Abstract
Arctic climate change is leading to an advance of plant phenology (the timing of life history events) with uncertain impacts on tundra ecosystems. Although the lengthening of the growing season is thought to lead to increased plant growth, we have few studies of how plant phenology change is altering tundra plant productivity. Here, we test the correspondence between 14 years of Salix arctica phenology data and radial growth on Qikiqtaruk–Herschel Island, Yukon Territory, Canada. We analysed stems from 28 individuals using dendroecology and linear mixed-effect models to test the statistical power of growing season length and climate variables to individually predict radial growth. We found that summer temperature best explained annual variation in radial growth. We found no strong evidence that leaf emergence date, earlier leaf senescence date, or total growing season length had any direct or lagged effects on radial growth. Radial growth was also not explained by interannual variation in precipitation, MODIS surface greenness (NDVI), or sea ice concentration. Our results demonstrate that at this site, for the widely distributed species S. arctica, temperature—but not growing season length—influences radial growth. These findings challenge the assumption that advancing phenology and longer growing seasons will increase the productivity of all plant species in Arctic tundra ecosystems.
Similar content being viewed by others
Introduction
The Arctic is warming three to four times faster than the rest of the planet (Meredith et al. 2019; You et al. 2021) and tundra plant communities are particularly sensitive to that warming (Elmendorf et al. 2015; Bjorkman et al. 2020). Climate change is resulting in a longer snow- and ice-free season, potentially facilitating longer growing seasons (Cleland et al. 2007; Khorsand Rosa et al. 2015; Prevéy et al. 2021; Frei and Henry 2021). Concurrent with these changes, shifts in distribution and abundance (Sturm et al. 2001; Elmendorf et al. 2012a), biomass (Hudson and Henry 2009), and phenology (timing of life history events) (Oberbauer et al. 2013) have been observed for species across the tundra. Previous research assumes that the altered phenology will correspond directly with increased growth of tundra plants (Myneni et al. 1997; Piao et al. 2007; Ernakovich et al. 2014; Park et al. 2016; Arndt et al. 2019; Kim et al. 2021). Arctic spectral greening trends from satellite vegetation indices, such as the Normalised Differential Vegetation Index (NDVI), are used as proxy metrics of tundra plant phenology (Piao et al. 2007; Zeng et al. 2013; Park et al. 2016) and plant productivity (Myneni et al. 1997; Kim et al. 2021). However, remote sensing studies of tundra phenology may not be capturing on-the-ground plant phenological and growth dynamics and may instead be influenced by other land surface changes such as community compositional shifts and snow cover (Helman 2018) particularly in Arctic systems (Arndt et al. 2019; Myers-Smith et al. 2020; Cao et al. 2020). Satellite (Myers-Smith et al. 2020) and in situ (Oberbauer et al. 2013) studies indicate widespread but variable phenological shifts in the Arctic. Remote sensing studies have linked phenology change with increased plant productivity in tundra ecosystems (Myneni et al. 1997; Park et al. 2016; Kim et al. 2021); however, in situ studies directly linking plant phenology change to plant growth are rare.
Plant phenology is changing throughout the tundra (Myers-Smith et al. 2019; Bjorkman et al. 2020; Prevéy et al. 2021), but the consequences on plant growth remain unclear. Phenology defines the bounds for plant activity, including photosynthesis, and has shifted around the Arctic due to warming (Assmann et al. 2019; Myers-Smith et al. 2020). The snow-free season across the Arctic has extended by 2–4 days per decade of warming (Piao et al. 2007; Barichivich et al. 2013; Park et al. 2016; Myers-Smith et al. 2019). Seasons are starting earlier and finishing earlier or later depending on the location and study metrics investigated (Piao et al. 2007; Zeng et al. 2011, 2013; Keenan and Richardson 2015; Park et al. 2016; Myers-Smith et al. 2019). Two key points in deciduous species’ phenology are leaf emergence and leaf senescence: the time between being the entire growing season. Leaf emergence and senescence are both shifting across the Arctic, leading to a longer, earlier growing season at many sites, although changing phenology is not uniform across sites or species (Oberbauer et al. 2013; Assmann et al. 2019; Myers-Smith et al. 2020). Earlier leaf emergence is associated with earlier snowmelt (Assmann et al. 2019; Myers-Smith et al. 2019) and declining sea ice (Post et al. 2009; Bhatt et al. 2010; Kerby and Post 2013), although some studies have identified trends towards later phenology in some species and locations usually aligning with later snowmelt (Wipf and Rixen 2010; Bjorkman et al. 2015). Earlier leaf emergence may expose individuals to late spring frost events (Wheeler et al. 2015) or other harsh conditions. Early senescence through deterministic leaf age (Oberbauer et al. 2013; Keenan and Richardson 2015), nutrient availability (Lim et al. 2007), or photoperiod (Arft et al. 1999) may also undermine any growth benefits of earlier leaf emergence. Whether plants can take advantage of an extended growing season to increase productivity and accumulate biomass is therefore uncertain.
At mid-latitudes of the Arctic, shrub growth can be particularly sensitive to climate (Myers-Smith et al. 2015a) and there is ground based and satellite evidence for rapid shrub expansion in the region (Fraser et al. 2011; Moffat et al. 2016; Myers-Smith et al. 2019). Dendroecology allows for the exploration of the growth history of shrubs based on the width of rings formed during seasonal woody tissue deposition (Myers-Smith et al. 2015b). Individual annual growth ring chronologies can be compared with environmental variables to reveal the climate sensitivity of radial growth over time. Through dendroecology, we can directly observe how changing conditions affect shrub growth, validating assumptions and models. Individual growth is a key element in our understanding of shrub expansion throughout the Arctic (Tape et al. 2006; Myers-Smith et al. 2011a; Elmendorf et al. 2012b; Myers-Smith et al. 2019; García Criado et al. 2020). Increasing shrub cover and canopy height alters ecosystem processes and species interactions (Myers-Smith et al. 2011a; Tape et al. 2016, 2018; Way and Lapalme 2021) through snow trapping (Myers-Smith and Hik 2013), shading (Blok et al. 2010), hydrology and albedo (Sturm et al. 2005; Pomeroy et al. 2006), food webs (Ravolainen et al. 2014)—including soil assemblages (DeMarco et al. 2014)—and habitat provision for wildlife (Boelman et al. 2015). Shrub encroachment has been linked to warming in studies using dendrochronology (Forbes et al. 2010), remote sensing (Myneni et al. 1997; Myers-Smith et al. 2020), field observations (Hudson and Henry 2009; Myers-Smith et al. 2011b), and experiments (Elmendorf et al. 2012a, 2015; Khorsand Rosa et al. 2015; Frei and Henry 2021). To accurately predict the future structure and function of northern ecosystems, we must understand how plant growth is changing (Myers-Smith et al. 2020), especially the role of phenology as ecological dynamics change under warming (Keenan and Richardson 2015; Myers-Smith et al. 2019; Bjorkman et al. 2020; Samplonius et al. 2020).
Arctic vegetation change plays a key role in regional and global feedback loops (Liston et al. 2002; Sturm et al. 2005; Pearson et al. 2013; Grosse et al. 2016) and carbon budgets (Piao et al. 2007; McGuire et al. 2009; Parker et al. 2021). As phenology changes, we expect compositional shifts and increased growth during longer growing seasons (Myneni et al. 1997; Ernakovich et al. 2014; Panchen and Gorelick 2017). Biome-wide shifts in growth rates and community composition could have profound implications for global carbon budgets through biomass accumulation (Piao et al. 2007) and decomposition (DeMarco et al. 2014). Warming drives earlier leaf emergence (Ernakovich et al. 2014; Park et al. 2016), which has been linked with increased plant productivity using remote sensing observations (Myneni et al. 1997). And thus, studies of satellite-derived spectral greening trends have linked changes in phenology to changes in plant productivity (Myneni et al. 1997; Park et al. 2016; Kim et al. 2021). Furthermore, accurate Earth system models depend on our understanding of plant growth–climate relationships and ecosystem–climate feedbacks (Sturm et al. 2005; Loranty and Goetz 2012; Richardson et al. 2013; Pearson et al. 2013; Fisher et al. 2018; Bonan and Doney 2018). Despite underpinning global models, uncertainty remains in the expected association between phenology and growth of Arctic plants and whether warmer temperatures or longer growing seasons are the primary drivers of increasing tundra plant productivity.
In this study, we use dendroecology to test the correspondence between in situ phenology observations, environmental factors, and radial growth of Salix arctica Pall. (Salicaceae) on Qikiqtaruk–Herschel Island in the Western Canadian Arctic. Salix arctica has a circum-Arctic distribution, woody tissues which enable dendrology, and exceptionally closely monitored phenology at the site (Myers-Smith et al. 2019), offering a suitable species for this study. At this site, S. arctica phenology has advanced in both spring and autumn, although autumn only marginally, overall lengthening the growing season by 2 days per decade (Myers-Smith et al. 2019). We test three questions: (1) Do longer growing seasons facilitate greater shrub radial growth? (2) Of phenological metrics, does leaf emergence date, senescence date, or growing season length best explain shrub radial growth? (3) Do climatic factors (air temperature, precipitation, sea ice concentration, or snowmelt), or maximum plant productivity (estimated through the spectral Normalised Difference Vegetation Index, NDVI), explain shrub radial growth better than phenology? We hypothesise that (1) a longer realised period of growth will increase radial growth; (2) growing season length will explain shrub radial growth better than leaf emergence or senescence date as it encompasses the cumulative change in the growth period; and (3) growing season length will best explain shrub radial growth relative to temperatures or other variables as short growing seasons in Arctic systems are limiting plant growth (Myers-Smith et al. 2019).
Methods
Study site
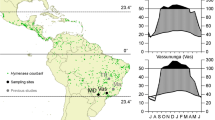
We studied S. arctica phenology and radial growth on Qikiqtaruk–Herschel Island, YT, Canada (69.57 °N, 138.90 °W) (Fig. 1). The island is approximately 100 km2 in area, with soils formed of marine and glacial deposits atop ice-rich permafrost (Burn and Zhang 2009). Qikiqtaruk sits at the northerly extent of tall shrubs, particularly Salix richardsonii Hook. (Salicaceae) (Myers-Smith et al. 2011b), which feature heavily in its flora alongside Eriophorum vaginatum L. (Cyperaceae) tussock tundra and dwarf shrub heath rich in S. arctica (Myers-Smith et al. 2019).
A map showing the location of Qikiqtaruk (69.57 °N, 138.90 °W) within Canada and Sentinel 2 false-colour images showing the location of the transects on Qikiqtaruk. The purple dots represent the ends of the five transects, and the purple box shows the area within which all samples were taken. The orange square shows the location of the phenology plots
Salix arctica sampling
As woody perennials, shrubs grow annual rings of wood which record radial growth over time (Myers-Smith et al. 2015b). Here, we focus on S. arctica, a prostrate willow with a circum-Arctic range which reaches as far north as the north coast of Greenland (Argus 2007). We collected 38 shrub samples on a coastal floodplain on the east side of the island in the Ice Creek watershed, a site of known vegetation change (Myers-Smith et al. 2019). The vegetation is dominated by Salix spp., defined by patches of S. richardsonii, a canopy-forming willow, with S. arctica at ground level. Cross-sections of 3–5 cm in length were taken from the base of the thickest stem of 6–8 individuals each along five parallel transects on the Qikiqtaruk floodplain in July 2016. Individuals were at least 10 m apart and transects were 50–100 m apart to lower the risk of sampling clones, particularly given the sprawling prostrate growth form of S. arctica (Argus 2007).
Dendroecological methods
We sliced thin sections (~ 25 µm) of each sample with a sledge microtome, then photographed (Online Resource 1), and measured the rings along four radii per sample using the ObjectJ package (1.04a) for ImageJ (2.0.0-rc-59/1.51j). We visually crossdated samples to check for partly missing rings, then averaged the radii for each individual, and crossdated again to check for entirely missing rings between samples.
We removed the first 2 years of radial growth data for each individual to account for age-related growth effects and the data from the year of sampling, as the growth for the season was not completed (Myers-Smith et al. 2015b). To maintain a minimum of 4 years of radial growth data after removing the data from 2016 and the first 2 years, the sample size was reduced to 28 individuals, running from 2002 to 2015 (Online Resource 1). Individuals with fewer than 7 years of radial growth were removed and we calculated the basal area increments from the ring width data. We then detrended the basal area increment data, fitting a smoothing spline (dplR package for R, f = 0.5, nyrs = 0.67), which removed variation in radial growth beyond interannual variation. We visually assessed different detrending methods, testing spline, negative exponential, and no detrending and found that spline detrending best removed individual growth trends to help focus our analyses on interannual variation in radial growth (Myers-Smith et al. 2015b). We therefore used the detrended basal area increment data for the main text analysis. We also conducted the same analysis using ring widths and the negative exponential detrending method and found similar results to our main text analysis, and these are presented in the supplementary materials (Online Resource 3). For the statistical analysis, all variables were normalised between 1 and − 1, so that effect sizes could be compared.
Phenology data
Phenological observations have been collected since 2001 by Yukon Parks Rangers on Qikiqtaruk every 2–3 days from April (snowmelt) until September (leaf senescence) along established transects (~ 250 m from our study site). The rangers record phenology throughout the growing season (Myers-Smith et al. 2019) in line with ITEX protocol (Prevéy et al. 2021). The phenological dates used in this study are the date of first leaf bud burst (leaf emergence) and the date of first yellowing of leaves (leaf senescence) averaged across the 20 S. arctica individuals in the observation transect. We calculated growing season length (GSL) as the number of days between leaf emergence and senescence. Please note that the individuals from the phenological modelling are not the same individuals (destructively) sampled for the dendroecological growth time-series. However, previous findings indicate that S. arctica phenology is generally consistent across individuals at the site (Myers-Smith et al. 2019; Assmann et al. 2020). Considering further the close proximity of the phenological monitoring sites and dendroecological transects we are therefore confident that the phenometrics from the phenological monitoring are representative for the individuals sampled for the dendroecological analysis.
Other environmental data
We also compared radial growth to seasonal air temperatures and precipitation, snowmelt, sea ice concentration, and productivity. The temperature data came from Environment Canada Qikiqtaruk–Herschel Island weather station (ID 1560) and precipitation data came from the ERA5-gridded dataset produced by Copernicus Climate Change Service and the European Centre for Medium-Range Weather Forecasts (Hersbach et al. 2020). We collated data into seasons (spring: April–May, summer: June–July, autumn: August–September, winter: October–March) including the lagged data for the preceding summer and autumn, as monthly resolution was higher than useful for this study. Snowmelt data are from the Qikiqtaruk phenology dataset, where the date at which transects are free of snow is recorded (Myers-Smith et al. 2019). We used the onset of sea ice melt data (Assmann et al. 2019), determined using the NOAA/NSIDC Climate Data Record (CDR) v3 Passive Microwave Sea Ice Concentrations (Meier et al. 2017). Productivity data are the annual maxima (estimated by smoothing trends in the data with a generalised additive model) of the MODIS MOD13A1v6 NDVI satellite dataset (Myers-Smith et al. 2020).
Statistical analysis
We selected our models a priori, using single-predictor models to compare individual variables and assess their predictive power on shrub radial growth. We used a Bayesian framework for our analyses including weakly informative priors of a half Student t prior with three degrees of freedom. The effect sizes of models were assessed relative to their credible intervals (95%). If the credible intervals for the estimated slope did not cross zero, we considered an effect to be significant. If the credible interval of the model slope sits at zero or fluctuates between overlapping zero and not overlapping zero, we consider the effect to be marginally significant.
To test the relationships between variables we used hierarchical linear mixed-effect models, with year, transect, and individual as random effects and individuals nested within transects. We used mixed models due the hierarchical structure of our data, caused by non-independence of individuals within transects and within a given year. Shrubs also shared conditions in each year, making them non-independent. The variability in absolute growth amongst individuals was high, as seen during crossdating, but due to sampling a single species within a relatively small area, similar relative growth responses were expected across groups. Thus, we chose not to use random slopes, only random intercepts. As a secondary analysis presented in the supplementary materials (Online Resource 2, Online Resource 3), we used a frequentist framework and compared models using AIC to see whether the models fit the data better than a null model using the conventional threshold (∆AICnull ≥ 2) (Akaike 1974). We also calculated conditional and marginal pseudo-R2 to test the absolute model fit including and excluding random effects, respectively (Nakagawa and Schielzeth 2013). We used models with maximum likelihood estimation for AIC comparisons and models with restricted maximum likelihood estimation for pseudo-R2 and effect size values. Residuals of models were visually assessed for normality with fitted residual plots and temporal autocorrelation (first- or second-order) with correlograms (Online Resource 2). We did not detect a signal of temporal autocorrelation, and residuals were similar across all models (Online Resource 2). We tested the correlation amongst all environmental and phenological variables and correlation coefficients varied between − 0.76 and 0.75 (Online Resource 1).
All statistical tests were carried out in R (3.6.3), via RStudio (1.2.1335), including the brms package for Bayesian analysis: Code and data are available at the following GitHub repository: https://github.com/ShrubHub/ShrubRingPhenoHub.
Results
Contrary to our first hypothesis that a longer realised period of growth will increase radial growth, we found that growing season length had no effect on S. arctica basal area increments, nor was there a lagged effect from the previous growing season (Table 1, Fig. 2). Contrary to our second hypothesis that growing season length will explain shrub radial growth better than leaf emergence or senescence date, it was leaf senescence date that was the best predictor of radial growth amongst these variables. Radial growth was significantly greater in years with an earlier leaf senescence date (negative effect), and we detected no relationship between radial growth and leaf emergence date, growing season length, or previous growing season length (Table 1, Fig. 2). Contrary to our third hypothesis that growing season length will best explain shrub radial growth relative to temperatures and other variables, we found that rather than growing season length, summer temperature was the best predictor amongst all variables. Summer temperature explained 2.8% of the variation in radial growth (Fig. 3, marginal pseudo-R2, Online Resource 2), with higher temperature coinciding with higher annual radial growth. However, the overall explanatory power of the models was low with no model explaining more than 2.8% of variation without including random effects (marginal pseudo-R2, Online Resource 2).
Radial growth corresponded weakly with phenological variables and more strongly with summer temperature. The relationships of radial growth with both leaf senescence and summer temperature are statistically significant. Scatter plots show the four phenological and two temperature variables we hypothesised to have relationships with radial growth (basal area increment, indexed) in a given year over the period 2002–2015. Trendlines are predictions from the hierarchical Bayesian models, dashed trendlines indicate a non-significant effect, and the shaded areas represent 95%, 80%, and 50% credible intervals of the model estimates
Only models of leaf senescence and summer temperature significantly explained variation in radial growth, and most variables showed no relationship to radial growth (Table 1). This plot shows standardised effect sizes (slopes) of hierarchical Bayesian models of phenological events (purple), seasonal temperature (red), seasonal precipitation (green), NDVI (yellow), minimum sea ice extent, sea ice concentration, and snowmelt date (all blue) on radial growth. The centre line is the effect and error bars are 95% credible intervals. For ease of comparison between effect sizes, explanatory variables in this analysis are variance scaled from − 1 to 1
All other variables aside from leaf senescence date and summer temperature were non-significant predictors of radial growth (Fig. 3). Leaf emergence date; current and previous years’ growing season length; temperatures from the winter, spring, autumn, and the previous year; all precipitation models; and snowmelt date did not explain variation in radial growth (Fig. 3). We used year as a random effect in our models (individual and transect level growth variation are accounted for during detrending and scaling of the radial growth index) and its effect was significant, indicating variation in radial growth amongst years beyond the effect of the bioclimatic variable of interest in that year (Online Resource 2, Online Resource 3). The model results agreed between the different detrending approaches, and although exact effect sizes differed slightly, the results were similar when models used detrended ring width data or used detrended basal area increment (Online Resource 3).
Discussion
Through a unique study of long-term in situ phenology monitoring and dendroecology, we compared interannual variation in phenology, environmental conditions, and NDVI to interannual variation in radial growth of S. arctica on Qikiqtaruk. We found that summer temperatures and leaf senescence—but not leaf emergence or growing season length—explained variation in radial growth for the widespread Arctic shrub S. arctica (Table 1, Fig. 3). Precipitation, sea ice, snowmelt, and NDVI did not correspond with variability in interannual radial growth in our study. Thus, we did not find support for the hypothesised relationship between phenology and radial growth. Our results suggest that factors other than the timing of the growing period from leaf emergence through senescence, such as temperature, can exert a larger influence on shrub growth in this tundra ecosystem. These findings ultimately have implications for how tundra shrub growth is modelled and thus the projection of Arctic carbon budgets.
Phenology
We found no evidence that earlier leaf emergence and longer growing seasons corresponded with increased radial growth in S. arctica, including growth in the following year (Table 1, Fig. 3). Results for preliminary analyses including other willow species from this and other sites have reached similar conclusions (Angers-Blondin 2019). Earlier leaf emergence did not result in greater S. arctica radial growth; however, we did find evidence of greater radial growth in years with earlier leaf senescence. Although we cannot identify a particular biological mechanism linking earlier leaf senescence with enhanced radial growth, early leaf senescence was correlated with warmer summer temperatures (Pearson’s product–moment correlation, df = 263, p < 0.001, ρ = − 0.60, Online Resource 1). Individuals may reach a threshold after intense early growth (Rumpf et al. 2014), allowing for early cessation of growth above ground. Alternatively, growth and leaf age could be deterministic (Oberbauer et al. 2013; Keenan and Richardson 2015; Semenchuk et al. 2016; Parker et al. 2017), with growth ending at a fixed time after growth begins each year. Or earlier leaf senescence could occur in years with warmer temperatures without a mechanistic link between the two variables. Our findings are in line with previous evidence that the timing of tundra plant senescence is driven at least in part by non-climatic factors (Arft et al. 1999; Oberbauer et al. 2013). Taken together, our results suggest that shifts to earlier shrub leaf emergence and longer growing seasons are not necessarily driving changes in tundra shrub growth, contrary to interpretations of satellite remote sensing data (Myneni et al. 1997; Zeng et al. 2011, 2013; Arndt et al. 2019) and reviews (Ernakovich et al. 2014).
Temperature
We found that higher summer temperatures increased the radial growth of S. arctica at our site. The summer is the peak season for growth and individuals are sensitive to warming in this period (Andreu-Hayles et al. 2020), as observed across the biome (Myers-Smith et al. 2015a; Myers-Smith and Hik 2018) from dendrochronology (Forbes et al. 2010; Blok et al. 2011; Myers-Smith et al. 2011a; Li et al. 2016; Weijers et al. 2018; Le Moullec et al. 2019; Prendin et al. 2022), repeat photography (Sturm et al. 2001; Tape et al. 2006), and experiments (Elmendorf et al. 2012a, 2015; Khorsand Rosa et al. 2015; Frei and Henry 2021). Temperature–growth relationships are heterogeneous across the tundra biome, with relatively low climate sensitivity observed on Qikiqtaruk compared with other mid-latitude tundra locations (Myers-Smith et al. 2015a). Growth response to temperature has decreased over time in another dwarf willow species, Salix polaris Wahlenb. (Salicaceae), at Bjørnøya, Svalbard (Owczarek et al. 2021), suggesting that growth responses may not be fixed over long timescales. The growth response to early leaf senescence suggests the importance of resource accumulation for growth in the following season, shrubs may senesce above ground but remain active below ground for longer periods. There is increasing evidence that above-ground phenology may be asynchronous with below-ground root growth (Blume‐Werry et al. 2016, 2017; Ögren 2017; Liu et al. 2022), though root phenology itself may not respond to autumn warming (Schwieger et al. 2018). Snow cover insulates shrubs from winter and spring temperatures (Kelsey et al. 2020; Rixen et al. 2022), and Krab et al. (2018) found diverging shrub radial growth responses to winter temperature, spring warming, and snowmelt amongst species. Vaccinium vitis‐idaea L. (Ericaceae) grew more with delayed snowmelt with a contrasting reduction in growth in Empetrum nigrum L. (Ericaceae). We, however, found no association between temperatures in the previous year and radial growth, and no relationship for winter, spring, and autumn temperatures and radial growth (Table 1, Fig. 3).
Hydrology
We did not find a strong influence of summer precipitation, sea ice, or snowmelt on interannual variation in radial growth S. arctica in this study. Growth of Arctic shrubs can be moisture limited (Keuper et al. 2012; Ackerman et al. 2017; Buchwal et al. 2020; Weijers 2022). Moisture sensitivity of growth can depend on temperature (Li et al. 2016) and can vary within (Thompson and Koenig 2018) and between sites (Myers-Smith et al. 2015a). Soils on Qikiqtaruk are frequently saturated, likely reducing the impacts of drought locally (Myers-Smith et al. 2019). We did not detect any influence of precipitation from summer rain, snowmelt, or cloud cover (Table 1, Fig. 3). Decreasing snow cover reduces soil insulation in winter and limits productivity increase under warming and earlier phenology in Alaska (Kelsey et al. 2020). The lack of a precipitation signal detected in our study could be influenced by our use of gridded climate datasets due to a lack of a complete local record for precipitation at this site. Gridded climate datasets poorly capture spatially variable precipitation, due to the paucity of Arctic meteorological stations and the high spatial variability of precipitation (Macias-Fauria et al. 2014; Myers‐Smith and Myers 2018). For sea ice, we found that lower annual minima and earlier melt are weakly associated with increased radial growth of S. arctica, although phenology for this species was not found to vary with sea ice extent (Assmann et al. 2019). Sea ice could influence plant growth and phenology through interactions with local climate (Post et al. 2009; Bhatt et al. 2010; Kerby and Post 2013; Macias-Fauria et al. 2017; Assmann et al. 2019) and drought-stress (Forchhammer 2017; Buchwal et al. 2020). We found no relationship between snowmelt date and radial growth, which is consistent with the primary mechanism of snowmelt controlling phenology and so influencing plant growth (Assmann et al. 2019; Myers-Smith et al. 2019). Taken together, our results suggest that temperature rather than growing season length, precipitation, or sea ice dynamics was the primary factor controlling S. arctica radial growth on Qikiqtaruk.
NDVI
We observed no correlation between NDVI and interannual variation in S. arctica radial growth, consistent with results for other shrub species at the site (Myers-Smith et al. 2019). Whilst satellite datasets do not entirely correspond with each other (Guay et al. 2014). and shrub biomass cannot be directly estimated from NDVI alone (Cunliffe et al. 2020), NDVI is easily scaled, well studied, and part of a broader picture of complex Arctic tundra vegetation change (Myers-Smith et al. 2020). Arctic shrubification has been linked with satellite-derived Arctic greening trends (Macias-Fauria et al. 2012), and comparing ground observations to spectral greening observed by satellites improves broad-scale interpretation of these trends (Myers-Smith et al. 2020). Correlation of NDVI and shrub growth has been found in some studies (Forbes et al. 2010; Macias-Fauria et al. 2012), but is not universal and varies with site and the time of year (Blok et al. 2011; Brehaut and Danby 2018; Andreu-Hayles et al. 2020). Taken together, these results suggest that satellite spectral greening indices are not capturing all of the variation in plant productivity, including the length of the snow-free season, indicated by analyses with shrub radial growth (Angers-Blondin 2019; Berner et al. 2020).
Study limitations
Whilst our findings bring together phenology and dendrochronology, two important fields of study of Arctic change, there are limitations. Sampling stem elongation (primary growth) and root collars rather than stems alone would improve the capture of interannual variation in shrub productivity. Primary and secondary (radial) growth can be driven by different controls (Bret-Harte et al. 2002; Campioli et al. 2012a, b), so study of annual stem increments or other measures of shrub growth in addition to radial growth would more robustly address questions of shrub growth (Myers-Smith et al. 2015b). Root collars show greater climate sensitivity (Ropars et al. 2017) and less response to individual conditions than stems (Sonesson and Callaghan 1991; Sadras and Denison 2009; Myers-Smith et al. 2015b), yet root collars are challenging to find and excavate in clonal species, such as S. arctica, and are more destructive to sample. We were not easily able to locate root collars consistently at this site (Angers-Blondin 2019). The destructive nature of dendrological sampling also prevented us from sampling the individuals in the long-term phenology transect directly, so we sampled nearby individuals as phenology is consistent across the site (Myers-Smith et al. 2019). Future research across different sites and species using localised climate and microenvironmental variables may shed more light on the relationships between plant phenology and growth.
Future study
Modern techniques facilitate below-ground monitoring of tundra plant phenology and root growth (Iversen et al. 2015; Sloan et al. 2016; Blume‐Werry et al. 2016, 2017), exposing an overlooked dimension of tundra dynamics. An increasing number of studies indicate phenological asynchrony above and below ground at sub-Arctic sites (Ögren 2017; Blume‐Werry et al. 2017), with below-ground root growth extending into the late summer and autumn in now thawed soils. Fungal symbiotes such as mycorrhizae can influence plant growth and carbon exchange in tundra shrubs which could be altering growth–climate interactions (Clemmensen et al. 2006; Compant et al. 2010; Deslippe et al. 2011). Iler et al. (2013) suggest that phenology responses to warming are reaching physiological limits in some Arctic and alpine species, potentially reducing the magnitude of future change. Collins et al. (2021) found that reproductive and vegetative phenologies are affected differently by experimental warming, which could alter ecosystem dynamics via trophic mismatches and resource allocation (Post and Forchhammer 2008; Clausen and Clausen 2013; Kerby 2015; Wheeler et al. 2015). There has been relatively little investigation of plant senescence and the drivers of the end of the growing season, creating uncertainty in our understanding of plant responses to warming across the growing season.
Innovative techniques such as drone-derived biomass estimates could also help with scaling up to landscape-wide analyses (Cunliffe et al. 2020). Newer approaches to studying tundra plant phenology such as time lapse cameras (a.k.a. phenocams) are overcoming inherent challenges of data collection in the Arctic (Westergaard-Nielsen et al. 2017; Richardson et al. 2018; Parmentier et al. 2021). Local observations of plant phenology and growth can be scaled up using drone and satellite data to bridge scale gaps and form a landscape perspective on tundra productivity change (Riihimäki et al. 2019; Assmann et al. 2019, 2020; Cunliffe et al. 2020). Challenges of scaling and data collection are being met by technological solutions, allowing us to see Arctic change from new angles and more clearly than ever before. Although further research is required, particularly for Arctic systems (Diepstraten et al. 2018), the increasing scope of monitoring of above- and below-ground plant responses encompassing phenology and growth allows for the investigation of key knowledge gaps about tundra ecosystem responses to global change.
Conclusion
Our findings demonstrate that plant phenology does not necessarily predict growth in an Arctic shrub, but that warmer temperatures in the summer are associated with increased annual radial growth. Interannual variation in precipitation, sea ice, snow cover, and MODIS NDVI for the landscape were not strongly related to radial growth. Our results indicate that future Arctic warming will likely enhance shrub growth and encroachment (Tape et al. 2006; Myers-Smith et al. 2011a; García Criado et al. 2020). Where this growth is not limited by water or nutrients (Mack et al. 2004; Myers-Smith et al. 2015a; Ackerman et al. 2017), there may be significant consequences for water, energy, and carbon fluxes (Loranty and Goetz 2012; Pearson et al. 2013; Parker et al. 2021). Taller shrub canopies could influence soil temperatures, litter decomposition rates, nutrient cycling, and ultimately the tundra carbon cycle (Sturm et al. 2005; Blok et al. 2010; Myers-Smith et al. 2011a; DeMarco et al. 2014; Way and Lapalme 2021). Whilst questions remain in these complex systems, studying shrub phenology and growth data for other sites and species—and incorporating a below-ground perspective on plant phenology (Iversen et al. 2015) and growth—will paint a clearer panarctic picture of plant responses to rapid Arctic warming (Myers-Smith et al. 2020). Investigating the magnitude and direction of change on the ground in tundra ecosystems is necessary to validate assumptions that underpin remote sensing studies (Myers-Smith et al. 2019; Piao et al. 2019; Cunliffe et al. 2020), strengthening our understanding of tundra plant responses to warming. Teasing apart the complex mechanisms between climate change and plant growth in tundra ecosystems is vital to improve projections of how Arctic vegetation change influences global climate.
Data availability
The code and data used for this study and generated during our analyses are available at the following GitHub repository: https://github.com/ShrubHub/ShrubRingPhenoHub
References
Ackerman D, Griffin D, Hobbie SE, Finlay JC (2017) Arctic shrub growth trajectories differ across soil moisture levels. Glob Change Biol 23:4294–4302. https://doi.org/10.1111/gcb.13677
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
Andreu-Hayles L, Gaglioti BV, Berner LT et al (2020) A narrow window of summer temperatures associated with shrub growth in Arctic Alaska. Environ Res Lett 15:105012. https://doi.org/10.1088/1748-9326/ab897f
Angers-Blondin S (2019) Reading between the rings: climatic and biotic controls of shrub growth and expansion in the tundra biome. PhD Thesis, University of Edinburgh, School of Geosciences
Arft AM, Walker MD, Gurevitch J et al (1999) Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecol Monogr 69:491. https://doi.org/10.2307/2657227
Argus GW (2007) Salix (Salicaceae) distribution maps and a synopsis of their classification in North America, North of Mexico. Harv Pap Bot 12:335–368. https://doi.org/10.3100/1043-4534(2007)12[335:SSDMAA]2.0.CO;2
Arndt KA, Santos MJ, Ustin S et al (2019) Arctic greening associated with lengthening growing seasons in Northern Alaska. Environ Res Lett 14:125018. https://doi.org/10.1088/1748-9326/ab5e26
Assmann JJ, Myers-Smith IH, Phillimore AB et al (2019) Local snow melt and temperature—but not regional sea ice—explain variation in spring phenology in coastal Arctic tundra. Glob Change Biol. https://doi.org/10.1111/gcb.14639
Assmann JJ, Myers-Smith IH, Kerby JT et al (2020) Drone data reveal heterogeneity in tundra greenness and phenology not captured by satellites. Environ Res Lett 15:125002. https://doi.org/10.1088/1748-9326/abbf7d
Barichivich J, Briffa KR, Myneni RB et al (2013) Large-scale variations in the vegetation growing season and annual cycle of atmospheric CO 2 at high northern latitudes from 1950 to 2011. Glob Change Biol 19:3167–3183. https://doi.org/10.1111/gcb.12283
Berner LT, Massey R, Jantz P et al (2020) Summer warming explains widespread but not uniform greening in the Arctic tundra biome. Nat Commun 11:4621. https://doi.org/10.1038/s41467-020-18479-5
Bhatt US, Walker DA, Raynolds MK et al (2010) Circumpolar arctic tundra vegetation change is linked to sea ice decline. Earth Interact 14:1–20. https://doi.org/10.1175/2010EI315.1
Bjorkman AD, Elmendorf SC, Beamish AL et al (2015) Contrasting effects of warming and increased snowfall on Arctic tundra plant phenology over the past two decades. Glob Change Biol 21:4651–4661. https://doi.org/10.1111/gcb.13051
Bjorkman AD, García Criado M, Myers-Smith IH et al (2020) Status and trends in Arctic vegetation: evidence from experimental warming and long-term monitoring. Ambio 49:678–692. https://doi.org/10.1007/s13280-019-01161-6
Blok D, Heijmans MM, Schaepman-Strub G et al (2010) Shrub expansion may reduce summer permafrost thaw in Siberian tundra. Glob Change Biol 16:1296–1305. https://doi.org/10.1111/j.1365-2486.2009.02110.x
Blok D, Sass-Klaassen U, Schaepman-Strub G et al (2011) What are the main climate drivers for shrub growth in Northeastern Siberian tundra? Biogeosciences 8:1169–1179. https://doi.org/10.5194/bg-8-1169-2011
Blume-Werry G, Wilson SD, Kreyling J, Milbau A (2016) The hidden season: growing season is 50% longer below than above ground along an arctic elevation gradient. New Phytol 209:978–986. https://doi.org/10.1111/nph.13655
Blume-Werry G, Jansson R, Milbau A (2017) Root phenology unresponsive to earlier snowmelt despite advanced above-ground phenology in two subarctic plant communities. Funct Ecol 31:1493–1502. https://doi.org/10.1111/1365-2435.12853
Boelman NT, Gough L, Wingfield J et al (2015) Greater shrub dominance alters breeding habitat and food resources for migratory songbirds in Alaskan arctic tundra. Global Change Biol 21:1508–1520. https://doi.org/10.1111/gcb.12761
Bonan GB, Doney SC (2018) Climate, ecosystems, and planetary futures: the challenge to predict life in earth system models. Science 359:eaam8328. https://doi.org/10.1126/science.aam8328
Brehaut L, Danby RK (2018) Inconsistent relationships between annual tree ring-widths and satellite-measured NDVI in a mountainous subarctic environment. Ecol Indic 91:698–711. https://doi.org/10.1016/j.ecolind.2018.04.052
Bret-Harte MS, Shaver GR, Chapin FS (2002) Primary and secondary stem growth in arctic shrubs: implications for community response to environmental change. J Ecol 90:251–267. https://doi.org/10.1046/j.1365-2745.2001.00657.x
Buchwal A, Sullivan PF, Macias-Fauria M et al (2020) Divergence of Arctic shrub growth associated with sea ice decline. Proc Natl Acad Sci 117:33334–33344. https://doi.org/10.1073/pnas.2013311117
Burn CR, Zhang Y (2009) Permafrost and climate change at Herschel Island (Qikiqtaruq), Yukon Territory. Canada J Geophys Res 114:F02001. https://doi.org/10.1029/2008JF001087
Campioli M, Leblans N, Michelsen A (2012a) Stem secondary growth of tundra shrubs: impact of environmental factors and relationships with apical growth. Arct Antarct Alp Res 44:16–25. https://doi.org/10.1657/1938-4246-44.1.16
Campioli M, Leblans N, Michelsen A (2012b) Twenty-two years of warming, fertilisation and shading of subarctic heath shrubs promote secondary growth and plasticity but not primary growth. PLoS ONE 7:e34842. https://doi.org/10.1371/journal.pone.0034842
Cao R, Feng Y, Liu X et al (2020) Uncertainty of vegetation green-up date estimated from vegetation indices due to snowmelt at northern middle and high latitudes. Remote Sens 12:190. https://doi.org/10.3390/rs12010190
Clausen KK, Clausen P (2013) Earlier Arctic springs cause phenological mismatch in long-distance migrants. Oecologia 173:1101–1112. https://doi.org/10.1007/s00442-013-2681-0
Cleland E, Chuine I, Menzel A et al (2007) Shifting plant phenology in response to global change. Trends Ecol Evol 22:357–365. https://doi.org/10.1016/j.tree.2007.04.003
Clemmensen KE, Michelsen A, Jonasson S, Shaver GR (2006) Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two arctic tundra ecosystems. New Phytol 171:391–404. https://doi.org/10.1111/j.1469-8137.2006.01778.x
Collins CG, Elmendorf SC, Hollister RD et al (2021) Experimental warming differentially affects vegetative and reproductive phenology of tundra plants. Nat Commun 12:3442. https://doi.org/10.1038/s41467-021-23841-2
Compant S, Van Der Heijden MGA, Sessitsch A (2010) Climate change effects on beneficial plant-microorganism interactions: climate change and beneficial plant-microorganism interactions. FEMS Microbiol Ecol. https://doi.org/10.1111/j.1574-6941.2010.00900.x
Cunliffe AM, Assmann JJ, Daskalova G et al (2020) Aboveground biomass corresponds strongly with drone-derived canopy height but weakly with greenness (NDVI) in a shrub tundra landscape. Environ Res Lett 15:125004. https://doi.org/10.1088/1748-9326/aba470
DeMarco J, Mack MC, Bret-Harte MS (2014) Effects of arctic shrub expansion on biophysical vs. biogeochemical drivers of litter decomposition. Ecology 95:1861–1875. https://doi.org/10.1890/13-2221.1
Deslippe JR, Hartmann M, Mohn WW, Simard SW (2011) Long-term experimental manipulation of climate alters the ectomycorrhizal community of Betula nana in Arctic tundra: climate change alters ectomycorrhizal fungi. Glob Change Biol 17:1625–1636. https://doi.org/10.1111/j.1365-2486.2010.02318.x
Diepstraten RAE, Jessen TD, Fauvelle CMD, Musiani MM (2018) Does climate change and plant phenology research neglect the Arctic tundra? Ecosphere 9:e02362. https://doi.org/10.1002/ecs2.2362
Elmendorf SC, Henry GHR, Hollister RD et al (2012a) Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time: warming effects on tundra vegetation. Ecol Lett 15:164–175. https://doi.org/10.1111/j.1461-0248.2011.01716.x
Elmendorf SC, Henry GHR, Hollister RD et al (2012b) Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat Clim Change 2:453–457. https://doi.org/10.1038/nclimate1465
Elmendorf SC, Henry GHR, Hollister RD et al (2015) Experiment, monitoring, and gradient methods used to infer climate change effects on plant communities yield consistent patterns. Proc Natl Acad Sci 112:448–452. https://doi.org/10.1073/pnas.1410088112
Ernakovich JG, Hopping KA, Berdanier AB et al (2014) Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob Change Biol 20:3256–3269. https://doi.org/10.1111/gcb.12568
Fisher RA, Koven CD, Anderegg WRL et al (2018) Vegetation demographics in earth system models: a review of progress and priorities. Glob Change Biol 24:35–54. https://doi.org/10.1111/gcb.13910
Forbes BC, Fauria MM, Zetterberg P (2010) Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Glob Change Biol 16:1542–1554. https://doi.org/10.1111/j.1365-2486.2009.02047.x
Forchhammer M (2017) Sea-ice induced growth decline in Arctic shrubs. Biol Lett 13:20170122. https://doi.org/10.1098/rsbl.2017.0122
Fraser RH, Olthof I, Carrière M et al (2011) Detecting long-term changes to vegetation in northern Canada using the landsat satellite image archive. Environ Res Lett 6:045502. https://doi.org/10.1088/1748-9326/6/4/045502
Frei ER, Henry GHR (2021) Long-term effects of snowmelt timing and climate warming on phenology, growth, and reproductive effort of Arctic tundra plant species. Arct Sci. https://doi.org/10.1139/as-2021-0028
García Criado M, Myers-Smith IH, Bjorkman AD et al (2020) Woody plant encroachment intensifies under climate change across tundra and savanna biomes. Glob Ecol Biogeogr 29:925–943. https://doi.org/10.1111/geb.13072
Grosse G, Goetz S, McGuire AD et al (2016) Changing permafrost in a warming world and feedbacks to the earth system. Environ Res Lett 11:040201. https://doi.org/10.1088/1748-9326/11/4/040201
Guay KC, Beck PSA, Berner LT et al (2014) Vegetation productivity patterns at high northern latitudes: a multi-sensor satellite data assessment. Glob Change Biol 20:3147–3158. https://doi.org/10.1111/gcb.12647
Helman D (2018) Land surface phenology: what do we really ‘see’ from space? Sci Total Environ 618:665–673. https://doi.org/10.1016/j.scitotenv.2017.07.237
Hersbach H, Bell B, Berrisford P et al (2020) The ERA5 global reanalysis. Q J R Meteorol Soc 146:1999–2049. https://doi.org/10.1002/qj.3803
Hudson JMG, Henry GHR (2009) Increased plant biomass in a High Arctic heath community from 1981 to 2008. Ecology 90:2657–2663. https://doi.org/10.1890/09-0102.1
Iler AM, Høye TT, Inouye DW, Schmidt NM (2013) Nonlinear flowering responses to climate: are species approaching their limits of phenological change? Philos Trans R Soc B Biol Sci 368:20120489. https://doi.org/10.1098/rstb.2012.0489
Iversen CM, Sloan VL, Sullivan PF et al (2015) The unseen iceberg: plant roots in arctic tundra. New Phytol 205:34–58. https://doi.org/10.1111/nph.13003
Keenan TF, Richardson AD (2015) The timing of autumn senescence is affected by the timing of spring phenology: implications for predictive models. Glob Change Biol 21:2634–2641. https://doi.org/10.1111/gcb.12890
Kelsey KC, Pedersen SH, Leffler AJ et al (2020) Winter snow and spring temperature have differential effects on vegetation phenology and productivity across plant communities. Glob Change Biol. https://doi.org/10.1111/gcb.15505
Kerby JT (2015) Phenology in a chancing Arctic: Linking trophic interactions across scales. PhD Thesis, The Pennsylvania State University, Intercollege Graduate Degree Program in Ecology
Kerby JT, Post E (2013) Advancing plant phenology and reduced herbivore production in a terrestrial system associated with sea ice decline. Nat Commun 4:2514. https://doi.org/10.1038/ncomms3514
Keuper F, Parmentier F-JW, Blok D et al (2012) Tundra in the rain: differential vegetation responses to three years of experimentally doubled summer precipitation in Siberian shrub and Swedish bog tundra. Ambio 41:269–280. https://doi.org/10.1007/s13280-012-0305-2
Khorsand Rosa R, Oberbauer SF, Starr G et al (2015) Plant phenological responses to a long-term experimental extension of growing season and soil warming in the tussock tundra of Alaska. Glob Change Biol 21:4520–4532. https://doi.org/10.1111/gcb.13040
Kim J, Kim Y, Zona D et al (2021) Carbon response of tundra ecosystems to advancing greenup and snowmelt in Alaska. Nat Commun 12:6879. https://doi.org/10.1038/s41467-021-26876-7
Krab EJ, Roennefarth J, Becher M et al (2018) Winter warming effects on tundra shrub performance are species-specific and dependent on spring conditions. J Ecol 106:599–612. https://doi.org/10.1111/1365-2745.12872
Le Moullec M, Buchwal A, Wal R et al (2019) Annual ring growth of a widespread high arctic shrub reflects past fluctuations in community-level plant biomass. J Ecol 107:436–451. https://doi.org/10.1111/1365-2745.13036
Li B, Heijmans MMPD, Berendse F et al (2016) The role of summer precipitation and summer temperature in establishment and growth of dwarf shrub Betula nana in northeast Siberian tundra. Polar Biol 39:1245–1255. https://doi.org/10.1007/s00300-015-1847-0
Lim PO, Kim HJ, Gil Nam H (2007) Leaf senescence. Annu Rev Plant Biol 58:115–136. https://doi.org/10.1146/annurev.arplant.57.032905.105316
Liston GE, Mcfadden JP, Sturm M, Pielke RA (2002) Modelled changes in arctic tundra snow, energy and moisture fluxes due to increased shrubs. Glob Change Biol 8:17–32. https://doi.org/10.1046/j.1354-1013.2001.00416.x
Liu H, Wang H, Li N et al (2022) Phenological mismatches between above- and belowground plant responses to climate warming. Nat Clim Change 12:97–102. https://doi.org/10.1038/s41558-021-01244-x
Loranty MM, Goetz SJ (2012) Shrub expansion and climate feedbacks in Arctic tundra. Environ Res Lett 7:011005. https://doi.org/10.1088/1748-9326/7/1/011005
Macias-Fauria M, Forbes BC, Zetterberg P, Kumpula T (2012) Eurasian Arctic greening reveals teleconnections and the potential for structurally novel ecosystems. Nat Clim Change 2:613–618. https://doi.org/10.1038/nclimate1558
Macias-Fauria M, Seddon AWR, Benz D et al (2014) Spatiotemporal patterns of warming. Nat Clim Change 4:845–846. https://doi.org/10.1038/nclimate2372
Macias-Fauria M, Karlsen SR, Forbes BC (2017) Disentangling the coupling between sea ice and tundra productivity in Svalbard. Sci Rep 7:8586. https://doi.org/10.1038/s41598-017-06218-8
Mack MC, Schuur EAG, Bret-Harte MS et al (2004) Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431:440–443. https://doi.org/10.1038/nature02887
McGuire AD, Anderson LG, Christensen TR et al (2009) Sensitivity of the carbon cycle in the Arctic to climate change. Ecol Monogr 79:523–555. https://doi.org/10.1890/08-2025.1
Meier WN, Fetterer F, Savoie M, et al (2017) NOAA/NSIDC Climate Data Record of Passive Microwave Sea Ice Concentration, Version 3
Meredith M, Sommerkorn M, Cassotta S, et al (2019) Chapter 3: polar regions. In: IPCC special report on the ocean and cryosphere in a changing climate. IPCC Intergovernmental Panel on Climate Change, Geneva, Switzerland, pp 313–414
Moffat ND, Lantz TC, Fraser RH, Olthof I (2016) Recent vegetation change (1980–2013) in the tundra ecosystems of the Tuktoyaktuk Coastlands, NWT, Canada. Arct Antarct Alp Res 48:581–597. https://doi.org/10.1657/AAAR0015-063
Myers-Smith IH, Hik DS (2013) Shrub canopies influence soil temperatures but not nutrient dynamics: an experimental test of tundra snow–shrub interactions. Ecol Evol 3:3683–3700. https://doi.org/10.1002/ece3.710
Myers-Smith IH, Hik DS (2018) Climate warming as a driver of tundra shrubline advance. J Ecol 106:547–560. https://doi.org/10.1111/1365-2745.12817
Myers-Smith IH, Myers JH (2018) Comment on “Precipitation drives global variation in natural selection.” Science 359:eaan5028. https://doi.org/10.1126/science.aan5028
Myers-Smith IH, Forbes BC, Wilmking M et al (2011a) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6:045509. https://doi.org/10.1088/1748-9326/6/4/045509
Myers-Smith IH, Hik DS, Kennedy C et al (2011b) Expansion of canopy-forming willows over the twentieth century on Herschel Island, Yukon territory, Canada. Ambio 40:610–623. https://doi.org/10.1007/s13280-011-0168-y
Myers-Smith IH, Elmendorf SC, Beck PSA et al (2015a) Climate sensitivity of shrub growth across the tundra biome. Nat Clim Change 5:887–891. https://doi.org/10.1038/nclimate2697
Myers-Smith IH, Hallinger M, Blok D et al (2015b) Methods for measuring arctic and alpine shrub growth: a review. Earth-Sci Rev 140:1–13. https://doi.org/10.1016/j.earscirev.2014.10.004
Myers-Smith IH, Grabowski MM, Thomas HJD et al (2019) Eighteen years of ecological monitoring reveals multiple lines of evidence for tundra vegetation change. Ecol Monogr 89:e01351. https://doi.org/10.1002/ecm.1351
Myers-Smith IH, Kerby JT, Phoenix GK et al (2020) Complexity revealed in the greening of the Arctic. Nat Clim Change 10:106–117. https://doi.org/10.1038/s41558-019-0688-1
Myneni RB, Keeling CD, Tucker CJ et al (1997) Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386:698–702. https://doi.org/10.1038/386698a0
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Oberbauer SF, Elmendorf SC, Troxler TG et al (2013) Phenological response of tundra plants to background climate variation tested using the international tundra experiment. Philos Trans R Soc B Biol Sci 368:20120481. https://doi.org/10.1098/rstb.2012.0481
Ögren A (2017) Is above- and belowground phenology of Eriophorum vaginatum in sync in a peatland underlain by permafrost? Master’s Thesis, Umeå University, Department of Ecology and Environmental Sciences
Owczarek P, Opała-Owczarek M, Migała K (2021) Post-1980s shift in the sensitivity of tundra vegetation to climate revealed by the first dendrochronological record from Bear Island (Bjørnøya), western Barents Sea. Environ Res Lett 16:014031. https://doi.org/10.1088/1748-9326/abd063
Panchen ZA, Gorelick R (2017) Prediction of Arctic plant phenological sensitivity to climate change from historical records. Ecol Evol 7:1325–1338. https://doi.org/10.1002/ece3.2702
Park T, Ganguly S, Tømmervik H et al (2016) Changes in growing season duration and productivity of northern vegetation inferred from long-term remote sensing data. Environ Res Lett 11:084001. https://doi.org/10.1088/1748-9326/11/8/084001
Parker TC, Tang J, Clark MB et al (2017) Ecotypic differences in the phenology of the tundra species Eriophorum vaginatum reflect sites of origin. Ecol Evol 7:9775–9786. https://doi.org/10.1002/ece3.3445
Parker TC, Thurston AM, Raundrup K et al (2021) Shrub expansion in the Arctic may induce large-scale carbon losses due to changes in plant-soil interactions. Plant Soil. https://doi.org/10.1007/s11104-021-04919-8
Parmentier F-JW, Nilsen L, Tømmervik H, Cooper EJ (2021) A distributed time-lapse camera network to track vegetation phenology with high temporal detail and at varying scales. Earth Syst Sci Data 13:3593–3606. https://doi.org/10.5194/essd-13-3593-2021
Pearson RG, Phillips SJ, Loranty MM et al (2013) Shifts in Arctic vegetation and associated feedbacks under climate change. Nat Clim Change 3:673–677. https://doi.org/10.1038/nclimate1858
Piao S, Friedlingstein P, Ciais P et al (2007) Growing season extension and its impact on terrestrial carbon cycle in the northern hemisphere over the past 2 decades: phenology and carbon cycle in NH. Glob Biogeochem Cycles. https://doi.org/10.1029/2006GB002888
Piao S, Liu Q, Chen A et al (2019) Plant phenology and global climate change: current progresses and challenges. Glob Change Biol 25:1922–1940. https://doi.org/10.1111/gcb.14619
Pomeroy JW, Bewley DS, Essery RLH et al (2006) Shrub tundra snowmelt. Hydrol Process 20:923–941. https://doi.org/10.1002/hyp.6124
Post E, Forchhammer MC (2008) Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philos Trans R Soc B Biol Sci 363:2367–2373. https://doi.org/10.1098/rstb.2007.2207
Post E, Forchhammer MC, Bret-Harte MS et al (2009) Ecological dynamics across the Arctic associated with recent climate change. Science 325:1355–1358. https://doi.org/10.1126/science.1173113
Prendin AL, Normand S, Carrer M et al (2022) Influences of summer warming and nutrient availability on Salix glauca L. growth in Greenland along an ice to sea gradient. Sci Rep 12:3077. https://doi.org/10.1038/s41598-022-05322-8
Prevéy J, Elmendorf S, Bjorkman A et al (2021) The tundra phenology database: more than two decades of tundra phenology responses to climate change. Arct Sci. https://doi.org/10.1139/AS-2020-0041
Ravolainen VT, Bråthen KA, Yoccoz NG et al (2014) Complementary impacts of small rodents and semi-domesticated ungulates limit tall shrub expansion in the tundra. J Appl Ecol 51:234–241. https://doi.org/10.1111/1365-2664.12180
Richardson AD, Keenan TF, Migliavacca M et al (2013) Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric For Meteorol 169:156–173. https://doi.org/10.1016/j.agrformet.2012.09.012
Richardson AD, Hufkens K, Milliman T et al (2018) Tracking vegetation phenology across diverse north American biomes using PhenoCam imagery. Sci Data 5:180028. https://doi.org/10.1038/sdata.2018.28
Riihimäki H, Luoto M, Heiskanen J (2019) Estimating fractional cover of tundra vegetation at multiple scales using unmanned aerial systems and optical satellite data. Remote Sens Environ 224:119–132. https://doi.org/10.1016/j.rse.2019.01.030
Rixen C, Høye TT, Macek P et al (2022) Winters are changing: snow effects on Arctic and alpine tundra ecosystems. Arct Sci. https://doi.org/10.1139/AS-2020-0058
Ropars P, Angers-Blondin S, Gagnon M et al (2017) Different parts, different stories: climate sensitivity of growth is stronger in root collars vs. stems in tundra shrubs. Glob Change Biol 23:3281–3291. https://doi.org/10.1111/gcb.13631
Rumpf SB, Semenchuk PR, Dullinger S, Cooper EJ (2014) Idiosyncratic responses of high Arctic plants to changing snow regimes. PLoS ONE 9:e86281. https://doi.org/10.1371/journal.pone.0086281
Sadras VO, Denison RF (2009) Do plant parts compete for resources? An evolutionary viewpoint. New Phytol 183:565–574. https://doi.org/10.1111/j.1469-8137.2009.02848.x
Samplonius JM, Atkinson A, Hassall C et al (2020) Strengthening the evidence base for temperature-mediated phenological asynchrony and its impacts. Nat Ecol Evol. https://doi.org/10.1038/s41559-020-01357-0
Schwieger S, Kreyling J, Milbau A, Blume-Werry G (2018) Autumnal warming does not change root phenology in two contrasting vegetation types of subarctic tundra. Plant Soil 424:145–156. https://doi.org/10.1007/s11104-017-3343-5
Semenchuk PR, Gillespie MAK, Rumpf SB et al (2016) High Arctic plant phenology is determined by snowmelt patterns but duration of phenological periods is fixed: an example of periodicity. Environ Res Lett 11:125006. https://doi.org/10.1088/1748-9326/11/12/125006
Sloan VL, Fletcher BJ, Phoenix GK (2016) Contrasting synchrony in root and leaf phenology across multiple sub-Arctic plant communities. J Ecol 104:239–248. https://doi.org/10.1111/1365-2745.12506
Sonesson M, Callaghan TV (1991) Strategies of survival in plants of the Fenoscandian tundra. Arctic 44:95–105. https://doi.org/10.14430/arctic1525
Sturm M, Racine C, Tape K (2001) Increasing shrub abundance in the Arctic. Nature 411:546–547. https://doi.org/10.1038/35079180
Sturm M, Douglas T, Racine C, Liston GE (2005) Changing snow and shrub conditions affect albedo with global implications. J Geophys Res 110:G01004. https://doi.org/10.1029/2005JG000013
Tape K, Sturm M, Racine C (2006) The evidence for shrub expansion in northern Alaska and the Pan-Arctic. Glob Change Biol 12:686–702. https://doi.org/10.1111/j.1365-2486.2006.01128.x
Tape KD, Christie K, Carroll G, O’Donnell JA (2016) Novel wildlife in the Arctic: the influence of changing riparian ecosystems and shrub habitat expansion on snowshoe hares. Glob Change Biol 22:208–219. https://doi.org/10.1111/gcb.13058
Tape KD, Jones BM, Arp CD et al (2018) Tundra be dammed: beaver colonization of the Arctic. Glob Change Biol 24:4478–4488. https://doi.org/10.1111/gcb.14332
Thompson JA, Koenig LS (2018) Vegetation phenology in Greenland and links to cryospheric change. Ann Glaciol 59:59–68. https://doi.org/10.1017/aog.2018.24
Way RG, Lapalme CM (2021) Does tall vegetation warm or cool the ground surface? Constraining the ground thermal impacts of upright vegetation in northern environments. Environ Res Lett 16:054077. https://doi.org/10.1088/1748-9326/abef31
Weijers S, Myers-Smith IH, LÖffler J (2018) A warmer and greener cold world: summer warming increases shrub growth in the alpine and high Arctic tundra. Erdkunde 72:63–85. https://doi.org/10.3112/erdkunde.2018.01.04
Weijers S (2022) Declining temperature and increasing moisture sensitivity of shrub growth in the Low-Arctic erect dwarf-shrub tundra of western Greenland. Preprints
Westergaard-Nielsen A, Lund M, Pedersen SH et al (2017) Transitions in high-Arctic vegetation growth patterns and ecosystem productivity tracked with automated cameras from 2000 to 2013. Ambio 46:39–52. https://doi.org/10.1007/s13280-016-0864-8
Wheeler HC, Høye TT, Schmidt NM et al (2015) Phenological mismatch with abiotic conditions—implications for flowering in Arctic plants. Ecology 96:775–787. https://doi.org/10.1890/14-0338.1
Wipf S, Rixen C (2010) A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Res 29:95–109. https://doi.org/10.1111/j.1751-8369.2010.00153.x
You Q, Cai Z, Pepin N et al (2021) Warming amplification over the Arctic pole and third pole: trends, mechanisms and consequences. Earth Sci Rev 217:103625. https://doi.org/10.1016/j.earscirev.2021.103625
Zeng H, Jia G, Epstein H (2011) Recent changes in phenology over the northern high latitudes detected from multi-satellite data. Environ Res Lett 6:045508. https://doi.org/10.1088/1748-9326/6/4/045508
Zeng H, Jia G, Forbes BC (2013) Shifts in Arctic phenology in response to climate and anthropogenic factors as detected from multiple satellite time series. Environ Res Lett 8:035036. https://doi.org/10.1088/1748-9326/8/3/035036
Acknowledgements
We would like to extend our sincere gratitude to the Inuvialuit people for the opportunity to visit and conduct research on their land. We thank John Godlee and Eleanor Walker for helping with sample collection. We thank the Herschel Island-Qikiqtaruk Territorial Park rangers for collecting the phenology measurements and the Aurora Research Institute for logistical support in the field with particular thanks to Richard Gordon, Cameron Eckert, and in particular the park rangers Edward McLeod, Sam McLeod, and Ricky Joe. We thank the research group of Hugues Lantuit at the Alfred Wegener Institute and the Aurora Research Institute for logistical support. We thank Heather Goodare for proof-reading versions of this manuscript and Dieter Piepenburg, Elsa Godtfredsen, and one anonymous reviewer for their constructive feedback. Research permits for this work include Yukon Researcher and Explorer permits (16-48S&E) and Yukon Parks Research permits (RE-Inu-02-16). Funding for this research was provided by NERC through the Shrub Tundra standard grant (NE/M016323/1) and an equipment loan from the NERC Geophysical Equipment Facility (GEF 1063).
Author information
Authors and Affiliations
Contributions
JB and IMS conceived and designed research. SAB collected the samples and JB conducted lab work, with all authors contributing to the analysis and additional data. The main script was adapted by JB and IMS from code written by SAB. JB and IMS wrote the manuscript, with JJA creating Fig. 1. All authors read and approved the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boyle, J.S., Angers-Blondin, S., Assmann, J.J. et al. Summer temperature—but not growing season length—influences radial growth of Salix arctica in coastal Arctic tundra. Polar Biol 45, 1257–1270 (2022). https://doi.org/10.1007/s00300-022-03074-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03074-9