Abstract
In Kongsfjorden (Spitsbergen), we quantified the zonation of visually dominant macroalgal taxa and of detached macroalgae from underwater videos taken in summer 2009 at six transects between 2 and 138 m water depth. For the first time, we provide information on the occurrence of deep water red algae below the kelp forest and of detached macroalgae at water depth > 30 m. The presence and depth distribution of visually dominant red algae were especially pronounced at the outer fjord, decreased with proximity to the glacial front and they were absent at the innermost locations. Deepest crustose coralline red algae and foliose red algae were observed at 72 and 68 m, respectively. Brown algae were distributed along the entire fjord axis at 2–32 m. Green algae were only present at the middle to inner fjord and at areas influenced by physical disturbance at water depths of 2–26 m. With proximity to the inner fjord the depth distribution of all macroalgae became shallower and only extended to 18 m depth at the innermost location. Major recipients of detached macroalgae were sites at the shallower inner fjord and at the middle fjord below the photic zone at depths to 138 m. They may either fuel deep water secondary production, decompose or support carbon sequestration. Univariate and community analyses of macroalgal classes including detached macroalgae across transects and over depths reveal a considerable difference in community structure between the outermost sites, the central part and the inner fjord areas, reflecting the strong environmental gradients along glacial fjords.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macroalgae, as the major primary producers and habitat builders along coastal rocky shores (Duarte and Cebrián 1996; Mann 2000; Christie et al. 2009; Tait and Schiel 2018), constitute a central part of marine shelf ecosystems worldwide. As sunlight is rapidly absorbed by the water column in the marine environment, primary production by macroalgae, microphytobenthos, seagrass or phytoplankton is restricted to the shallow euphotic zone (Gattuso et al. 2006; Ramirez-Llodra et al. 2010).
Besides build-up of biomass, temperate to polar macroalgal forests, which are normally dominated by brown algae of the order Laminariales or Desmarestiales (Lüning 1990), produce macroalgal detritus through incremental blade erosion, detachment of old blades or removal of whole thalli (Krumhansl and Scheibling 2012; Pedersen et al. 2020). The detached macroalgal biomass may therefore fuel secondary production (Ramirez-Llodra et al. 2016) or enter the detrital food chain and carbon cycle via different processes: it may persist as bigger thallus parts or as whole detached thalli where the general form or taxon affiliation of the seaweeds is still visible. We consider both as ‘macroalgal detritus’ in the current investigation. The biomass may further fragment into small particulate organic material (POM) where taxon identity is no longer detectable or dissolve into organic carbon (DOC). This macroalgal detritus may become exported to neighbouring or distant habitats (Fredriksen 2003; Britton-Simmons et al. 2012; Pedersen et al. 2020) contributing to the carbon export into the deep sea (Dierssen et al. 2009; Krause-Jensen and Duarte 2016). Depending on its rate of decomposition or burial it may contribute to carbon sequestration (Krause-Jensen et al. 2018) and act as a marine carbon reservoir (Blue carbon; Nellemann et al. 2009; Macreadie et al. 2019; Ortega et al. 2019).
Kongsfjorden in western Spitsbergen is a deep Arctic glacial and sedimentary fjord partially characterized by a steep rocky coast well populated by macroalgae. Macroalgal species composition, biomass and distribution in shallow zones above 30 depths are well known (Hop et al. 2012, 2016; Fredriksen et al. 2014, 2019; Bartsch et al. 2016). Almost 40% of the coastal middle to inner zone of Kongsfjorden between 0 and 30 m depth is covered by macroalgae (Kruss et al. 2017). In total 84 macroalgal species were reported for Kongsfjorden (Fredriksen et al. 2019) and brown algae, especially kelps of the order Laminariales, constitute the majority of the biomass in a depth range between 0 and 20 m (Hop et al. 2012, 2016; Bartsch et al. 2016). There is a clear distributional gradient along the fjord axis varying with substrate type and distance to the glacial fronts. Species with a wider biogeographical distribution are typically broadly spread along the fjord axis (Hop et al. 2016). Whilst the majority of macroalgae communities is restricted to hard bottom locations, in the inner bay of Kongsfjorden some soft bottom areas support macroalgae, primarily on drop stones (Hop et al. 2016; Fredriksen et al. 2019).
Whilst most green algae grow in the littoral zone, the majority of brown algae are located in the upper sublittoral followed by red algae which dominate greater depths (Hop et al. 2012) although lowest depth extension has not been revealed yet. In contrast to this detailed information, the depth distribution of macroalgae > 30 m and of the amount and occurrence of detrital macroalgae is virtually unknown, mostly due to the inaccessibility of the habitat and of diving depth limits. Whilst detached macroalgae may strongly influence the underlying soft bottom communities, the accumulation rate of detached macroalgae at deeper parts of the fjord is unknown as is the effect on the food web structure (Molis et al. 2019).
The objectives of our study thus was (i) to gain knowledge on the distribution of visually dominant macroalgal species or taxon groups beyond the depth limit of 30 m and (ii) to determine the amount and spatial occurrence of macroalgal detritus in Kongsfjorden. A remote video approach via a systematic analysis of georeferenced video transects was accomplished. The footage was recorded across the shore at different locations along the fjord axis, dominated by rocky substrate and also soft sediments and spanning a depth range between 2 and 138 m. Two of the selected sites are located at the northern shorelines and had previously been sampled by Hop et al. (2012, 2016) and Bartsch et al. (2016), but only at shallower depths. At the southern shore four video transect sites are located. Part of this area has been investigated before by air-borne hyperspectral imaging and acoustic imaging providing a different resolution and not being able to detect macroalgal detritus (Volent et al. 2007; Kruss et al. 2017). All information was collated into a geographical information system (GIS) and resulting shape files are accessible on PANGEA and may serve for future applications.
Materials and methods
Study area
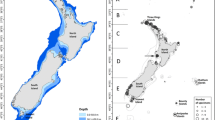
The study was performed in Kongsfjorden (78° 59 N, 11–12° E) at the western coast of Spitsbergen (Fig. 1). The coastal habitats of Kongsfjorden contain a mixture of hard and soft bottom substrates. Central and outer Kongsfjorden is dominated by outcrops of bedrock with a thin-sediment cover (Howe et al. 2003). At the inner part of the fjord, a large glacial complex contributes freshwater influx through melting and calving. Along with the raise in freshwater input by the glacial run-off, the concentration of suspended inorganic matter is increasing towards the inner fjord resulting in soft bottom-dominated areas (Svendsen et al. 2002). All deep fjord sites below 250 m are sedimentary in nature (Howe et al. 2003).
Data collection
In June 2009, the remotely operated vehicle (ROV) ACHILLE M4 (Institut Polaire Français Paul Emile Victor IPEV, France) recorded video transects at six coastal locations of Kongsfjorden, Spitsbergen stretching between 2 and maximal 138 m (Fig. 1). Four transects were located at the south shore along the length axis of the fjord (Kongsfjordneset, Brandal, Prince Heinrich Island, Tyskahytta). Two further transects investigated the steep bedrock of Hansneset with a west–east orientation 50 m apart from each other (Hansneset 1 and Hansneset 2). The ROV was operated on-board the research vessel Teisten (Kings Bay AS, Norway) connected by a cable as described in Laudien and Orchard (2012). The ship’s navigation was recorded every two seconds by a GPS in WGS 84 (EPSG 4326). During the ROV dives, the ship drifted with a speed limited to 0.5 knots. The ROV operated along the same vector and at about the same forward speed as the ship. The video transects were georeferenced by means of the ship coordinates. Although offset between ship and ROV was not exactly quantifiable, we assume only a negligible inaccuracy, since the ROV was operated with the same speed as the ship. Sample points were removed, when the ship drifted slower than the ROV, e.g. when the ship was turning or passing locations with steep bedrock.
The video camera SONY HVR-A1E HDV 1080i fitted with a 300-m Extreme Vision Lens was equipped with two halogen lights providing the broad illumination. On most ROV images, three laser pointers were visible, two arranged in a 3-cm horizontal distance and one in a 2-cm vertical distance resulting in a marked area of 6 cm2 providing the spatial reference of the captured images. When laser pointers were not visible, an estimation of the area was achieved by comparing frames with a similar distance to the camera. In case the distance also was not directly visible, the scale was subjectively estimated through size of dominant flora or fauna on the images. Unfortunately, laser spots were switched off at Kongsfjordneset and Hansneset 1. Since Hansneset 2 was filmed with a similar estimated distance from the sea floor, the mean area per frame from this transect was used for area estimation of Kongsfjordneset and Hansneset 1. As a precise spatial reference was not possible over all transects, we analysed the percent cover of the frames without a relation to the ground area. The mean frame size was approx. 70–80 × 70–80 cm. Besides the video footage, the ROV measured water depth every other second. The accuracy of the depth sensor is ± 1 m or maximum ± 2 m at deeper points (D. Fleury, IPEV, personal communication).
Spatial analysis
The georeferenced data (date, depth, coordinates) of all transects were linked to the timecode of the video and imported into the geographical coordinate system (GIS) ArcGIS 10.6.1 (ESRI 2011) by an R-Script (Pehlke) and projected to North Pole Lambert Azimuthal Equal Area (EPSG 102017). The course of the ship and hence the spatial orientation as well as the length of transects were evaluated and illustrated on a map (Online Resource 2). The ROV-born depth data were used to map elevation profiles for transects and to analyse correlation between macroalgal distribution and depth. Gaps in GPS positioning and depth data (e.g. when the sea bed was steep) were closed by linear interpolation. Furthermore, a digital elevation model of Kongsfjorden was created from a point shape file given by the Norwegian Mapping Authority with a resolution of 10 × 10 m. With the help of this digital elevation model ROV depth data were cross -validated before start of analysis.
Every 5 m we extracted coordinates from transects (R script Pehlke) to achieve a representative sampling pattern of the seabed. The corresponding video timecode was extracted with the help of GIS. This considerably facilitated data evaluation and improved the systematic sampling of the video frames.
We consider the Kongsfjorden GIS as a start to establish a geodatabase which encloses geodata and metadata of any formats. It provides useful background information for other researchers who would like to see where transects were located and the geographical information may enhance the repeatability of the investigation in the future.
Image analysis
Each transect was analysed every 5 m. At Tyskahytta (see Fig. 1), we only considered macroalgal coverage every 10 m, since the environment and the biodiversity of the benthic cover was rather constant over distance. The Software Avidemux 2.7 (Mean, France) was used for deinterlacing, video frame extraction, sharpening (Avidemux filter Yadif) and formatting the frames (images) to bitmap files. When the frame was unsuitable due to turbulence of the seabed or the camera was covered by macroalgae thalli, the next appropriate frame was used. The substitute image was scrutinized to ensure it represented a comparable area of the sea bed. The extracted images were analysed using ImageJ 2 software and its plugin GRID (Rueden et al. 2017). A raster was placed over each frame such that every grid cell was covering 5% of the frame (20,736 pixel2). Each frame was scored according to presence/absence and percent cover of visually dominant macroalgal taxa and categorical groups, detached macroalgae or part of them (macroalgal detritus) and visible substrate type. Macroalgal taxa were identified after Klekowski and Weslawski (1995) and Pedersen (2011). As many species or groups of species could not be determined to species level on the images, we estimated the coverage in the next broader categories: kelp (Laminariales), filamentous brown algae, foliose red algae, filamentous red algae, crustose coralline red algae, foliose green algae [this is probably Ulvaria obscura which is the only blade like Ulvales species reported for Kongsfjorden (Fredrisken et al. 2019)] and filamentous green algae (Acrosiphonia spp.). In addition to these categories, whenever possible kelp was further discriminated into Alaria esculenta, digitate kelp spp. [Laminaria digitata and Hedophyllum nigripes are grouped together as they are not distinguishable by their external morphology (Dankworth et al. 2020)], Laminaria solidungula and Saccharina latissima. In the case of filamentous brown algae Chordariaceae spp., which may comprise Dictyosiphon foeniculaceus, Chordaria flagelliformis and Stictyosiphon tortilis were distinguished from the two Desmarestia species (D. aculeata and D. viridis) and from Halosiphon tomentosus. Foliose red algae were further discriminated into Ptilota spp. (may comprise Ptilota gunneri and P. serrata according to Fredriksen et al. 2014), Coccotylus truncatus and Phycodrys rubens (for details see Table 2 in Results). Macroalgae were classified as detached when a free holdfast was visible, they were overturned and not in their usual orientation or when only fragments of the thalli were present. The presence and cover of sea urchins were recorded separately. Substrate was broadly classified into soft bottom, soft bottom with drop stones, hard bottom and hard bottom with silt. Whenever visible, the percent cover of rocky substrate (hard bottom substrate or drop stones) was estimated. Locations with high macroalgal cover, where it was impossible to estimate the substrate type, were assigned to hard bottom by default as a dense macroalgal vegetation does not grow on soft bottom (Wulff et al. 2009). The percent cover of all groups and substrate classes was estimated in 5% increments with 1% cover for minimum coverage: 0%, 1%, 5%, 10%, 15%, etc. up to 100%.
Elevation profiles for each transect were generated and categorized into 2-m-depth zones (0–2 m, > 2–4 m, > 4–6 m, etc.) and frames from one depth zone and transect were considered as pseudo-replicated images. Depending on the slope of the sea ground, the number of replicates per depth zone n was varying (n = 0–41). For every depth zone and transect mean percent cover for macroalgae in total as well as for each algae group (kelp, filamentous brown algae, foliose red algae, filamentous red algae, coralline red algae, foliose green algae and filamentous green algae) along with the cover of detached macroalgae was calculated.
Statistical analysis
Multivariate analysis
A cluster analysis based on the mean cover data per 5-m- depth zones of macroalgae groups (kelp, filamentous brown algae, foliose red algae, filamentous red algae, coralline red algae, foliose green algae and filamentous green algae) and detached macroalgae per transect was performed using a Bray–Curtis similarity matrix from square root-transformed data (Bray and Curtis 1957; Clarke et al. 2014). A depth of 45 m was chosen as a lower limit, since macroalgal vegetation was mainly constricted to shallower areas. Results are presented in form of a non-metric multi-dimensional scaling (nMDS) plot. To identify the key groups which contributed most to the dissimilarity between depth zones and transects a SIMPER analysis was performed (PRIMER v5; Plymouth Marine Laboratory, Clarke and Gorley 2006).
Univariate analysis
Differences in percentage kelp and detached macroalgae cover, as the major classes we were interested in, were tested. As transects were not replicated, 2-factor ANOVA’s without replication were performed with arcsine-transformed data with fixed factors, Transect and Depth, followed by Tukey HSD post hoc tests (R, R Core Team 2021). As homogeneity of variances was not achieved throughout (Levene’s test), we decreased the significance level to p = 0.01 to decrease the possibility of a Type I error. As data were not uniformly present for both cover classes over all transects and depths, we only compared those transects and depths with matching data. In conclusion, differences in mean percentage kelp cover were tested between 6 and 30 m categorized in 2-m-depth zones for the transects Kongsfjordneset, Hansneset 1 and 2 and Brandal. For mean percentage cover of detached macroalgae, differences were tested for shallower depths between 6 and 22 m for Kongsfjordneset, Hansneset 1 and 2, Brandal and Tyskahytta and for greater depths between 24 and 56 m for the transects Kongsfjordneset, Hansneset 1 and 2 and Brandal.
Results
Transect metadata
The lengths of the six georeferenced video transects varied between 560 and 1485 m (Table 1). The transect at Kongsfjordneset covered the longest distance up to the inner glacial front (24 km), followed by Hansneset 1 and 2 (16 km), Brandal (15 km), Prince Heinrich Island (11 km) and Tyskahytta (2 km). Depths ranged between 2 and 138 m, with the deepest transect being at Brandal and the shallowest at Tyskahytta (Table 1). In total, 907 images have been analysed covering an area of approx. 520 m2. The resulting analysed area of each depth range as well as the number of replicates per depth interval per transect are listed in Online Resource 1.
Macroalgal taxa
Overall, 16 macroalgal taxa or groups were identified from video frames along the six transects in Kongsfjorden which are depicted in Fig. 2. The depth distribution across transects is given in Table 2. Foliose green alga occurred only at the inner fjord mainly in the upper range of the sublittoral down to 16 m depth. Filamentous green algae (Acrosiphonia spp.) were present at Prince Heinrich Island, Tyskahytta, and Hansneset 1 and at Brandal between 2 and 26 m. The perennial kelp Alaria esculenta and Saccharina latissima as well as the filamentous brown algae Desmarestia spp. were present in all transects from the shallow sublittoral down to 30 m and 32 m, respectively. Perennial digitate kelps (2–24 m) were observed at Hansneset 1 and 2 and Kongsfjordneset only. The annual brown algae Chordariaceae spp. and Halosiphon tomentosus occurred exclusively at Brandal in shallow waters down to 10 m or 14 m, respectively. Laminaria solidungula, the only Arctic endemic kelp species, was only observed at Prince Heinrich Island between 22 and 24 m depth. The perennial red algae Coccotylus truncatus (12–48 m) and Phycodrys rubens (2–68 m) occurred only at Hansneset 1 and 2 and Prince Heinrich Island. The pseudo-perennial red alga Devaleraea ramentacea (4–14 m) and perennial Ptilota spp. (20–34 m) were just present at Hansneset. A filamentous red alga, possibly of the genus Polysiphonia, occurred exclusively at Kongsfjordneset and Prince Heinrich Island between 10 and 40 m. Crustose coralline red algae were visible at Kongsfjordneset and Hansneset at almost every depth (4–72 m) and occasionally at Prince Heinrich Island. In conclusion, 10 macroalgal taxa or groups were found at Hansneset 1, followed by Prince Heinrich Island (9) and by Hansneset 2 (8), Brandal (7) and Kongsfjordneset (6) and fewest number of macroalgal taxa/groups were observed at Tyskahytta (5).
Representative images of identified macroalgal taxa and detached macroalgae from videos at the different transects in Kongsfjorden 2009: a Filamentous green algae (possibly Acrosiphonia sp.), b bladed Ulvales sp. (possibly Ulvaria obscura), c Desmarestia sp., d pile of detached macroalgae (mostly kelp and filamentous brown algae) at Brandal at a depth of approximately 130 m, e Halosiphon tomentosus, f Alaria esculenta (characteristic midrib clearly visible), g digitate kelp (= Laminaria digitata and/or Hedophyllum nigripes), h Chordariaceae spp. (potential mixture of Dictyosiphon sp., Chordaria sp. and Stictyosiphon tortilis), i Laminaria solidungula, j Saccharina latissima, k Crustose coralline red algae, l Coccotylus truncatus, m Phycodrys rubens, n filamentous red alga, o Devaleraea ramentacea (red arrow) and p Ptilota sp. (red arrow)
Depth distribution of macroalgal groups and detached macroalgae across transects
The mean macroalgal cover varied throughout the fjord and the two major depth ranges above and below 30 m. The relative contribution of macroalgae groups and detached macroalgae is depicted in Fig. 3. The coverage of macroalgae, visible hard bottom substrate or drop stones was evaluated in classes along depth gradients for each transect. Figure 4 shows the mean macroalgal percentage cover of the sea floor as well as the cover of hard bottom in 2-m-depth intervals and Online Resource 1 provides mean values.
Above: digital elevation model of Kongsfjorden with 100-m interval isobaths (Norwegian Mapping Authority) and sampling sites: Brandal (BL), Hansneset 1 (HN1), Hansneset 2 (HN2), Kongsfjordneset (KN), Prince Heinrich Island (PHI) and Tyskahytta (TH). Below: total macroalgal cover of the sea floor (mean ± standard deviation, n = 25–163) is given per transect separated by 2 depth horizons (< and > 30 m). Relative percentage distribution of macroalgal groups and detached macroalgae is exhibited by pie charts
Coverage of visually dominant macroalgal groups and detached macroalgae along six transects in Kongsfjorden in 2009: a Kongsfjordneset, b Hansneset 1, c Hansneset 2, d Brandal, e Prince Heinrich Island, f Tyskahytta; mean percentage coverage per transect and 2-m-depth intervals (the values at the x-axis mark the respective lower depth value of the depth intervals) as well as mean cover of hard bottom substrate or drop stones (grey points). n: no data, cna: cover of crustose coralline red algae was not possible to quantify. Number of replicates per depth interval and per transect is listed in Online Resource 3
At the outer location, Kongsfjordneset and the more exposed northern locations Hansneset 1 and 2, the total macroalgal cover at 2–30 m depth was highest (53–57%), whilst the southern and inner locations Brandal, Prince Heinrich Island and Tyskahytta had a low coverage (23, 37, 7%, respectively) in the depth range 4-(24)30 m. Mean cover of attached macroalgae > 30 m depth was generally low with a maximum of 10% at Kongsfjordneset and a minimum at Brandal, where no attached macroalgae were found > 30 m. Filamentous brown algae were distributed along the entire fjord axis < 30 m (4–11%) and even occurred > 30 m at Kongsfjordneset, Hansneset 1 and 2 and Prince Heinrich Island (< 2%). Filamentous brown algae were also present at Hansneset between 54 and 60 m. However, attachment to the substrate was not verifiable. Whilst kelp were only present < 30 m depth in all transects with an overall mean cover at this depth range of 2–35%, visible red algal cover (foliose and filamentous red algae together) and crustose coralline red algae increased relative to the total macroalgal cover > 30 m depth (Fig. 3). At Kongsfjordneset, the rocky substrate was densely covered by visible crustose coralline red algae due to a lack of cover algae. Kelp was only growing in patches encircled by filamentous brown algae (Desmarestia spp.). In shallower waters (< 15 m), sea urchins were highly abundant surrounding those kelp patches. The Hansneset sites showed a high cover of filamentous and foliose red algae (HN1: 9% < 30 m, 1% > 30 m, HN2: 9% < 30 m, 3% > 30 m), whilst at the inner sites a low cover of red algae was observed (Fig. 3).
Green algae were only observed in the middle to inner fjord (Hansneset 1, Brandal and Tyskahytta) < 30 m depth and were highest at Brandal with 5%. The detailed qualitative description of the depth distribution of macroalgal groups and taxa exhibited in Fig. 4 is available in Online Resource 3.
In contrast to the low coverage of attached macroalgae > 30 m depth, detached macroalgae were observed at each transect in this depth range (Fig. 2), but also with lower coverages < 30 m at middle to inner transects Hansneset 1, Brandal, Prince Heinrich Island and Tyskahytta. Cover of detached macroalgae showed a clear gradient between outer (absent) and inner fjord (increasing) and generally increasing with depths. At Hansneset 1 and 2, a mean maximum cover of 8% of detached algae (mostly kelp, filamentous brown algae and foliose red algae) was observed between 4 and 72 m, whilst at Kongsfjordneset, detached macroalgae were nearly absent with only 1% coverage at 56–58 m (Fig. 4). At Prince Heinrich Island, detached macroalgae (mostly kelp and filamentous brown algae) were present with up to 13% coverage at 16–46 m. At Tyskahytta, detached macroalgae (mostly kelp) were observed at every depth and coverage was relatively high (4–11%) compared to that of attached macroalgae. Most detached macroalgae (mostly kelp and filamentous brown algae) were present at Brandal where they covered a remarkable area of the sea floor ≥ 24 m depth occasionally reaching 40% cover and increasing to 66% at 132 m. Figure 2d depicts an example of piles of detached and disintegrating kelp material visible at several sites at Brandal.
The differences in kelp cover as a proxy for a mature kelp forest and of detached macroalgae as a proxy for detritus pathways were also evaluated quantitatively between transects and along depths. The mean kelp cover was significantly different over the depth gradient and between transects (Table 3). The kelp cover at Hansneset 2 over all depths was highest reaching 100% between 6 and 14 m and therefore was significantly higher than at all other transects where kelp cover was maximally 62.5% at Hansneset 1, 30% at Brandal and 7.5% at Kongsfjordneset (Tukey post hoc test, p < 0.01). Interestingly, the kelp cover at Prince Heinrich Island at 14–16 m was unusually high with 98% but was excluded from statistical analysis due to missing data at shallower depths. Although kelp cover was significantly different over the depth gradient (Table 3), no significant differences amongst depth zones were revealed in the post hoc test.
The mean coverage of detached macroalgae between 6 and 22 m and between 24 and 56 m depth across transects were significantly different between transects, but not over the depth range (Tables 4 and 5). There were almost no detached macroalgae at Kongsfjordneset, Hansneset 1 and 2 and Brandal between 6 and 22 m, whilst the mean cover of detached macroalgae at Tyskahytta in this depth zone was significantly higher compared to all other transects reaching up to 10% (Tukey post hoc test, p < 0.01). At both Hansneset sites and Brandal, detached macroalgae mostly occurred below the kelp forest deeper than 24 m with a significantly higher cover at Brandal reaching up to 33% (Tukey post hoc test, p < 0.01). Prince Heinrich Island was not included in this analysis due to insufficient depth data for this transect.
Community analysis
Similarity of macroalgal group composition (kelp, filamentous brown algae, foliose red algae, filamentous red algae, coralline red algae, foliose green algae and filamentous green algae) along 9 depth horizons between 0 and 45 m per transect is visualized via a non-metric multi-dimensional scaling (nMDS) plot in Fig. 5 and support the qualitative description given above. All depth zones of Kongsfjordneset are clearly separated from all other transects and depth horizons. Results of a SIMPER analysis (all details in Online Resource 4) show that the mean high cover of visible coralline red algae contributed with 35–52% to the differences of Kongsfjordneset to all other transects. The soft bottom locations Brandal and Tyskahytta are also clearly separated and more similar to each other than to the other transects. SIMPER results show that cover of macroalgal detritus and kelp contributed mainly to the dissimilarity of Brandal and Tyskahytta to the other transects (15–31%, 13–33%, respectively).
Non-metric multi-dimensional scaling (nMDS) plot exhibiting similarity of the mean cover of macroalgae groups (kelp, filamentous brown algae, foliose red algae, filamentous red algae, coralline red algae, foliose green algae and filamentous green algae) and of detached macroalgae per transect and 5-m-depth zones using a Bray–Curtis similarity matrix from square root-transformed data. Brandal (BL), Hansneset 1 (HN1), Hansneset 2 (HN2), Kongsfjordneset (KN), Prince Heinrich Island (PHI) and Tyskahytta (TH). Stress = 0.1. The grey lines help to visualize the different relative community compositions of outermost KN and of sedimentary influenced BL and TH to central transects HN1, HN2 and PHI
The nMDS plot furthermore reveals that macroalgal composition, even in the applied broad groups, varies along depth gradients for every transect (Fig. 5). The SIMPER analysis shows that mean cover of brown algae (kelp and filamentous brown algae) was the primary factor to explain the dissimilarity between shallower sites (0–20 m) characterized by high brown algae coverage and deep sites down to 45 m with low coverages (19–37%, Online Resource 4). The deeper sites (20–45 m) are mostly differentiated by the dissimilarity of cover of detached macroalgae and visibility of coralline red algae (23–45%, 24–27% contribution to dissimilarity, respectively).
Discussion
Macroalgal distribution along the depth gradient and fjord axis
This study for the first time investigated the coastal benthic macroalgal community and the distribution of macroalgal detritus of Kongsfjorden over a vast area at greater depth complementing the detailed information available for the shallow sublittoral of the fjord.
The inclination of the sun angle and strong seasonality of the light in the Arctic (Pavlov et al. 2019) lead to a relatively lower annual sum of available light for macroalgae compared to temperate regions (Kirk 1994). However, macroalgae can grow at great depths here. Thus far, the deepest record of kelp in the Arctic was deeper than 60 m in Disko Bay (Krause-Jensen et al. 2019), Greenland. The deepest unidentified abundant macroalga growing attached to drop stones at a depth of 166 m was reported by Meyer and Sweetman (2015) in outer Raudfjorden, northern Spitsbergen. For Kongsfjorden, coralline red algae were reported to grow at 75 m depth in 2009 (Laudien and Orchard 2012). Our observations stretching over continuous wider areas confirm that these depth values are not single observations, but a vast red algal vegetation may exist below the shallow sublittoral kelp forest at sites where rocky substrate is available.
Apparently, the location along the fjord axis has an important influence on macroalgal cover and species composition which was shown here and has been reported earlier for the shallow sublittoral (Hop et al. 2016). The inner location Tyskahytta is greatly influenced by glacial melt limiting both light penetration (Pavlov et al. 2019) and available hard substrata. Sedimentation is one major factor inhibiting algal growth and recruitment (Eriksson and Johansson 2005; Zacher et al. 2016) and changing osmotic conditions due to freshwater discharge (Hanelt et al. 2004) require a high physiological acclimation potential of macroalgae (Karsten 2007; Zacher et al. 2009; Spurkland and Iken 2011b).
Green algae were most prominent at the middle to inner fjord at the upper sublittoral corresponding to the study of Hop et al. (2016) from 1996 to 1998. In our study, especially Acrosiphonia spp. was present in areas of high physical disturbance either through sedimentation (inner fjord: Tyskahytta) or ice scour (Hansneset). Acrosiphonia is an annual opportunistic species, known to be well adapted to ice-scouring. It can survive the winter as microscopic stage or as rhizoidal cushion and quickly recolonizes ice free areas when light levels increase in spring (Hop et al. 2002).
The cover of brown algae (Hop et al. 2016; Kruss et al. 2017) highly varied amongst transects and across the depth gradients. They formed especially dense forests in the middle fjord down to 20 m and therefore probably contributed most to the amount of detached macroalgae or only occurred as single individuals at locations influenced by glacial discharge. Whilst digitate kelps needed rocky substrate, Desmarestia spp., S. latissima and A. esculenta were also observed at soft bottom locations on drop stones. In sub-Arctic Alaska S. latissima was the only kelp able to grow on soft substrates as they can attach to small gravel (Spurkland and Iken 2011a; Hop et al. 2012).
The only Arctic endemic species present in the fjord, L. solidungula, had a very narrow distribution and was just found as small single individuals at Prince Heinrich Island. In studies 11–12 years before and 3–4 years after our investigation, this species was only present in the middle zone including the station Hansneset < 10 m in the understory of big kelps (Bartsch et al. 2016; Hop et al. 2016). Thus, L. solidungula is not abundantly growing in Kongsfjorden, whilst in northern and north-eastern Svalbard (Hinlopenstredet, Nordaustlandet) many specimens < 1 m total length were abundantly growing in between other kelps between 5 and 30 m (Belseth 2012). As this species requires low light, low temperature and high salinities for the completion of its life cycle (Tom Dieck 1992; Roleda 2016; Muth et al. 2021), it may possibly disappear in Kongsfjorden if seawater temperatures and freshwater input will further rise.
Interestingly, in our study no Saccorhiza dermatodea was visually obvious. This annual kelp-like species was commonly present down to 13 m depth in 1996/98 (Hop et al. 2012) and was also recorded in 2012/13 at Hansneset (Bartsch et al. 2016). Its absence in our investigation rather suggests confusion with Saccharina latissima or less physical disturbance in 2009 than an upward shift. The general shift of the lower depth distribution of kelps to shallower depth detected between 1996/1998 and 2014 at Hansneset (Bartsch et al. 2016) was not yet noticeable in our study. We identified dominant digitate kelp even at 16 m depth and also abundant S. latissima at depth to 30 m.
At the outermost and exposed site Kongsfjordneset which is characterized by steady bedrock, a dense kelp bed was expected (Lüning 1990). The observed barren ground with a high percentage of crustose coralline red algae was regarded as a proxy for grazing pressure by sea urchins. One key organism substantially feeding on macroalgae is the green sea urchin Strongylocentrotus droebachiensis. It rapidly increased in density at outer Kongsfjorden since 1993 (Hop et al. 2002; Beuchel and Gulliksen 2008) and was also observed abundantly (1–20%) at Kongsfjordneset in this study. The only bigger brown algae present at Kongsfjordneset in our study were pockets of A. esculenta and Desmarestia sp. As Desmarestia viridis produces and stores sulphuric acid, it effectively deters grazing by sea urchins, thereby creating a local refuge for kelp and associated communities (Molis et al. 2009).
The slow growing low light tolerating coralline red algae (Vásquez-Elizondo and Enríquez 2017) have only recently been recognized as important ecosystem engineers for the Arctic (e.g. Jørgensbye and Halfar 2017). Their depth distribution recorded for Kongsfjorden is similar to the northernmost coralline algae of Svalbard, recorded at Nordaustlandet in 78 m depth (Teichert et al. 2012) or to those from Greenland recorded at 77 m depth (Jørgensbye and Halfar 2017). As video recordings may hinder detection of coralline red algae or other small macroalgae beneath a dense canopy of cover algae, we can only assume that sites with abundant kelp or red algal cover are potentially rocky and also provide habitats for crustose coralline red algae. Data interpretation thus must be seen in this context.
Dense red algal vegetation at Hansneset and the low cover of foliose and filamentous red algae at all other stations at the south coast may indicate that this bushy red algae require hard substrate and a low sedimentation environment. For cold temperate zones, information on the depth distribution of Phycodrys or other foliose red algae is scarce. Reported depth limits range between 23 and 50 m (Sears and Cooper 1978; Vadas and Steneck 1988; Pedersén and Snoeijs 2001; Mikhaylova 2019) but recordings of exact lower depth limits are often hampered by depth limitations of SCUBA diving. Thus, these deep water observations in Kongsfjorden are of special significance and may have an overlooked ecological importance. Schmidt et al. (2021) hypothesize that deep water foliose red algae mats in the Mediterranean Sea are a yet undescribed ecosystem engineer that provides a multitude of ecological niches. If we consider that Phycodrys becomes up to 4 years old, a tetrasporophyte may release up to 550,000 tetraspores or female gametophytes up to 120,000 carpospores per individual (Schoschina 1996) and houses up to 7583 individuals of epifaunal species per 100 g fresh weight (Lippert et al. 2001), the role of deep water foliose red algae for the Arctic food web and macroalgal carbon cycling might be considerable, but remains unknown.
Detached macroalgae
The majority of local macroalgal primary production in fjord systems is exported to deep sites in northern Norway (Filbee-Dexter et al. 2018; Pedersen et al. 2020). In the Arctic, in western Spitsbergen deep water, macrobenthic fauna primarily relies on macroalgal detritus (Sokołowski et al. 2014; Renaud et al. 2015) but the extent of macroalgal detritus export has never been quantified for Kongsfjorden. The extensive kelp beds observed in the middle of the fjord and also at coastal sites such as Kapp Mitra (Hop et al. 2016) regularly produce at least the annual biomass of the kelp blades as macroalgal detritus. The kelp blades become either detached in spring when new growth is initiated (Lüning 1990), continuously erode over the year (Krumhansl and Scheibling 2011; Pedersen et al. 2020), or biomass is released in the form of millions of spores over winter from several kelp species in Kongsfjorden (Bartsch, pers. observation). Besides that, kelp and macroalgal thalli in general may become detached through wave action after storms (Krumhansl and Scheibling 2012) or through ice-scouring. Although there exist some mapping studies (Volent et al. 2007; Kruss et al. 2017), the precise extent of kelp stands along the fjord axis and thus the amount exported to connected sedimentary habitats is unknown. As the total fresh standing stock biomass of kelps at Hansneset ranged between approx. 2 and 13 kg fresh weight m−2 in 2012/13 (Bartsch et al. 2016), this might be in the same range as reported from other more temperate sites. In eastern Canada, the annual detrital production from the erosion of kelp blades increased linearly with kelp bed biomass and ranged from 0.5 to 1.71 kg dry weight m−2 (150–513 g C m−2) across sites (Krumhansl und Scheibling 2011). In the Northeast Atlantic, high export rates (> 98% across the study sites) of the annual production of kelp detritus were demonstrated (approx. 4.70 kg fresh weight m−2 or 301 g C m−2, Smale et al. 2021). As kelp blades only slowly decompose, this suggests a great potential for long distance transport of this biomass (de Bettignies et al. 2020; Pedersen et al. 2021).
During this study we demonstrated for the first time that considerable detrital macroalgal biomass is present in deep recipient sites below the kelp zone in Kongsfjorden distant from their production sites; this was especially pronounced at Brandal. The inner shallow location of Tyskahytta seems to be an additional recipient of detached macroalgae. The transportation of kelp material into deep sites and the inner fjord by currents probably is a continuous process as video frames often showed material with different signs of degradation. Due to the rotational Coriolis effects, Atlantic and Arctic water masses enter fjords on west Spitsbergen along the southern shores. In our case study, incoming water masses have to pass Brandal and have to exit along the northern shore (Howe et al. 2003; Pawłowska et al. 2017). As deep trenches facilitate the transport of detached macroalgae to the deep sea (Vetter and Dayton 1998), the deep central fjord basin, which is located in proximity to the Brandal transect (Howe et al. 2003) and which is clearly visible in the newly established digital elevation model for Kongsfjorden (Fig. 3), may be an ideal location for deposition of detritus.
Outlook
The Arctic contains a vast potential habitat for marine macrophytes. The impact of climate change is predicted to be dramatic for the Arctic due to the increased absorption of solar radiation accompanied by sea ice loss and the inflow of ocean heat from lower latitudes (Meredith et al. 2019). Increasingly warmer conditions may result in the northward expansion of boreal macroalgae and fauna (Arrigo et al. 2008; Krause-Jensen and Duarte 2014) and may also fuel primary production of many Arctic macroalgae which currently often thrive at sub-optimal temperature conditions (Iniguez et al. 2016; Franke et al. 2021) except for Arctic endemic species (e.g. Roleda 2016). As the ongoing glacier melting will increase turbidity and sedimentation in the near future, productivity of the macroalgal communities in Arctic fjords is expected to first become reduced (Traiger and Konar 2018; Filbee-Dexter et al. 2019). All these processes are expected to impact the carbon subsidy of deep sea Arctic areas, since kelp forests and deep fjord habitats are closely linked by the seasonal production of algal detritus (Filbee-Dexter et al. 2018).
Data availability
The videos recorded and analysed during the current study as well as a shape file with all georeferenced data points of the transects with the information on bottom substrate, cover of macroalgal groups and detached macroalgae are available on PANGEA (https://doi.pangaea.de/10.1594/PANGAEA.927141). Both R scripts to link the georeferenced data of all transects to the timecode of the video and import it into the geographic coordinate system as well as to extract coordinates from transects are available in the GitHub repository of the FACE-IT project.
References
Arrigo KR, van Dijken G, Pabi S (2008) Impact of a shrinking Arctic ice cover on marine primary production. Geophys Res Lett 35:L19603. https://doi.org/10.1029/2008GL035028
Bartsch I, Paar M, Fredriksen S, Schwanitz M, Daniel C, Hop H, Wiencke C (2016) Changes in kelp forest biomass and depth distribution in Kongsfjorden, Svalbard, between 1996–1998 and 2012–2014 reflect Arctic warming. Polar Biol 39:2021–2036. https://doi.org/10.1007/s00300-015-1870-1
Belseth E (2012) Eco-physiology of the Arctic kelp Laminaria solidungula. MSc thesis, NTNU, Trondheim, Norway
Beuchel F, Gulliksen B (2008) Temporal patterns of benthic community development in an Arctic fjord (Kongsfjorden, Svalbard): results of a 24-year manipulation study. Polar Biol 31:913–924. https://doi.org/10.1007/s00300-008-0429-9
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349. https://doi.org/10.2307/1942268
Britton-Simmons KH, Rhoades AL, Pacunski RE, Galloway AWE, Lowe AT, Sosik EA, Dethier MN, Duggins DO (2012) Habitat and bathymetry influence the landscape-scale distribution and abundance of drift macrophytes and associated invertebrates. Limnol Oceanogr 57:176–184. https://doi.org/10.4319/lo.2012.57.1.0176
Christie H, Norderhaug KM, Fredriksen S (2009) Macrophytes as habitat for fauna. Mar Ecol Prog Ser 396:221–233. https://doi.org/10.3354/meps08351
Clarke KR, Gorley RN (2006) Primer: user manual/tutorial. PRIMER-E, Plymouth
Clarke KR, Gorley RN, Somerfield PJ, Warwick RM (2014) Change in marine communities: an approach to statistical analysis and interpretation, 3rd edn. PRIMER-E, Plymouth
Dankworth M, Heinrich S, Fredriksen S, Bartsch I (2020) DNA barcoding and mucilage ducts in the stipe reveal the presence of Hedophyllum nigripes (Laminariales, Phaeophyceae) in Kongsfjorden (Spitsbergen). J Phycol 56:1245–1254. https://doi.org/10.1111/jpy.13012
de Bettignies F, Dauby P, Thomas F, Gobet A, Delage L, Bohner O, Loisel S, Davoult D (2020) Degradation dynamics and processes associated with the accumulation of Laminaria hyperborea (Phaeophyceae) kelp fragments: an in situ experimental approach. J Phycol 56:1481–1492
Dierssen HM, Zimmerman RC, Drake LA, Burdige DJ (2009) Potential export of unattached benthic macroalgae to the deep sea through wind-driven Langmuir circulation. Geophys Res Lett 36:L04602. https://doi.org/10.1029/2008GL036188
Duarte CM, Cebrián J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41:1758–1766. https://doi.org/10.4319/lo.1996.41.8.1758
Eriksson B, Johansson G (2005) Effects of sedimentation on macroalgae: species-specific responses are related to reproductive traits. Oecologia 143:438–448. https://doi.org/10.1007/s00442-004-1810-1
ESRI (2011) ArcGIS Desktop: release 10. Environmental Systems Research Institute, Redlands
Filbee-Dexter K, Wernberg T, Norderhaug KM, Ramirez-Llodra E, Pedersen MF (2018) Movement of pulsed resource subsidies from kelp forests to deep fjords. Oecologia 187:291–304. https://doi.org/10.1007/s00442-018-4121-7
Filbee-Dexter K, Wernberg T, Fredriksen S, Norderhaug KM, Pedersen MF (2019) Arctic kelp forests: diversity, resilience and future. Glob Planet Change 172:1–14. https://doi.org/10.1016/j.gloplacha.2018.09.005
Franke K, Liesner D, Heesch S, Bartsch I (2021) Looks can be deceiving: contrasting temperature characteristics of two morphologically similar kelp species co-occurring in the Arctic. Bot Mar 64:163–175. https://doi.org/10.1515/bot-2021-0014
Fredriksen S (2003) Food web studies in a Norwegian kelp forest based on stable isotope (delta C-13 and delta N-15) analysis. Mar Ecol Prog Ser 260:71–81. https://doi.org/10.3354/meps260071
Fredriksen S, Bartsch I, Wiencke C (2014) New additions to the benthic marine flora of Kongsfjorden, western Svalbard, and comparison between 1996/1998 and 2012/2013. Bot Mar 57:203–216. https://doi.org/10.1515/bot-2013-0119
Fredriksen S, Karsten U, Bartsch I, Woelfel J, Koblowsky M, Moy SR, Steneck RS, Wiktor JM, Hop H, Wiencke C (2019) Biodiversity of benthic macro- and microalgae from Svalbard with special focus on Kongsfjorden. In: Hop H, Wiencke C (eds) The ecosystem of Kongsfjorden, Svalbard, advances in polar ecology, vol 2. Springer, Cham
Gattuso JP, Gentili B, Duarte C, Kleypas J, Middelburg J, Antoine D (2006) Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences 3:489–513. https://doi.org/10.5194/bg-3-489-2006
Hanelt D, Bischof K, Wiencke C (2004) The radiation, temperature and salinity regime in Kongsfjorden. Ber Polarforsch Meeresforsch 492:14–25
Hop H, Pearson T, Hegseth EN, Kovacs KM, Wiencke C, Kwasniewski S, Slawek K et al (2002) The marine ecosystem of Kongsfjorden, Svalbard. Polar Res 21:167–208. https://doi.org/10.3402/polar.v21i1.6480
Hop H, Wiencke C, Vögele B, Kovaltchouk NA (2012) Species composition, zonation, and biomass of marine benthic macroalgae in Kongsfjorden, Svalbard. Bot Mar 55:399–414. https://doi.org/10.1515/bot-2012-0097
Hop H, Kovaltchouk NA, Wiencke C (2016) Distribution of macroalgae in Kongsfjorden, Svalbard. Polar Biol 39:2037–2051. https://doi.org/10.1007/s00300-016-2048-1
Howe JA, Moreton SG, Morri C, Morris P (2003) Multibeam bathymetry and the depositional environments of Kongsfjorden and Krossfjorden, western Spitsbergen, Svalbard. Polar Res 22:301–316. https://doi.org/10.3402/polar.v22i2.6462
Iñiguez C, Carmona R, Lorenzo MR, Niell FX, Wiencke C, Gordillo FJ (2016) Increased temperature, rather than elevated CO2, modulates the carbon assimilation of the Arctic kelps Saccharina latissima and Laminaria solidungula. Mar Biol 163:248. https://doi.org/10.1007/s00227-016-3024-6
Jørgensbye HIØ, Halfar J (2017) Overview of coralline red algal crusts and rhodolith beds (Corallinales, Rhodophyta) and their possible ecological importance in Greenland. Polar Biol 40:517–531. https://doi.org/10.1007/s00300-016-1975-1
Karsten U (2007) Research note: Salinity tolerance of Arctic kelps from Spitsbergen. Phycol Res 55:257–262. https://doi.org/10.1111/j.1440-1835.2007.00468.x
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems, 2nd edn. Cambridge University Press, Cambridge
Klekowski RZ, Weslawski J (1995) Atlas of the marine flora of southern Spitsbergen. IO PAN, Gdansk
Krause-Jensen D, Duarte CM (2014) Expansion of vegetated coastal ecosystems in the future. Front Mar Sci 1:77. https://doi.org/10.3389/fmars.2014.00077
Krause-Jensen D, Duarte CM (2016) Substantial role of macroalgae in marine carbon sequestration. Nat Geosci 9:737–742. https://doi.org/10.1038/ngeo2790
Krause-Jensen D, Lavery P, Serrano O, Marbà N, Masque P, Duarte CM (2018) Sequestration of macroalgal carbon: the elephant in the blue carbon room. Biol Lett 14:20180236. https://doi.org/10.1098/rsbl.2018.0236
Krause-Jensen D, Sejr MK, Bruhn A, Rasmussen MB, Christensen PB, Hansen JLS, Duarte CM, Bruntse G, Wegeberg S (2019) Deep penetration of kelps offshore along the west coast of Greenland. Front Mar Sci 6:2296–7745. https://doi.org/10.3389/fmars.2019.00375
Krumhansl KA, Scheibling RE (2011) Detrital production in Nova Scotian kelp beds: patterns and processes. Mar Ecol Prog Ser 421:67–82. https://doi.org/10.3354/meps08905
Krumhansl KA, Scheibling RE (2012) Production and fate of kelp detritus. Mar Ecol Prog Ser 467:281–302. https://doi.org/10.3354/meps09940
Kruss A, Tęgowski J, Tatarek A, Wiktor J, Blondel P (2017) Spatial distribution of macroalgae along the shores of Kongsfjorden (West Spitsbergen) using acoustic imaging. Pol Polar Res 38:205–229. https://doi.org/10.1515/popore-2017-0009
Laudien J, Orchard JB (2012) The significance of depth and substratum incline for the structure of a hard bottom sublittoral community in glacial Kongsfjorden (Svalbard, Arctic)—an underwater imagery approach. Polar Biol 35:1057–1072. https://doi.org/10.1007/s00300-011-1153-4
Lippert H, Iken K, Rachor E, Wiencke C (2001) Macrofauna associated with macroalgae in the Kongsfjord (Spitsbergen). Polar Biol 24:512–522. https://doi.org/10.1007/s003000100250
Lüning K (1990) Seaweeds: their environment, biogeography and ecophysiology. Wiley, New York
Macreadie PI, Anton A, Raven JA et al (2019) The future of blue carbon science. Nat Commun 10:3998. https://doi.org/10.1038/s41467-019-11693-w
Mann KH (2000) Ecology of coastal waters: with implications for management, 2nd edn. Wiley-Blackwell, Hoboken
Meredith M, Sommerkorn M, Cassotta S, Derksen C, Ekaykin A, Hollowed A, Kofinas G, Mackintosh A, Melbourne-Thomas J, Muelbert MMC, Ottersen G, Pritchard H, Schuur EAG (2019) Polar regions. In: Pörtner H-O, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A, Petzold J, Rama B, Weyer NM (eds) IPCC special report on the ocean and cryosphere in a changing climate. Cambridge University Press, Cambridge, pp 203–320
Meyer KS, Sweetman AK (2015) Observation of a living macroalga at 166 m in a high Arctic fjord. Mar Biodivers Rec 8:e58. https://doi.org/10.1017/S175526721500038X
Mikhaylova TA (2019) Vegetation of the red algal belt of the White Sea (European Arctic, Russia). Novosti Sistematiki Nizshikh Rastenii 53:39–65. https://doi.org/10.31111/nsnr/2019.53.1.39
Molis M, Wessels H, Hagen W, Karsten U, Wiencke C (2009) Do sulphuric acid and the brown alga Desmarestia viridis support community structure in Arctic kelp patches by altering grazing impact, distribution patterns, and behaviour of sea urchins? Polar Biol 32:71–82. https://doi.org/10.1007/s00300-008-0504-2
Molis M, Beuchel F, Laudien J, Wlodarska-Kowalczuk M, Buschbaum C (2019) Ecological drivers of and responses by Arctic benthic communities, with an emphasis on Kongsfjorden, Svalbard. In: Hop H, Wiencke C (eds) The ecosystem of Kongsfjorden, Svalbard. Advances in polar ecology, vol 2. Springer, Cham
Muth AF, Bonsell C, Dunton KH (2021) Inherent tolerance of extreme seasonal variability in light and salinity in an Arctic endemic kelp (Laminaria solidungula). J Phycol 57:1557–1562. https://doi.org/10.1111/jpy.13187
Nellemann C, Corcoran E, Duarte CM, Valdes L, De Young C, Fonseca L, Grimsditch G (2009) Blue carbon. A UNEP rapid response assessment. United Nations Environment Programme, GRID-Arendal, Arendal
Ortega A, Geraldi NR, Alam I, Kamau AA, Acinas SG, Logares R, Gasol JM, Massana R, Krause-Jensen D, Duarte CM (2019) Important contribution of macroalgae to oceanic carbon sequestration. Nat Geosci 12:748–754. https://doi.org/10.1038/s41561-019-0421-8
Pavlov AK, Leu E, Hanelt D, Bartsch I et al (2019) The underwater light climate in Kongsfjorden and its ecological implications. In: Hop H, Wiencke C (eds) The ecosystem of Kongsfjorden, Svalbard. Advances in polar ecology, vol 2. Springer, Cham
Pawłowska J, Zajączkowski M, Szczuciński W, Zaborska A, Kucharska M, Jernas PE, Forwick M (2017) The influence of Coriolis force driven water circulation on the palaeoenvironment of Hornsund (S Spitsbergen) over the last century. Boreas 46:737–749. https://doi.org/10.1111/bor.12249
Pedersen PM (2011) Grønlands havalger. Epsilon, Kopenhagen
Pedersen MF, Filbee-Dexter K, Norderhaug KM, Fredriksen S, Frisk NL, Fagerli CW, Wernberg T (2020) Detrital carbon production and export in high latitude kelp forests. Oecologia 192:227–239. https://doi.org/10.1007/s00442-019-04573-z
Pedersen MF, Filbee-Dexter K, Frisk NL, Sárossy Z, Wernberg T (2021) Carbon sequestration potential increased by incomplete anaerobic decomposition of kelp detritus. Mar Ecol Prog Ser 660:53–67. https://doi.org/10.3354/meps13613
Pedersén M, Snoeijs PJM (2001) Patterns of macroalgal diversity, community composition and long-term changes along the Swedish west coast. Hydrobiologia 459:83–102. https://doi.org/10.1023/A:1012506507322
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramirez-Llodra E, Brandt A, Danovaro R, De Mol B, Escobar E, German CR, Levin LA, Martinez Arbizu P, Menot L, Buhl-Mortensen P, Narayanaswamy BE, Smith CR, Tittensor DP, Tyler PA, Vanreusel A, Vecchione M (2010) Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences 7:2851–2899. https://doi.org/10.5194/bg-7-2851-2010
Ramirez-Llodra E, Rinde E, Gundersen H, Christie H, Fagerli CW, Fredriksen S, Gitmark JK, Norling K, Walday MG, Norderhaug KM (2016) A snap shot of the short-term response of crustaceans to macrophyte detritus in the deep Oslofjord. Sci Rep 6:23800. https://doi.org/10.1038/srep23800
Renaud PE, Løkken TS, Jørgensen LL, Berge J, Johnson BJ (2015) Macroalgal detritus and food-web subsidies along an Arctic fjord depth-gradient. Front Mar Sci 2:31. https://doi.org/10.3389/fmars.2015.00031
Roleda MY (2016) Stress physiology and reproductive phenology of Arctic endemic kelp Laminaria solidungula J. Agardh. Polar Biol 39:1967–1978. https://doi.org/10.1007/s00300-015-1813-x
Rueden CT, Schindelin J, Hiner MC et al (2017) Image J2: ImageJ for the next generation of scientific image data. BMC Bioinform 18:529. https://doi.org/10.1186/s12859-017-1934-z
Schmidt N, El-Khaled YC, Roßbach FI, Wild C (2021) Fleshy red algae mats influence their environment in the Mediterranean Sea. Front Mar Sci 8:2296–7745. https://doi.org/10.3389/fmars.2021.721626
Schoschina E (1996) Seasonal and age dynamics of growth and reproduction of Phycodrys rubens (Rhodophyta) in the Barents and White Seas. Aquat Bot 55:13–30. https://doi.org/10.1016/0304-3770(96)01055-8
Sears JR, Cooper RA (1978) Descriptive ecology of offshore, deep-water, benthic algae in the temperate western North Atlantic Ocean. Mar Biol 44:309–314
Smale DA, Pessarrodona A, King N, Moore PJ (2021) Examining the production, export, and immediate fate of kelp detritus on open-coast subtidal reefs in the Northeast Atlantic. Limnol Oceanogr. https://doi.org/10.1002/lno.11970
Sokołowski A, Szczepańska A, Richard P, Kędra M, Wołowicz M, Węsławski JM (2014) Trophic structure of the macrobenthic community of Hornsund, Spitsbergen, based on the determination of stable carbon and nitrogen isotopic signatures. Polar Biol 37:1247–1260. https://doi.org/10.1007/s00300-014-1517-7
Spurkland T, Iken K (2011a) Kelp bed dynamics in estuarine environments in subarctic Alaska. J Coast Res 27:133–143. https://doi.org/10.2112/JCOASTRES-D-10-00194.1
Spurkland T, Iken K (2011b) Salinity and irradiance effects on growth and maximum photosynthetic quantum yield in subarctic Saccharina latissima (Laminariales, Laminariaceae). Bot Mar 54:355–365. https://doi.org/10.1515/bot.2011.042
Svendsen H, Beszczynska-Møller A, Hagen JO, Lefauconnier B, Tverberg V, Gerland S, Ørbæk JB, Bischof K, Papucci C, Zajaczkowski M, Azzolini R, Bruland O, Wiencke C, Winther J-G, Dallmann W (2002) The physical environment of Kongsfjorden-Krossfjorden, an Arctic fjord system in Svalbard. Polar Res 21:133–166. https://doi.org/10.3402/polar.v21i1.6479
Tait LW, Schiel DR (2018) Ecophysiology of layered macroalgal assemblages: importance of subcanopy species biodiversity in buffering primary production. Front Mar Sci 5:444. https://doi.org/10.3389/fmars.2018.00444
Teichert S, Woelkerling W, Rüggeberg A, Wisshak M, Piepenburg D, Meyerhöfer M, Form A, Büdenbender J, Freiwald A (2012) Rhodolith beds (Corallinales, Rhodophyta) and their physical and biological environment at 80°31′N in Nordkappbukta (Nordaustlandet, Svalbard Archipelago, Norway). Phycologia 51:371–390. https://doi.org/10.2216/11-76.1
Tom Dieck I (1992) North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): hybridization experiments and temperature responses. Phycologia 31:147–163. https://doi.org/10.2216/i0031-8884-31-2-147.1
Traiger SB, Konar B (2018) Mature and developing kelp bed community composition in a glacial estuary. J Exp Mar Biol Ecol 501:26–35. https://doi.org/10.1016/J.JEMBE.2017.12.016
Vadas RL, Steneck RS (1988) Zonation of deep water benthic algae in the Gulf of Maine. J Phycol 24:338–346. https://doi.org/10.1111/j.1529-8817.1988.tb04476.x
Vásquez-Elizondo RM, Enríquez S (2017) Light absorption in coralline algae (Rhodophyta): a morphological and functional approach to understanding species distribution in a coral reef lagoon. Front Mar Sci 4:2296–7745. https://doi.org/10.3389/fmars.2017.00297
Vetter EW, Dayton PK (1998) Macrofaunal communities within and adjacent to a detritus rich submarine canyon system. Deep Sea Res II 45:25–54
Volent Z, Johnsen G, Sigernes F (2007) Kelp forest mapping by use of airborne hyperspectral imager. J Appl Remote Sens. https://doi.org/10.1117/1.282261
Wulff A, Iken K, Quartino M, Al-Handal A, Wiencke C, Clayton M (2009) Biodiversity, biogeography and zonation of benthic micro- and macroalgae in the Arctic and Antarctic. Bot Mar 52:491–507. https://doi.org/10.1515/BOT.2009.072
Zacher K, Rautenberger R, Hanelt D, Wulff A, Wiencke C (2009) The abiotic environment of polar marine benthic algae. Bot Mar 52:483–490. https://doi.org/10.1515/BOT.2009.082
Zacher K, Bernard M, Bartsch I, Wiencke C (2016) Survival of early life history stages of Arctic kelps (Kongsfjorden, Svalbard) under multifactorial global change scenarios. Polar Biol 39:2009–2020. https://doi.org/10.1007/s00300-016-1906-1
Acknowledgements
This work was performed at the International Arctic Environmental Research and Monitoring Facility in Ny-Ålesund, Spitsbergen, Norway. We are grateful to the AWIPEV station and Kings Bay personnel in Ny-Ålesund for their support of our research. Our deepest thanks are expressed for support during the fieldwork by the captain of the research boat Teisten Arne Kristover Olstad (Kings Bay AS, Norway) and the two ROV pilots Dominique Fleury and Alain Pottier (IPEV, France). We would like to thank Stein Fredriksen, Margaret O. Amsler and Morten F. Pedersen for their constructive comments on earlier versions of the manuscript.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the priority programme ‘Antarctic Research with comparative investigations in Arctic ice areas’ by a grant Za735/1-1. The finalization of this study was enabled in the frame of the project FACE-IT (The Future of Arctic Coastal Ecosystems – Identifying Transitions in Fjord Systems and Adjacent Coastal Areas). FACE-IT has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 869154. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CW planned and conducted the data collection. Data analysis was performed by KS. KZ and IB contributed analytical tools and statistics. KJ and HP contributed to GIS. HP contributed to R. The first draft of the manuscript was written by KS and all authors commented on previous versions of the manuscript. The revised version was rewritten by KS and IB and all authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schimani, K., Zacher, K., Jerosch, K. et al. Video survey of deep benthic macroalgae and macroalgal detritus along a glacial Arctic fjord: Kongsfjorden (Spitsbergen). Polar Biol 45, 1291–1305 (2022). https://doi.org/10.1007/s00300-022-03072-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03072-x