Abstract
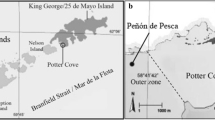
Prior understanding of Harpagifer antarcticus reproductive biology was based exclusively on macroscopic observations of its gonadal maturation cycle. Our present study with H. antarcticus specimens collected in summer of 2019 at Paradise Bay (PB), Western Antarctic Peninsula (WAP), provides a) additional reproductive parameters, and b) for first time, a histological analysis of the gonads, clarifying understanding of the spawning period of the species in the area. In females the gonado-somatic index was 5.3–19.6%, leading clutch oocytes was 0.94–1.95 mm, and total fecundity ranged between 408 and 1723 oocytes/female (X ± SD = 794 ± 328, n = 22). The histology allowed the identification of gravid females with oocytes in late vitellogenesis with evidence of ongoing hydration processes and spent females with post-ovulatory follicles. The presence of spermatozoa in mature testes indicated that the males were in developing and ripe condition. Our reproductive data for H. antarcticus at PB, validated with histology, indicate that the species was in spawning during summer, in agreement with a previous report of a late spring–summer spawning for the species at the same site. Literature data indicate that at the South Orkney Islands, H. antarcticus spawns three months earlier (May–June), probably associated with differential environmental factors between this area and the WAP. Our findings support the hypothesis that the life cycle characteristics of H. antarcticus—e.g., low fecundity and short pelagic phase—promote the isolation of populations as reflected in differences in the reproductive timing.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Code availability
Not applicable.
References
Agnew DJ, Heaps L, Jones C, Watson A, Berkieta K, Pearce J (1999) Depth distribution and spawning pattern of Dissostichus eleginoides at South Georgia. CCAMLR Science 6:19–36
Barrera-Oro E (2002) Review: the role of fish in the Antarctic marine food web: differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarct Sci 14:293–309
Barrera-Oro E, La Mesa M, Moreira E (2014) Early life history timings in marbled rockcod (Notothenia rossii) fingerlings from the South Shetland Islands as revealed by otolith microincrement. Polar Biol 37:1099–1109
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish 3:52–70
Burren PJ (1988) Reproductive biology of Harpagifer sp. at Signy Island, South Orkney Islands. MSc Thesis, University of North Wales, 52
Casaux RJ (1998) The contrasting diet of Harpagifer antarcticus (Notothenioidei, Harpagiferidae) at two localities of the South Shetland Islands, Antarctica. Polar Biol 19:283–285
Daniels RA (1978) Nesting behaviour of Harpagifer bispinis in Arthur Harbour, Antarctic Peninsula. J Fish Biol 12:465–474
Daniels RA (1979) Nest guard replacement in the Antarctic fish Harpagifer bispinis: possible altruistic behavior. Science 205:831–833
Daniels RA (1983) Demographic characteristics of an Antarctic plunderfish, Harpagifer bispinis antarcticus. Mar Ecol Progr Ser 13:181–187
Duhamel G, Hulley PA, Causse R, Koubbi P, Vacchi M, Pruvost P, Vigetta S, Irisson JO, Moreméde S, Belchier M, Dettai A, Detrich HW, Gutt J, Jones CD, Kock KH, Lopez Abellan LJ, Van de Putte AP, Biogeographic patterns of fish. En: De Broyer C, Koubbi P, Griffiths HJ, Raymond B, Udekem d’Acoz C. d’, Van de Putte AP, Danis B, David B, Grant S, Gutt J, Held C, Hosie G, Huettmann F, Post A, Ropert-Coudert Y (eds) (2014) Biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 328–362
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107
Eastman JT (2017) Bathymetric distributions of notothenioid fishes. Polar Biol 40:2077–2095
Eastman JT (2019) An analysis of maximum body size and designation of size categories for notothenioid fishes. Polar Biol 42:1131–1145
Eastman JT, Eakin RR (2021) Checklist of the species of notothenioid fishes. Antarct Sci. https://doi.org/10.1017/S0954102020000632
Ghigliotti L, Ferrando S, Carlig E, Di Blasi D, Gallus L, Pisano E, Hancet S, Vacchi M (2017) Reproductive features of the Antarctic silverfish (Pleuragramma antarctica) from the western Ross Sea. Polar Biol 40:199–211
Gon O, Heemstra PC (eds) (1990) Fishes of the Southern Ocean. J. L. B. Smith Institute of Ichthyology, Grahamstown, 462 pp. 12 pls
Hourigan TF, Radtke RL (1989) Reproduction of the Antarctic fish Nototheniops nudifrons. Mar Biol 100:277–283
Hüne M, González-Wevar C, Poulin E, Mansilla A, Fernández DA, Barrera-Oro E (2015) Low level of genetic divergence between Harpagifer fish species (Perciformes: Notothenioidei) suggests a Quaternary colonization of Patagonia from the Antarctic Peninsula. Polar Biol 38:607–617
Hureau JC (1990) Harpagiferidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 357–363
Kjesbu O (2009) Applied fish reproductive biology: contribution of individual reproductive potential to recruitment and fisheries management. Wiley, Hoboken
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioids fish. Antarct Sci 3:125–150
La Mesa M, Caputo V, Rampa R, Vacchi M (2003) Macroscopic and histological analyses of gonads during the spawning season of Chionodraco hamatus (Pisces, Channichthyidae) of Terra Nova Bay, Ross Sea, Southern Ocean. Polar Biol 26:621–628
La Mesa M, Vera-Duarte J, Landaeta MF (2017) Early life history traits of Harpagifer antarcticus (Harpagiferidae, Notothenioidei) from the South Shetland Islands during austral summer. Polar Biol 40:1699–1705
La Mesa M, Llompart F, Riginella E, Eastman JT (2020) Parental care and reproductive strategies in notothenioid fishes. Fish Fish 22:356–376
Llompart F, Fernandez D, Aureliano D, La Mesa M (2020) Life-history traits of the Magellan plunderfish Harpagifer bispinis (Forster, 1801) in the Beagle Channel (Tierra del Fuego, South America). Polar Biol 43:1643–1654
Macchi G, Barrera-Oro E (1995) Histological study on the ovarian development of mackerel icefish (Champsocephalus gunnari) from the South Georgia Islands. CCAMLR Sci 2:35–49
Meneghesso C, Riginella E, La Mesa M, Donato F, Mazzoldi C (2017) Age and reproduction in two Antarctic plunderfishes (Artedidraconidae) from the Weddell Sea. Polar Biol 40:13–24
Murua H, Motos L (1998) Reproductive modality and batch fecundity of the European hake (Merluccius merluccius L.) in the Bay of Biscay. Calif Coop Res Rep 39:196–203
Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S (2003) Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J Northwest Atl Fish Sci 33:33–54
Novillo M, Moreira E, Macchi G, Barrera-Oro E (2019) Reproductive effort in Chaenocephalus aceratus validated by gonadal histology: inshore sites serve as spawning grounds for some notothenioids. Polar Biol 42:1959–1972
Piacentino G, Moreira E, Barrera Oro E (2018) Early stages of notothenioid fish from Potter Cove, South Shetland Islands. Polar Biol 41:2607–2613. https://doi.org/10.1007/s00300-018-2366-6
Rae GA, Calvo J (1995) Fecundity and reproductive habits in Patagonothen tessellata (Richardson, 1845) from the Beagle Channel, Argentina. Antarct Sci 7:235–240
Rae GA, Calvo J (1996) Histological analysis of gonadal development in Patagonotothen tessellata (Richardson 1845) (Nototheniidae: Pisces) from the Beagle Channel, Argentina. J Appl Ichthyol 12:31–38
Saborido-Rey F, Junquera S (1998) Histological assessment of variations in sexual maturity of cod (Gadus morhua) at the Flemish Cap (north-west Atlantic). ICES J Mar Sci 55:515–521
Tomo AP (1981) Contribución a1 conocimiento de la fauna ictiologica del sector Antártico Argentino. Contribución Científica, Instituto Antártico Argentino 14:1–242
Van der Molen S, Matallanas J (2004) Reproductive biology of female Antarctic spiny plunderfish Harpagifer spinosus (Notothenioidei: Harpagiferidae), from Îles Crozet. Antarct Sci 16:99–105
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleost. Am Zool 21:325–343
West G (1990) Methods of assessing ovarian development in fishes: a review. Aust J Mar Fresh Res 41:199–222
White MG, Burren PJ (1992) Reproduction and larval growth of Harpagifer antarcticus Nybelin (Pisces, Notothenioidei). Antarct Sci 4:421–430
Yates P, Ziegler P, Welsford D, McIvor J, Farmer B, Woodcook E (2018) Spatio-temporal dynamics in maturation and spawning of Patagonian toothfish Dissostichus eleginoides on the sub-Antarctic Kerguelen Plateau. J Fish Biol 92:34–54
Acknowledgements
We are grateful to Carlos Bellisio and Agustin Zarco for their help in field activities and laboratory tasks and to Marta Estrada and Hugo Brachetta for the histological sections preparation. Prof. Joseph Eastman provided useful advice on a previous version of this work. We thank Drs. Vladimir Laptikhovsky, Christopher D. Jones, and one anonymous reviewer whose comments improved the quality of our paper. INIDEP contribution N°2253.
Funding
This work was supported by grants from Dirección Nacional del Antártico, Instituto Antártico Argentino [Grant number PICTA 0100], Consejo Nacional de Investigaciones Científicas y Técnicas [Grant number PIP2017-2019: 11220170100219CO, Resolution 2018–8-APN-DIR#CONICET], and Fondo para la Investigación Científica y Tecnológica [Grant number PICT 2018:03310, Resolution 401/19].
Author information
Authors and Affiliations
Contributions
MN and EBO planned and designed the research. MN conducted fieldwork and carried out laboratory work. MN and EM performed statistical analyses. GM and MN analyzed the histological slides. MN and EBO led the writing of the manuscript with contributions from GM and EM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to participate
The authors declare consent to participate.
Consent for publication
The authors declare consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Novillo, M., Moreira, E., Macchi, G. et al. Histological analysis provides further insights into Harpagifer antarcticus reproductive biology at the western Antarctic Peninsula. Polar Biol 44, 2165–2175 (2021). https://doi.org/10.1007/s00300-021-02953-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02953-x