2020) found plastic particles identified as PE and PP in Atlantic cod (Gadus morhua) and saithe (Pollachius virens) collected around Iceland. Akoueson et al. (2020) further report microplastics in European plaice (Pleuronectes platessa) that were identified as PE and PET (polyethylene terephthalate). Kühn et al. (2021) confirm that both PE and PP as well as ABS are also commonly found in fulmars from Iceland. Polymer type details of plastics ingested by biota outside the Atlantic part of the Arctic are scarce. Two studies report polymer types in Belugas (Delphinapterus leucas) and their prey fish from the central Canadian Arctic. These items were dominated by polyester, however their samples consisted of mainly fibres (Moore et al. 2019, 2022).
Linking diet and anthropogenic debris uptake in Arctic foxes
The diet of the Arctic foxes generally showed a high variety in prey items, which is consistent with previous Arctic fox diet studies done in Iceland (Hersteinsson and Macdonald 1996; Helgason, 2008). It is known that Arctic foxes can prey on a large variety of species, and their diet is subject to seasonal and geographical changes (Hersteinsson and Macdonald 1996; Ellgutter et al. 2020). Bird and terrestrial plant remains dominated the diet of the Arctic foxes investigated. However, estimating a clear MNI for plant material is difficult and may have caused a disproportionally large number of this prey item to appear present in the faecal samples compared to the other items. Despite that a few differences can be seen in the diet from different regions, such as benthic prey only occurring in samples from the Westfjords, there were no statistical differences in the diet composition of the faecal and precursor samples investigated. This is likely due to (1) the high variety of prey items found in samples from all regions, (2) to the relatively small number of the different prey items generally found in the samples and (3) to the variation in sample size between the regions.
Kittiwakes (Rissa tridactyla) and fulmars are among the foxes’ main prey items (Hersteinsson et al. 2000; Pálsson et al. 2015). Between 2018 and 2020, 67% out of 121 fulmars in Iceland had plastic in their stomach (Snæþórsson 2021), however, no data for Icelandic kittiwakes is available. Therefore, it stands to reason that anthropogenic debris found in Arctic foxes might have been ingested via their prey. The co-occurrence of anthropogenic debris with the same type of prey remain could have been indicative to the origin of the anthropogenic debris particle (Hammer et al. 2016). Unfortunately, within this study, the feather remains in the faeces could not be identified to species level. In addition, occurrences of anthropogenic debris could not be associated with a certain type of prey. The equal distribution of anthropogenic litter in Arctic fox faeces also indicates no additional plastic load from seabirds during the breeding season in summer, however, sample sizes from winter were small in this study.
Suitability of Arctic foxes as a monitoring species for anthropogenic debris in the Arctic
Bioindicators are organisms which reflect the overall state of health of the environment with regards to pollution and other anthropogenic disturbances in the environment and can, therefore be used as monitoring tool. Their response to changing conditions in the environment is regarded as representative of the state of the environment as a whole targeting e.g. anthropogenic stressor such as plastic pollution. Among the factors that contribute to a suitable monitoring species, availability and accessibility of biota samples play an important role. Availability of samples in large enough numbers increases statistical confidence levels and trends can be detected more easily. In fulmars for example, an annual sample size of 40 birds is advised (Van Franeker et al. 2011), but in species with a lower frequency of occurrence of anthropogenic debris a larger sample size is needed (MSFD 2013). As a circumpolar species, the distribution range of the Arctic fox is suitable for monitoring purposes (Berteaux et al. 2017). In Iceland, 4400–6400 Arctic foxes were legally hunted between 2017 and 2019 (Umhverfisstofnun 2020), therefore carcasses are available in sufficient numbers and faeces can be collected non-invasively. In other Arctic regions, where hunting of Arctic fox is prohibited, faeces could still be collected in sufficient numbers. The results of the current study demonstrate a low uptake of anthropogenic debris, but at the same time a large variety of anthropogenic debris items in terms of size, shape and polymer type and show that this species might be adequate to examine anthropogenic debris of different categories.
However, due to the fact that Arctic foxes find their prey in both marine and terrestrial environments, the examination of targeted anthropogenic debris entry points is impossible. It also remains unclear whether the lack of temporal and geographical trends reflects a stable situation of anthropogenic debris pollution during the sampling period and in different sampling locations, or if the Arctic fox is unsuitable to indicate any changes. Ingestion rates of anthropogenic debris should be high enough to enable comparable measurements and the extraction of statistical trends regarding changes in time, per region and in anthropogenic debris composition (UNEP/MAP 2018). The current study demonstrates that anthropogenic debris ingestion by Arctic foxes occurred during three decennials and across different regions of Iceland, however no changes in time or region were detected. These results overlap with previous studies on debris ingestion by Arctic foxes that report comparable uptake in different regions and time periods.
Conclusion
The amount of litter found in Arctic fox faecal matter was similar to those in other studies in the Atlantic Arctic region. No regional differences within Iceland and no temporal trend could be detected within the three decades covered in this study. To better understand anthropogenic debris uptake and pathways across the food web and ecosystems, additional research is needed using a protocol where the entire digestive tract as well as faecal samples of terrestrial mammals are analysed. The lack of a terrestrial indicator species for anthropogenic debris in the Arctic shows the need for further research to find a species suitable to monitor anthropogenic debris in Arctic food webs and in the Arctic environment. Increasing tourist numbers in Arctic regions could increase the pollution levels even in remote areas such as the Westfjords. To evaluate whether the opportunistic Arctic fox may or may not be a suitable anthropogenic debris monitoring species, further studies are recommended. In Iceland, the northern fulmar is already used to monitor plastic in the marine environment, but an additional monitoring scheme for terrestrial regions could be beneficial.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Akoueson F, Sheldon LM, Danopoulos E, Morris S, Hotten J, Chapman E, Li J, Rotchell JM (2020) A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environ Pollut 263:114452. https://doi.org/10.1016/j.envpol.2020.114452
AMAP (2021) Litter and microplastics monitoring guidelines. Arctic Monitoring and Assessment Programme (AMAP), Tromsø
Arthur C, Baker J, Bamford H (2009) Proceedings of the international research workshop on the occurrence, effects, and fate of microplastic marine debris, September 9–11, 2008. In: Arthur C, Baker J, Bamford H (eds) NOAA technical memorandum NOS-OR&R-30. University of Washington Tacoma, USA
Baak JE, Provencher JF, Mallory ML (2020a) Plastic ingestion by four seabird species in the Canadian Arctic: comparisons across species and time. Mar Pollut Bull 158:111386. https://doi.org/10.1016/j.marpolbul.2020.111386
Baak JE, Linnebjerg JF, Barry T, Gavrilo MV, Mallory ML, Price C, Provencher JF (2020b) Plastic ingestion by seabirds in the circumpolar Arctic: a review. Env Rev 28:506–516. https://doi.org/10.1139/er-2020-0029
Baxter A (2009) Report on the blue whale stranding northwest coast of the South Island, New Zealand. Department of Conservation, Nelson
Beach RJ, Newby TC, Larson RO, Pedersen M, Juris J (1976) Entanglement of an Aleutian reindeer in a Japanese fish net. The Murrelet 57:66–66
Bergmann M, Collard F, Fabres J, Gabrielsen GW, Provencher JF, Rochman CM, Van Sebille E, Tekman MB (2022) Plastic pollution in the Arctic. Nat Rev Earth Environ 3:323–337. https://doi.org/10.1038/s43017-022-00279-8
Berteaux D, Casajus N, Angerbjörn A, Fuglei E (2017) Foreword to supplement 1: research on a polar species—the Arctic fox. Polar Res 10(1080/17518369):1347411
Bravo Rebolledo EL, Van Franeker JA, Jansen OE, Brasseur SM (2013) Plastic ingestion by harbour seals (Phoca vitulina) in The Netherlands. Mar Pollut Bull 67:200–202. https://doi.org/10.1016/j.marpolbul.2012.11.035
Camphuysen CJ, Henderson JR, Velilla E, Kühn S, Leopold MF (2017) North Sea Fish and their remains. Royal Netherlands Institute of Sea Research and Pisces Conservation Ltd, Texel
Carlsson P, Singdahl-Larsen C, Lusher AL (2021) Understanding the occurrence and fate of microplastics in coastal Arctic ecosystems: the case of surface waters, sediments and walrus (Odobenus rosmarus). Sci Total Environ 792:148308. https://doi.org/10.1016/j.scitotenv.2021.148308
Clarke KR, Warwick R (2001) Change in marine communities. An approach to statistical analysis and interpretation 2nd editions, Plymouth
Collard F, Ask A (2021) Plastic ingestion by Arctic fauna: a review. Sci Total Environ 786:147462. https://doi.org/10.1016/j.scitotenv.2021.147462
Cózar A, Martí E, Duarte CM, García-de-Lomas J, Van Sebille E, Ballatore TJ, Eguíluz VM, González-Gordillo JI, Pedrotti ML, Echevarría F, Troublè R, Irigoien X (2017) The Arctic Ocean as a dead end for floating plastics in the North Atlantic branch of the Thermohaline circulation. Sci Adv 3:e1600582. https://doi.org/10.1126/sciadv.1600582
De Vries AN, Govoni D, Árnason SH, Carlsson P (2020) Microplastic ingestion by fish: body size, condition factor and gut fullness are not related to the amount of plastics consumed. Mar Pollut Bull 151:110827. https://doi.org/10.1016/j.marpolbul.2019.110827
Ellgutter JAC, Ehrich D, Killengreen ST, Ims RA, Unnsteinsdóttir ER (2020) Dietary variation in Icelandic arctic fox (Vulpes lagopus) over a period of 30 years assessed through stable isotopes. Œcol 192:403–414. https://doi.org/10.1007/s00442-019-04580-0
Ferðamálastofa (2021) Foreign tourists in Iceland. In: Ferðamálastofa - Icelandic tourism board. https://www.maelabordferdathjonustunnar.is/en/tourists-to-iceland/by-flight. Accessed 11 May 2022
Garrott RA, Eberhardt LE, Hanson WC (1983) Summer food habits of juvenile arctic foxes in northern Alaska. J Wildl Manag 47:540–545. https://doi.org/10.2307/3808533
GESAMP (2019) Guidelines or the monitoring and assessment of plastic litter and microplastics in the ocean. In: Kershaw PJ, Turra A, Galgani F (eds) Joint group of experts on the scientific aspects of marine environmental protection. United Nations environment programme, Nairobi
Hallanger IG, Ask A, Fuglei E (2022) Occurrence of ingested human litter in winter arctic foxes (Vulpes lagopus) from Svalbard. Norway Environ Pollut 303:119099. https://doi.org/10.1016/j.envpol.2022.119099
Hammer S, Nager R, Johnson P, Furness R, Provencher J (2016) Plastic debris in great skua (Stercorarius skua) pellets corresponds to seabird prey species. Mar Poll Bull 103:206–210. https://doi.org/10.1016/j.marpolbul.2015.12.018
Härkönen T (1986) Guide to the otoliths of the bony fishes of the Northeast Atlantic. Biological Consultants, Hellerup
Harris MP, Leopold MF, Jensen JK, Meesters EH, Wanless S (2015) The winter diet of the Atlantic Puffin Fratercula arctica around the Faroe Islands. Ibis 157:468–479. https://doi.org/10.1111/ibi.12272
HELCOM (2021) Baltic sea action plan—2021 update. Helsinki Commission—HELCOM, Helsinki, Finland, pp 31. https://helcom.fi/media/publications/Baltic-Sea-Action-Plan-2021-update.pdf
Helgason HH (2008). Fæða refa (Vulpes lagopus) á hálendi Íslands að vetrarlagi [Feeding foxes (Vulpes lagopus) in the highlands of Iceland during the winter]. Msc Thesis, Department of Biology, Háskola Íslands, p. 12
Hersteinsson P, Macdonald DW (1996) Diet of arctic foxes (Alopex lagopus) in Iceland. J Zool 240:457–474. https://doi.org/10.1111/j.1469-7998.1996.tb05298.x
Hersteinsson P, Björnsson T, Unnsteinsdóttir ER, Olafsdóttir AH, Sigþórsdóttir H, Eiríksson Þ (2000) Refir á Hornströndum [Arctic foxes in Hornstrandir]. Náttúrufræðingurinn 69:313–142
ICES (2018) Interim report of the working group on marine litter (WGML). ICES Headquarters, Copenhagen
Kapel CMO (1999) Diet of Arctic Foxes (Alopex lagopus) in Greenland. Arctic 52:289–293
Kühn S, Van Franeker JA (2012) Plastic ingestion by the northern fulmar (Fulmarus glacialis) in Iceland. Mar Pollut Bull 64:1252–1254. https://doi.org/10.1016/j.marpolbul.2012.02.027
Kühn S, Van Franeker JA (2020) Quantitative overview of marine debris ingested by marine megafauna. Mar Pollut Bull 151:110858. https://doi.org/10.1016/j.marpolbul.2019.110858
Kühn S, Bravo Rebolledo EL, Van Franeker JA (2015) Deleterious effects of litter on marine life. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer, Cham
Kühn S, Schaafsma FL, Van Werven B, Flores H, Bergmann M, Egelkraut-Holtus M, Tekman MB, Van Franeker JA (2018) Plastic ingestion by juvenile polar cod (Boreogadus saida) in the Arctic Ocean. Polar Biol 41:1269–1278. https://doi.org/10.1007/s00300-018-2283-8
Kühn S, Booth AM, Sørensen L, Van Oyen A, Van Franeker JA (2020) Transfer of additive chemicals from marine plastic debris to the stomach oil of northern fulmars. Front Environ Sci 8:138. https://doi.org/10.3389/fenvs.2020.00138
Kühn S, Van Oyen A, Bravo Rebolledo EL, Ask AV, Van Franeker JA (2021) Polymer types ingested by northern fulmars (Fulmarus glacialis) and southern hemisphere relatives. Environ Sci Polar Res 28:1643–1655. https://doi.org/10.1007/s11356-020-10540-6
Lusher AL, Provencher JF, Baak JE, Hamilton BM, Vorkamp K, Hallanger IG, Pijogge L, Liboiron M, Bourdages M, Hammer S (2022) Monitoring litter and microplastics in Arctic mammals and bird. Arc Sci. https://doi.org/10.1139/AS-2021-0058
Mai L, Bao LJ, Wong CS, Zeng EY (2018) Microplastics in the terrestrial environment. In: Zeng EY (ed) Microplastic contamination in aquatic environments. Elsevier, Amsterdam
Malinen A (2021) Microplastic ingestion by Atlantic mackerel and blue whiting in Icelandic waters. Master’s thesis, University of the Westfjords, Ísafjörður, Iceland, p. 65 http://hdl.handle.net/1946/39152
Mallory ML (2006) The Northern Fulmar (Fulmarus glacialis) in Arctic Canada: ecology, threats, and what it tells us about marine environmental conditions. Environ Rev 14:187–216. https://doi.org/10.1139/a06-003
Mallory ML (2008) Marine plastic debris in northern fulmars from the Canadian high Arctic. Mar Pollut Bull 56:1501–1504. https://doi.org/10.1016/j.marpolbul.2008.04.017
Marquard-Petersen U (1998) Food habits of Arctic wolves in Greenland. J Mammal 79:236–244. https://doi.org/10.2307/1382859
Moore RC, Loseto L, Noel M, Etemadifar A, Brewster JD, MacPhee S, Bendell L, Ross PS (2019) Microplastics in beluga whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar Pollut Bull 150:110723. https://doi.org/10.1016/j.marpolbul.2019.110723
Moore RC, Noel M, Etemadifar A, Loseto L, Posacka AM, Bendell L, Ross PS (2022) Microplastics in beluga whale (Delphinapterus leucas) prey: an exploratory assessment of trophic transfer in the Beaufort Sea. Sci Tot Env 806:150201. https://doi.org/10.1016/j.scitotenv.2021.150201
MSFD (2013) Guidance on monitoring of marine litter in European seas. A guidance document within the common implementation strategy for the marine strategy framework directive. Technical subgroup on marine litter. Publications Office of the European Union, Luxembourg
Nashoug BF (2017) Sources of marine litter. Workshop report Svalbard. Report nr 1017. Svolvær, Lofoten. pp. 3–18 https://pame.is
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara R, Simpson G, Solymos P (2020) Vegan: community ecology package. R package version 2.5-6. 2019
Pálsson S, Hersteinsson P, Unnsteinsdóttir ER, Nielsen ÓK (2015) Population limitation in a non-cyclic arctic fox population in a changing climate. Œecol 180:1147–1157. https://doi.org/10.1007/s00442-015-3536-7
PAME (2019) Desktop study on marine litter including microplastics in the Arctic. 11th Arctic council ministerial meeting, Rovaniemi, Finland, p. 138 http://hdl.handle.net/11374/2389
PlasticsEurope (2020) An analysis of European plastics production, demand, and waste data. PlasticsEurope—Association of plastics manufacturers, Brussels, Belgium, p. 64 https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts-2020
Prestrud P (1992) Food habits and observations of the hunting behaviour of Arctic foxes, Alopex lagopus, in Svalbard. Can Field Nat 106:225–236
Provencher JF, Gaston AJ, Mallory M (2009) Evidence for increased ingestion of plastics by northern fulmars (Fulmarus glacialis) in the Canadian Arctic. Mar Pollut Bull 58:1078–1096. https://doi.org/10.1016/j.marpolbul.2009.04.002
Puskic PS, Lavers JL, Bond AL (2020) A critical review of harm associated with plastic ingestion on vertebrates. Sci Total Environ 743:140666. https://doi.org/10.1016/j.scitotenv.2020.140666
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raubenheimer K, Urho N (2020) Possible elements of a new global agreement to prevent plastic pollution. Nordic Council of Ministers, Denmark, p 148
Rillig MC (2012) Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol 46:6453–6454. https://doi.org/10.1021/es302011r
Rochman CM, Hoellein T (2020) The global odyssey of plastic pollution. Science 368:1184–1185. https://doi.org/10.1126/science.abc4428
Ryan PG (2016) Ingestion of plastics by marine organisms. In: Takada H, Karapanagioti H (eds) Hazardous chemicals associated with plastics in the marine environment. Springer, Cham, pp 235–266
Savory GA, Hunter CM, Wooller MJ, O’Brien DM (2014) Anthropogenic food use and diet overlap between red foxes (Vulpes vulpes) and arctic foxes (Vulpes lagopus) in Prudhoe Bay, Alaska. Can J Zool 92:657–663. https://doi.org/10.1139/cjz-2013-0283
Skúladóttir E (2019) Plast í meltingarvegi refa (Vulpes lagopus) á Íslandi. [Gastrointestinal plastic in Arctic fox (Vulpes lagopus) in Iceland] Umhverfisstofnun, Report number NÍ-19015, Reykjavik, Iceland, p. 17. http://hdl.handle.net/10802/21764
Snæþórsson AÖ (2021) Plast í meltingarvegi fýla við Ísland árið 2020 [Plastic in the gastrointestinal tract of fulmars in Iceland in 2020]. Náttúrustofa Norðausturlands, Húsavík
Tanaka K, Takada H, Yamashita R, Mizukawa K, Watanuki FM, Y, (2015) Facilitated leaching of additive-derived PBDEs from plastic by seabirds’ stomach oil and accumulation in tissues. Environ Sci Technol 49:11799–11807. https://doi.org/10.1021/acs.est.5b01376
Tanaka K, Watanuki Y, Takada H, Ishizuka M, Yamashita R, Kazama M, Hiki N, Kashiwada F, Mizukawa K, Mizukawa H, Hyrenbach D, Hester M, Nakayama IY, SMM, (2020) In vivo accumulation of plastic-derived chemicals into seabird tissues. Curr Biol 30:723–728. https://doi.org/10.1016/j.cub.2019.12.037
Tekman ML, Walther BA, Peter C, Gutow L, Bergmann M (2022) Impacts of plastic pollution in the oceans on marine species, biodiversity and ecosystems. WWF GermanyBerlin, Germany, p 221
Trevail AM, Gabrielsen GW, Kühn S, Van Franeker JA (2015) Elevated levels of ingested plastic in a high Arctic seabird, the northern fulmar (Fulmarus glacialis). Pol Biol 38:975–981. https://doi.org/10.1007/s00300-015-1657-4
Umhverfisstofnun (2020) Uppgjör áætlunar um refaveiðar 2017–2019 [Settlement of the fox hunting plan]. Baldursson SRB (ed), Umhverfisstofnun, Reykjavik, Iceland, pp 11. https://www.ust.is/library/Skrar/Einstaklingar/Veidi/Refur/Uppgj%C3%B6r%20%C3%A1%C3%A6tlunar%20um%20refavei%C3%B0ar%202017_2019-UST-0570.pdf
UNEP/MAP (2018) Defining the most representative species for IMAP candidate indicator 24. SPA/RAC. Galgani, F. (ed.), Tunis, Tunisia, pp 42. https://www.rac-spa.org/sites/default/files/doc_marine_litter/imap_eng_web.pdf
Van Franeker JA, Blaize C, Danielsen J, Fairclough K, Gollan J, Guse N, Hansen PL, Heubeck M, Jensen JK, Le Guillou G, Olsen B, Olsen KO, Pedersen J, Stienen EW, Turner DM (2011) Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ Pollut 159:2609–2615. https://doi.org/10.1016/j.envpol.2011.06.008
Vestfjarðastofa (2022) Westfjords regional development office—Vestfjarðastofa. https://www.vestfirdir.is/is/eng. Accessed 27 June 2022
Yu SP, Cole M, Chan BKK (2020) Effects of microplastic on zooplankton survival and sublethal responses. Oceanogr Mar Biol 58:351–394
Acknowledgements
We would like to thank Ingeborg Hallanger and one anonymous reviewer for their valuable comments which have helped to improve the manuscript very much.
Funding
BT was partially funded by the Environment Agency of Iceland, Umhverfisstofnun, Suðurlandsbraut 24, 108 Reykjavík, Iceland under its OSPAR agreement Research Task on plastic in the Atlantic.
Author information
Authors and Affiliations
Contributions
BT Conceptualization, sample collection (2020), laboratory analysis, original draft preparation. EU Conceptualization, sample collection (1999–2018), supervision. FS Statistical analyses, supervision. SK Conceptualization, laboratory analysis, supervision. All authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest and have approved the final version of the manuscript.
Ethical approval
All international, national, and institutional guidelines for sampling of organisms in the polar regions have been followed.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Technau, B., Unnsteinsdóttir, E.R., Schaafsma, F.L. et al. Plastic and other anthropogenic debris in Arctic fox (Vulpes lagopus) faeces from Iceland. Polar Biol 45, 1403–1413 (2022). https://doi.org/10.1007/s00300-022-03075-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03075-8