Abstract
Defining the impact of anthropogenic stressors on Antarctic wildlife is an active aim for investigators. Telomeres represent a promising molecular tool to investigate the fitness of wild populations, as their length may predict longevity and survival. We examined the relationship between telomere length and human exposure in Adélie penguin chicks (Pygoscelis adeliae) from East Antarctica. Telomere length was compared between chicks from areas with sustained human activity and on neighboring protected islands with little or no human presence. Adélie penguin chicks from sites exposed to human activity had significantly shorter telomeres than chicks from unexposed sites in nearby protected areas, with exposed chicks having on average 3.5% shorter telomeres than unexposed chicks. While sampling limitations preclude our ability to draw more sweeping conclusions at this time, our analysis nonetheless provides important insights into measures of colony vulnerability. More data are needed both to understand the proximate causes (e.g., stress, feeding events) leading to shorter telomeres in chicks from human exposed areas, as well as the fitness consequences of reduced telomere length. We suggest to further test the use of telomere length analysis as an eco-indicator of stress in wildlife among anthropized sites throughout Antarctica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite being one of the most remote environments on Earth, Antarctica is subject to increasing levels of human activity (Hughes 2018). Activities such as pedestrian approach and vehicle operations may detrimentally impact wildlife by causing population declines, redistribution of breeding sites, or changes in individual behavior (SCAR 2008). While onshore human activity in Antarctica was historically limited to scientific research programs, commercial tourism has also developed in the past several decades (Tin et al. 2014). Tourism is one of the key issues for which the Antarctic Treaty System is seeking scientific input, as highlighted in question 78 of the 1st Horizon Scan of the Scientific Committee on Antarctic Research (“How will regulatory mechanisms evolve to keep pace with Antarctic tourism?”) (Kennicutt 2014). Human activities have been shown to have a small negative overall impact on Antarctic wildlife, though a large amount of heterogeneity exists among studies due to variations in locations, species, and types of activities (Coetzee and Chown 2016).
Penguins have shown variable responses to human activities since research on the topic began thirty years ago. At the population level, negative impacts were noted on breeding success and chick survival in Adélie (Pygoscelis adeliae) (Wilson et al. 1990; Giese 1996; Bricher et al. 2008) and Gentoo (Pyogoscelis papua) penguins (Trathan et al. 2008; Lynch et al. 2010). However, for Adélie and Gentoo penguins at other sites, exposure to human activities was associated with either no difference or even increases in population size (Micol and Jouventin 2001) and breeding success (Cobley and Shears 1999; Holmes et al. 2006; Carlini et al. 2007; Lynch et al. 2010). At the behavioral level, heightened vigilance in Emperor (Aptenodytes forsteri) (Giese and Riddle 1999; Burger and Gochfeld 2007) and Gentoo penguins (Holmes et al. 2006) was observed in response to various types of human activities. Even when no behavioral responses were observed, physiological measures indicated that animals were nonetheless affected. For instance, while Adélie penguins from both tourist-visited and non-visited areas along the Antarctic Peninsula exhibited no differences in breeding success or behavior in response to human activities, birds from tourist-visited sites did exhibit an increased heart rate response (Culik et al. 1990; Carlini et al. 2007). While no relationship was found between corticosterone levels measured from the guano of Gentoo penguins and tourist landings (Lynch et al. 2019), newly-hatched chicks of Magellanic penguins (Spheniscus magellanicus) living in tourist-visited areas exhibited elevated corticosterone levels (Walker et al. 2005; Villanueva et al. 2012), indicative of a higher sensitivity to stress (Angelier and Wingfield 2013). This emphasizes the importance of employing physiological measures of response in order to best assess the impacts of exposure to human activities.
Telomere length is an important example of a physiological measure that has been successfully employed as a proxy for fitness (Bauch et al. 2013; Angelier et al. 2019) and life expectancy (Wilbourn et al. 2018). Telomeres are nucleoprotein complexes located at the extremities of chromosomes, whose function is to protect and ensure the replication of coding DNA (Blackburn 2000; Young 2018). Composed of repetitive non-coding DNA sequences, they are dynamic structures regulated by enzymatic activity vulnerable to changes in the cellular environment (e.g., oxidative stress). Telomeres are thus subject to the condition and life history trade-offs of an organism (Young 2018), particularly environmental stress (Angelier et al. 2018; Chatelain et al. 2020). While telomere length will vary over an organisms’ life history (Bize et al. 2009), telomeres generally decrease in length throughout the life of the organism, with the rate of telomere loss conserved phylogenetically across families (Tricola 2018, including Adélie penguins). Telomere length during early life and development can strongly predict longevity (Heidinger et al. 2012), and reduction in telomere length is often greatest during early life (Salomons et al. 2009). Multiple intrinsic factors may influence the increased rate of telomere length loss during early life (Salomons et al. 2009), such as higher rates of cell replication (Monaghan and Ozanne 2018), greater oxidative stress during development (Reichert and Stier 2017), or even the properties of longer telomeres themselves, which increase their vulnerability to oxidative damage (Oikawa et al. 2001). This increased reduction rate renders telomeres particularly susceptible to extrinsic factors such as environmental stressors during early life (Boonekamp et al. 2014; Gil et al. 2019). Experimental manipulation of traffic noise in nestling wild sparrows (Passer domesticus) was associated with a significant reduction in telomere length (Meillère et al. 2015), evidence that environmental stress in early life can produce long-term consequences (Angelier et al. 2018). Impacts of exposure to human activities have been observed on early life stages in (1) Emperor penguins, in the form of increased energy expenditure (Regel and Pütz 1997) and transitory stress behavior (Giese and Riddle 1999) and in (2) Magellanic penguins, in the form of increased corticosterone levels in response to stress exposure (Walker et al. 2005). However, telomere length has never been measured in relation to exposure to human activities in penguins. The potential for telomere length to serve as an eco-indicator of populations suffering from the negative consequences of environmental stress was recently shown in the common lizard (Zootoca vivipara), where shorter telomere length was associated with populations facing higher extinction risk (Dupoué 2017). From the examples above, telomere shortening can be considered as a proxy that transcends individual and intergenerational impacts, providing the opportunity to assess fitness pressure on populations exposed to human activities (Giraudeau et al. 2019).
In the present study, we tested the hypothesis that exposure to human activities as a result of living in close proximity to an active research station in Antarctica would be associated with a reduction in telomere length. The aforementioned relationship between early life telomere length and survival (Heidinger et al. 2012) coupled with the particular sensitivity of telomeres to environmental stressors during development (Salomons et al. 2009), emphasizes the importance of investigating the consequences of exposure to human activities on Adélie penguins during early life stages. We thus compared telomere lengths of Adélie penguin chicks living near a site exposed to human activities, the Dumont d’Urville station on Petrels Island, to those of chicks living in protected areas on a neighboring unexposed site. We predicted that Adélie penguin chicks from the exposed site would have shorter telomeres than chicks from the unexposed site if they are affected by exposure to human activities.
Materials and methods
Study sites and sampling
The study was carried out at two sites approximately 2 km apart in the Pointe Géologie Archipelago along Terre Adélie, East Antarctica between January 27th and February 1st during the 2016 austral summer (Fig. 1). The “exposed” site next to the station and defunct airstrip of Dumont d’Urville on Petrels Island was characterized by its proximity to station buildings, human and vehicular traffic, and associated noise levels. The “unexposed” site was located in the protected area on Lamarck and Bernard Islands (French ZPS, “Zone de Protection Spéciale”), which experiences minimal to no human incursion. Adélie penguin nests were randomly sampled from the perimeter of the colony in order to minimize disturbance. Nests had one to two chicks. Given that bird phenology is similar on all islands of the Pointe Géologie archipelago, hatching timings at exposed and unexposed sites are considered synchronous. Because sampling was carried out at the same time among sites over a 5-day period, the age of recently hatched chicks was presumed to be comparable. Indeed, body size can provide a robust estimate of age in seabird chicks (Dehnhard et al. 2011). Chicks were weighed using an electronic balance (resolution, ± 2 g; Ohaus, Giessen, Germany) and the length of the left flipper measured to the nearest mm to calculate a “scaled mass index” (SMI) (Peig and Green 2009) to adjust the mass of individuals to that expected if their body sizes were constant. Flipper length was used as it had a strong positive correlation with mass (r = 0.347, p < 0.001). The SMI was calculated as:

copyright CNES 2016, Distribution Airbus DS, Pléiades Satellite Image 03/03/2016, resolution 50 cm (CNES 2016). b Photos of the exposed site on PI and c the unexposed site taken on BI (copyright Timothée Poupart). Dumont d’Urville station DDU; Petrels Island PI; Bernard Island BI; Lamarck Island LI
Study area at Pointe Géologie Archipelago along Adélie Land, East Antarctica. a Map of study area. The exposed site is represented by the dark yellow blob within the area of principal human activity delineated by dashed dark yellow lines. The purple blob indicates the unexposed site within protected areas delineated by dashed purple lines. Basemap photo
where Mi and Li are the body mass and flipper length of each individual i, L0 is the arithmetic mean of the flipper length of all individuals included in the study (L0 = 17.96 cm, n = 26), and b is the slope estimate of the regression of log-transformed body mass on log-transformed flipper length (b = 2.11).
For all individuals, a blood sample was collected from the alar vein (2-mL heparinized syringe, 25-gauge needle). Blood samples were spun (5000 rpm for 10 min at 4 °C), and then stored at − 20 °C for further analyses. Sex was determined by PCR amplification of the CHD gene according to standard procedures (Fridolfsson and Ellegren 1999; Weimerskirch et al. 2005).
Due to the protected status of the location of the unexposed colonies, it was not possible to obtain data related to age, phenology, or brood size, given the strict regulations that govern sampling in protected areas in order to minimize human impacts.
Telomere length analysis
Data from n = 15 chicks from exposed sites, and n = 11 chicks from unexposed sites were processed for the final analysis. While there were overall more females than males in the study (n = 17 females, n = 11 males), there was a comparable proportion of males and females at both site types (exposed site: n = 10 (67%) females, n = 5 males (33%); unexposed site: n = 7 females (64%), n = 4 males (36%)). Telomere length was obtained from DNA in red blood cells using a Southern blotting method (Nussey 2014). Genomic DNA was extracted using the DNeasy Blood and Tissue kit (Qiagen) following the manufacturer’s protocol. Concentration and quality of the extracted DNA (260/280 nm and 260/230 nm) was checked using a NanoDrop UV–Vis spectrophotometer (Thermo Scientific). Telomere restriction fragment (TRF) analysis was carried out using the TeloTAGGG Telomere Length Assay (Roche) as previously described with minor modifications (Blévin, 2016). In short, TRF analysis involves: (i) digestion of genomic DNA by restriction enzymes, (ii) separation by gel electrophoresis of the digested DNA, (iii) transfer of separated DNA to a denaturing nylon membrane, (iv) hybridization with telomere-specific probes, (v) incubation with antibodies capable of producing a chemiluminescent signal, (vi) image analysis of chemiluminescent telomere smear density in order to estimate telomere length (Nussey et al. 2014). A more detailed description of the methods used can be found in the Electronic Supplementary Material.
Statistical analyses
Statistical tests were performed in R 3.5.0 (R Development Core Team 2016). The normality of the distribution of data among each of the variables was tested (flipper length, mass, SMI, TRF length) using the Shapiro–Wilk’s Normality test, both over all site types (exposed and unexposed) and within site types. All normality tests showed no significant deviation from normal distribution of the data from all variables tested except for flipper length among exposed sites, which had a significantly left-skewed distribution (Supplementary Fig. S1, Supplementary Fig. S2). Individual data for all variables can be found in Table S1. Generalized linear models (GLMs; normal errors and identity link functions, R package stats) were fitted to test whether descriptive covariates (flipper length, mass, and SMI) differed by site type or sex (two-level factors, exposed or unexposed and female or male, respectively). GLMs were chosen to account for non-normality in the distribution of dependent variables. Models were selected in a stepwise approach starting from full models including all response variables. Non-significant (p > 0.05) factors and covariates were removed one at a time until the most parsimonious model was reached. Homoscedasticity and normality of residuals were checked in all models.
Results
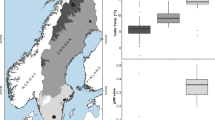
Flipper length, mass, and body condition were neither affected by site type (Fig. 2) nor by sex, thus supporting the comparability of Adélie chicks between site types and among sexes (flipper length, GLM: site type effect: F1, 24 = 0.688, p = 0.415, sex effect: F1, 24 = 0.300, p = 0.589; mass, GLM: site type effect: F1, 24 = 3.475, p = 0.075, sex effect: F1, 24 = 0.030, p = 0.865; SMI, GLM: site type effect: F1, 24 = 2.766, p = 0.109).
Box plots displaying the relationship between explanatory variables and site type (two-level factor: exposed and unexposed). Plots are shown for a flipper length, b mass, and c scaled mass index (SMI). Boxes are bound by the first and third quartile, and bold black lines within boxes indicate the median. Whiskers extend to the furthest data point from the first and third quartiles, respectively, within 1.5 times the interquartile distance. Data points more than 1.5 times the interquartile distance are individually represented as open circles. No relationships were significant (p > 0.05 for all GLMs)
Site type (Fig. 3a, Table S2a) and mass (Fig. 3d, Table S2b) were the only explanatory variables to have a significant (p < 0.05) negative effect on telomere length (GLM: site type effect: F1, 24 = 6.683, p = 0.016; GLM: mass effect: F1, 24 = 4.290, p = 0.0493). Sex (Fig. 3b, Table S2c), flipper length (Fig. 3c, Table S2d), and SMI (Fig. 3e, Table S2e) had no significant effect on telomere length (GLM: sex effect: F1, 24 = 0.012, p = 0.915; GLM: flipper length effect: F1, 24 = 0.797, p = 0.381; GLM: SMI effect: F1, 24 = 3.245, p = 0.0842). The most parsimonious model was selected based on removal of nonsignificant variables and reduction in AIC (Table S3). The most parsimonious GLM to explain variation in telomere length ultimately included only site type (Table S3). The average TRF length of Adélie chicks from exposed sites (14.42 kilobases) was reduced by 3.5% in comparison to Adélie chicks from unexposed sites (14.94 kilobases).
Effect of explanatory variables on telomere length. a Site type (exposed, unexposed) b sex (F, M) c flipper length d mass e scaled mass index (SMI). Boxplots (a, b) are bound by the first and third quartile, and bold black lines within boxes indicate the median. Whiskers extend to the furthest data point from the first and third quartiles, respectively, within 1.5 times the interquartile distance. Scatter plots (c, d, e) contain regression lines in black, with dark yellow and purple data points deriving from exposed and unexposed sites, respectively. R2 values are indicated on the lower right of each plot, and were calculated using the R stats function lm for the indicated variables. p values derived from GLMs are indicated above each plot, with significant effects (p < 0.05) highlighted with an asterisk (*)
Discussion
In this study, we examined for the first time the impact of anthropogenic activities on telomere length in an Antarctic species. Importantly, significantly shorter telomeres were observed in Adélie penguin chicks from exposed sites in the vicinity of the active research station Dumont d’Urville compared to telomeres from chicks from unexposed sites in nearby protected areas. This result supports the hypothesis that exposure to human activities is associated with reduced telomere length in penguin chicks. Decreased telomere length was found in chicks from exposed sites despite evidence of behavioral and adrenotropic habituation to exposure to human activities in closely related species (Walker et al. 2006; Viblanc et al. 2012; Villanueva et al. 2012).
Decreased telomere length is associated with increased mortality risk (Wilbourn et al. 2018), and populations with shorter telomeres have been shown to experience an increased risk of extinction (Dupoué et al. 2017). Telomere length is often a proxy of individual quality (Bauch et al. 2013; Angelier et al. 2019), and telomere length during early life is a strong predictor of longevity (Heidinger et al. 2012). This then begs the question as to whether reduced telomere length in Adélie chicks in sites exposed to human activities is a harbinger for future reductions in population size and fitness. Unfavorable environmental conditions were associated with reduced telomere length in other long-lived bird species, and were significant predictors of survival in early life (Watson et al. 2015). Such outcomes are further supported by evidence for the possible heritability of telomere length from generation to generation (Dugdale and Richardson 2018; Giraudeau et al. 2019), which would further exacerbate the impacts of telomere length reductions in early life. While the telomere length reduction observed in European storm petrels (Hydrobates pelagicus) in Watson et al. (2015) was much higher than that observed in Adélie penguins in the present study (22% versus 3.5%), it is difficult to use this discrepancy to infer biological significance or lack thereof. First, telomere dynamics are species specific, thus reduction rates cannot be compared between species (Ingles and Deakin 2016). Second, it is possible that the larger differences observed in telomere lengths of European storm petrels are related to the fact Watson et al. (2015) compared telomere lengths between chicks based on their survival. Indeed, if we were to only compare chicks that did not survive past a certain life stage, it could be possible to observe greater differences in telomere length reduction between groups. We cannot know the biological significance of the telomere reduction observed among exposed chicks in the present study, but future longitudinal studies will be critical to assess the extent to which telomere length reductions can predict mortality in Adélie penguins.
There exist multiple causal explanations for the observed reductions in telomere length in Adélie chicks from exposed sites. Human activities may be a direct stressor for chicks, resulting in cascading physiological impacts (i.e., stress hormones levels) that cause the observed reductions in telomere length (reviewed in Angelier et al. 2018). Indeed, Angelier et al. (2019) showed a negative correlation between levels of the stress hormone corticosterone and telomere length in Black-browed albatrosses (Thalassarche melanophris). Alternatively, anthropogenic stressors may impact the level of parental care adults offer to chicks, thus resulting in the same observed telomere length reductions because developmental nutritional constraints are often associated with a fast rate of telomere attrition (reviewed in Angelier et al. 2018). In addition, sibling competition has been correlated with reductions in telomere length, and can impact chick growth (Mizutani et al. 2016; Young 2017). Indeed, human disturbance, parental care, and sibling rivalry may act synergistically on telomeres. However, our finding that SMI of chicks did not vary between site types supports our hypothesis that differences in exposure to human activities, and not family dynamics related to parental care and sibling competition, could have contributed to the observed differences in telomere length. Future studies applying telomere length as an eco-indicator would benefit from the inclusion of nest occupancy data to better control for the influence of parental care and sibling competition in observed telomere length differences.
Size and body condition generally have an inverse relationship with telomere length in wild vertebrates, particularly during growth and development (Angelier et al. 2018 and references contained therein). Similarly, in the present study, a significant negative correlation was found between telomere length and mass (Fig. 3d), and while not significant, negative relationships were found between telomere length and flipper length (Fig. 3c) and SMI (Fig. 3e). This inverse relationship reflects the complex balance between life history trade-offs and telomere attrition (Young 2018), reflected in the value of fledging weight as a predictor of chick longevity, wherein heavier chicks are more likely to survive (Salihoglu et al. 2001; Ainley 2018). These life history trade-offs can be related to the increased energy expenditure and cell division associated with growth, resulting in increased oxidative stress (Monaghan and Ozanne 2018). While on the cellular level, a clear relationship between increased oxidative stress and decreased telomere length has been established (Kawanishi and Oikawa 2004), that relationship is more equivocal in vivo (Boonekamp et al. 2017; Reichert and Stier 2017). Indeed, Beaulieu et al. (2013) found no relationship between oxidative damage and population changes in Gentoo and Adélie penguins, while antioxidant defense capacity, in contrast, strongly correlated with population trends. In future studies, it would be useful to integrate multiple physiological markers (stress hormones, markers of oxidative stress, telomere length), in order to attain a more holistic assessment of individual quality and tease apart the role of the relevant life history factors and their underlying mechanisms. While in the present study we found that all morphological measures did not vary significantly between site types, a non-significant trend of exposed sites containing larger chicks was observed (Fig. 2b, Fig. 2c). Further detailed monitoring and the inclusion of more individuals will be necessary to assess the validity of these non-significant trends, and thus tease apart the influence of growth and nutritional conditions from human activities on telomere length.
Environmental factors related to climate, extreme weather events, and variable conditions have also been associated with telomere reduction in birds (Mizutani et al. 2013; Watson et al. 2015). Adélie penguins in the Pointe Géologie Archipelago live under particularly severe environmental constraints (Ropert-Coudert et al. 2018). However, environmental exposure is unlikely to vary among sites due to their proximity to one another along the Pointe Géologie Archipelago (exposed and unexposed sites are separated by less than 3 km). Both sites are oriented from east to west, with no obstacles between the nests and the sea. These similarities in geographic proximity and access conditions indicate that environmental conditions are unlikely to explain the observed difference in telomere length between sites.
The small-scale analysis presented here highlights a potential vulnerability for penguin colonies located close to human activities. Yet, further studies with—if possible—larger sample sizes are needed in order to (1) confirm this relationship between exposure to human activities and telomere length in chicks, (2) better understand the proximate mechanisms leading to telomere reduction, (3) evaluate the fitness consequences of reduced telomere length for Adélie penguin chicks, and (4) control whenever possible for confounding variables such as age, brood size, colony breeding success, and environmental exposure. While only chicks from the perimeter of the colonies in question were sampled to minimize disturbance, this also represents a potential bias in our sample, as penguins nesting at the edge of the colony may differ in their response and exposure to human activities. Although the difference in exposure to human activities was clear between the unexposed and the exposed sites, quantification of human exposure (e.g. noise level and proximity to human activities) would be useful in future studies, so that changes in telomere length can be understood with respect to varying levels of human activity exposure. We thus suggest to further test the use of telomere length analysis as an eco-indicator of stress in chicks of Adélie penguins, and other penguin species, among anthropized sites throughout Antarctica. Telomere size could indeed be a relatively easy-to-use marker to inform the Committee for Environmental Protection at the Antarctic Treaty System on the impact that Antarctic stations or sites recurrently visited by tourists have on bird colonies.
Data availability
Data are available from the corresponding author upon request.
Code availability
R Code used in the statistical analyses are available from the corresponding author upon request.
References
Ainley DG et al (2018) Post-fledging survival of Adélie penguins at multiple colonies: chicks raised on fish do well. Mar Ecol Prog Ser 601:239–251. https://doi.org/10.3354/meps12687
Angelier F, Wingfield JC (2013) Importance of the glucocorticoid stress response in a changing world: theory, hypotheses and perspectives. Gen Comp Endocrinol 190:118–128. https://doi.org/10.1016/j.ygcen.2013.05.022
Angelier F, Costantini D, Blévin P, Chastel O (2018) Do glucocorticoids mediate the link between environmental conditions and telomere dynamics in wild vertebrates? A review. Gen Comp Endocrinol 256:99–111. https://doi.org/10.1016/j.ygcen.2017.07.007
Angelier F, Weimerskirch H, Barbraud C, Chastel O (2019) Is telomere length a molecular marker of individual quality? Insights from a long-lived bird. Funct Ecol. https://doi.org/10.1111/1365-2435.13307
Bauch C, Becker PH, Verhulst S (2013) Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2012.2540
Beaulieu M, Thierry A-M, González-Acuña D, Polito MJ (2013) Integrating oxidative ecology into conservation physiology. Conserv Physiol. https://doi.org/10.1093/conphys/cot004
Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P (2009) Telomere dynamics rather than age predict life expectancy in the wild. Proc R Soc B Biol Sci 276:1679. https://doi.org/10.1098/rspb.2008.1817
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53. https://doi.org/10.1038/35040500
Blévin P et al (2016) Exposure to oxychlordane is associated with shorter telomeres in arctic breeding kittiwakes. Sci Total Environ 563–564:125–130. https://doi.org/10.1016/j.scitotenv.2016.04.096
Boonekamp JJ, Mulder GA, Salomons HM, Dijkstra C, Verhulst S (2014) Nestling telomere shortening, but not telomere length reflects developmental stress and predicts survival in wild birds. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2013.3287
Boonekamp JJ, Bauch C, Mulder E, Verhulst S (2017) Does oxidative stress shorten telomeres? Biol Let. https://doi.org/10.1098/rsbl.2017.0164
Bricher PK, Lucieer A, Woehler EJ (2008) Population trends of Adélie penguin (Pygoscelis adeliae) breeding colonies: a spatial analysis of the effects of snow accumulation and human activities. Polar Biol 31:1397–1407. https://doi.org/10.1007/s00300-008-0479-z
Burger J, Gochfeld M (2007) Responses of Emperor penguins (Aptenodytes forsteri) to encounters with ecotourists while commuting to and from their breeding colony. Polar Biol 30:1303–1313. https://doi.org/10.1007/s00300-007-0291-1
Carlini AR, Coria NR, Santos MM, Libertelli MM, Donini G (2007) Breeding success and population trends in Adélie penguins in areas with low and high levels of human disturbance. Polar Biol 30:917–924. https://doi.org/10.1007/s00300-006-0251-1
Chatelain M, Drobniak SM, Szulkin M (2020) The association between stressors and telomeres in non-human vertebrates: a meta-analysis. Ecol Lett 23:381–398. https://doi.org/10.1111/ele.13426
CNES (2016) Distribution Airbus DS, Pléiades Satellite Image—Dumont d’Urville Station 03/03/2016. https://www.intelligence-airbusds.com/en/5751-image-gallery-details?img=42566#.XA_V7ql7nE5. Accessed 12 Feb 2019.
Cobley ND, Shears JR (1999) Breeding performance of gentoo penguins (Pygoscelis papua) at a colony exposed to high levels of human disturbance. Polar Biol 21:355–360. https://doi.org/10.1007/s003000050373
Coetzee BWT, Chown SL (2016) A meta-analysis of human disturbance impacts on Antarctic wildlife. Biol Rev 91:578–596. https://doi.org/10.1111/brv.12184
Culik B, Adelung DJ, Woakes A (1990) The effect of disturbance on the heart rate and behaviour of Adélie Penguins (Pygoscelis adeliae) during the breeding season. In: Kerry KR, Hempel G (eds) Antarctic ecosystems. Springer, Berlin, pp 177–182
Dehnhard N, Poisbleau M, Demongin L, Chastel O, van Noordwijk HJ, Quillfeldt P (2011) Leucocyte profiles and corticosterone in chicks of southern rockhopper penguins. J Comp Physiol B 181:83–90. https://doi.org/10.1007/s00360-010-0508-4
Dugdale HL, Richardson DS (2018) Heritability of telomere variation: it is all about the environment! Phil Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2016.0450
Dupoué A et al (2017) Shorter telomeres precede population extinction in wild lizards. Sci Rep 7:16976. https://doi.org/10.1038/s41598-017-17323-z
Fridolfsson A-K, Ellegren H (1999) A Simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121. https://doi.org/10.2307/3677252
Giese M (1996) Effects of human activity on Adélie penguin Pygoscelis adeliae breeding success. Biol Cons 75:157–164. https://doi.org/10.1016/0006-3207(95)00060-7
Giese M, Riddle M (1999) Disturbance of Emperor penguin Aptenodytes forsteri chicks by helicopters. Polar Biol 22:366–371. https://doi.org/10.1007/s003000050430
Gil D, Alfonso-Iñiguez S, Pérez-Rodríguez L, Muriel J, Monclús R (2019) Harsh conditions during early development influence telomere length in an altricial passerine: Links with oxidative stress and corticosteroids. J Evol Biol 32:111–125. https://doi.org/10.1111/jeb.13396
Giraudeau M, Angelier F, Sepp T (2019) Do Telomeres Influence Pace-of-Life-Strategies in Response to Environmental Conditions Over a Lifetime and Between Generations? BioEssays 41:1800162. https://doi.org/10.1002/bies.201800162
Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. Proc Natl Acad Sci 109:1743–1748. https://doi.org/10.1073/pnas.1113306109
Holmes ND, Giese M, Achurch H, Robinson S, Kriwoken LK (2006) Behaviour and breeding success of gentoo penguins Pygoscelis papua in areas of low and high human activity. Polar Biol 29:399–412. https://doi.org/10.1007/s00300-005-0070-9
Hughes KA et al (2018) Antarctic environmental protection: Strengthening the links between science and governance. Environ Sci Policy 83:86–95. https://doi.org/10.1016/j.envsci.2018.02.006
Ingles ED, Deakin JE (2016) Telomeres, species differences, and unusual telomeres in vertebrates: presenting challenges and opportunities to understanding telomere dynamics. AIMS Genet 3:1–24. https://doi.org/10.3934/genet.2016.1.1
Kawanishi S, Oikawa S (2004) Mechanism of Telomere Shortening by Oxidative Stress. Ann N Y Acad Sci 1019:278–284. https://doi.org/10.1196/annals.1297.047
Kennicutt MC et al (2014) Polar research: Six priorities for Antarctic science. Nature 512:23–25. https://doi.org/10.1038/512023a
Lynch HJ, Fagan WF, Naveen R (2010) Population trends and reproductive success at a frequently visited penguin colony on the western Antarctic Peninsula. Polar Biol 33:493–503. https://doi.org/10.1007/s00300-009-0726-y
Lynch MA, Youngflesh C, Agha NH, Ottinger MA, Lynch HJ (2019) Tourism and stress hormone measures in Gentoo penguins on the Antarctic Peninsula. Polar Biol 42:1299–1306. https://doi.org/10.1007/s00300-019-02518-z
Meillère A, Brischoux F, Ribout C, Angelier F (2015) Traffic noise exposure affects telomere length in nestling house sparrows. Biol Let. https://doi.org/10.1098/rsbl.2015.0559
Micol T, Jouventin P (2001) Long-term population trends in seven Antarctic seabirds at Pointe Géologie (Terre Adélie) Human impact compared with environmental change. Polar Biol 24:175–185. https://doi.org/10.1007/s003000000193
Mizutani Y, Tomita N, Niizuma Y, Yoda K (2013) Environmental perturbations influence telomere dynamics in long-lived birds in their natural habitat. Biol Let. https://doi.org/10.1098/rsbl.2013.0511
Mizutani Y, Niizuma Y, Yoda K (2016) How do growth and sibling competition affect telomere dynamics in the first month of life of long-lived seabird? PLoS ONE 11:e0167261. https://doi.org/10.1371/journal.pone.0167261
Monaghan P, Ozanne SE (2018) Somatic growth and telomere dynamics in vertebrates: relationships, mechanisms and consequences. Phil Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2016.0446
Nussey DH et al (2014) Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol Evol 5:299–310. https://doi.org/10.1111/2041-210X.12161
Oikawa S, Tada-Oikawa S, Kawanishi S (2001) Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry 40:4763–4768. https://doi.org/10.1021/bi002721g
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891. https://doi.org/10.1111/j.1600-0706.2009.17643.x
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Regel J, Pütz K (1997) Effect of human disturbance on body temperature and energy expenditure in penguins. Polar Biol 18:246–253. https://doi.org/10.1007/s003000050185
Reichert S, Stier A (2017) Does oxidative stress shorten telomeres in vivo? A review. Biol Lett 13: 20170463. https://doi.org/10.1098/rsbl.2017.0463
Ropert-Coudert Y et al (2018) Two Recent Massive Breeding Failures in an Adélie Penguin Colony Call for the Creation of a Marine Protected Area in D'Urville Sea/Mertz. Front Mar Sci 5:264. https://doi.org/10.3389/fmars.2018.00264
Salihoglu B, Fraser WR, Hofmann EE (2001) Factors affecting fledging weight of Adélie penguin (Pygoscelis adeliae) chicks: a modeling study. Polar Biol 24:328–337. https://doi.org/10.1007/s003000000215
Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S (2009) Telomere shortening and survival in free-living corvids. Proc R Soc B Biol Sci 276:3157. https://doi.org/10.1098/rspb.2009.0517
SCAR (2008) Human disturbance to wildlife in the broader Antarctic region: a review of findings. Paper presented at the Antarctic Treaty Consultative Meeting XXXI, Kyiv, Ukraine, 2–13 June 2008
Tin T, Lamers M, Liggett D, Maher PT, Hughes KA (2014) Setting the scene: human activities, environmental impacts and governance arrangements in Antarctica. In: Tin T, Liggett D, Maher PT, Lamers M (eds) Antarctic futures: human engagement with the Antarctic environment. Springer Netherlands, Dordrecht, pp 1–24
Trathan PN, Forcada J, Atkinson R, Downie RH, Shears JR (2008) Population assessments of gentoo penguins (Pygoscelis papua) breeding at an important Antarctic tourist site, Goudier Island, Port Lockroy, Palmer Archipelago, Antarctica. Biol Cons 141:3019–3028. https://doi.org/10.1016/j.biocon.2008.09.006
Tricola GM et al (2018) The rate of telomere loss is related to maximum lifespan in birds. Phil Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2016.0445
Viblanc VA, Smith AD, Gineste B, Groscolas R (2012) Coping with continuous human disturbance in the wild: insights from penguin heart rate response to various stressors. BMC Ecol 12:10. https://doi.org/10.1186/1472-6785-12-10
Villanueva C, Walker BG, Bertellotti M (2012) A matter of history: effects of tourism on physiology, behaviour and breeding parameters in Magellanic Penguins (Spheniscus magellanicus) at two colonies in Argentina. J Ornithol 153:219–228. https://doi.org/10.1007/s10336-011-0730-1
Walker BG, Dee Boersma P, Wingfield JC (2005) Physiological and Behavioral Differences in Magellanic Penguin Chicks in Undisturbed and Tourist-Visited Locations of a Colony. Conserv Biol 19:1571–1577. https://doi.org/10.1111/j.1523-1739.2005.00104.x
Walker BG, Dee Boersma P, Wingfield JC (2006) Habituation of Adult Magellanic Penguins to Human Visitation as Expressed through Behavior and Corticosterone Secretion. Conserv Biol 20:146–154. https://doi.org/10.1111/j.1523-1739.2005.00271.x
Watson H, Bolton M, Monaghan P (2015) Variation in early-life telomere dynamics in a long-lived bird: links to environmental conditions and survival. J Exp Biol 218:668–674. https://doi.org/10.1242/jeb.104265
Weimerskirch H, Lallemand J, Martin J (2005) Population sex ratio variation in a monogamous long-lived bird, the wandering albatross. J Anim Ecol 74:285–291. https://doi.org/10.1111/j.1365-2656.2005.00922.x
Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ (2018) The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2016.0447
Wilson KJ, Taylor RH, Barton KJ (1990) The impact of man on Adélie penguins at Cape Hallett, Antarctica. In: Kerry KR, Hempel G (eds) Antarctic ecosystem. Springer Berlin Heidelberg, Berlin, pp 183–190
Young AJ (2018) The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Phil Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2016.0452
Young RC et al (2017) Effects of developmental conditions on growth, stress and telomeres in black-legged kittiwake chicks. Mol Ecol 26:3572–3584. https://doi.org/10.1111/mec.14121
Acknowledgements
This work was supported by the French Polar Institute Paul-Emile Victor (IPEV) through the polar program 1091, the WWF-UK (especially Rod Downie for his continuous support throughout the years), and the Programme Zone Atelier de Recherches sur l’Environnement Antarctique et Subantarctique (i-LTER). We thank Mathieu Casado (AWI, Potsdam) for his assistance in creation of the map figure. This work was completed while JAC was completing her PhD in Evolution, Ecology, and Conservation at the University of Padua, with funding from a Cariparo Fellowship for foreign students and additional support from an Antarctic Science International (ASI) Bursary, a Scientific Committee on Antarctic Research (SCAR) Fellowship, and an Erasmus+ Student Traineeship. JAC now acknowledges support from the Alexander von Humboldt Foundation in the form of a Humboldt Research Fellowship for Postdoctoral Researchers funding her current research at the Alfred Wegener Institute Helmholtz Center for Polar and Marine Research, the Berlin Center for Genomics in Biodiversity Research, and the Leibniz Institute for Zoo and Wildlife Research. We are indebted to Ursula Ellenberg and one anonymous reviewer who considerably enhanced the quality of the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the French Polar Institute Paul-Emile Victor (IPEV) through the polar program 1091, the WWF-UK, and the Programme Zone Atelier de Recherches sur l’Environnement Antarctique et Subantarctique (i-LTER). This work was completed while JAC was completing her PhD in Evolution, Ecology, and Conservation at the University of Padua, with funding from a Cariparo Fellowship for foreign students and additional support from an Antarctic Science International (ASI) Bursary, a Scientific Committee on Antarctic Research (SCAR) Fellowship, and an Erasmus + Student Traineeship. JAC now acknowledges support from the Alexander von Humboldt Foundation in the form of a Humboldt Research Fellowship for Postdoctoral Researchers funding her current research at the Alfred Wegener Institute Helmholtz Center for Polar and Marine Research, the Berlin Center for Genomics in Biodiversity Research, and the Leibniz Institute for Zoo and Wildlife Research.
Author information
Authors and Affiliations
Contributions
FA, TP, and TR collected the samples; FA, YRC, and JAC conceived the ideas; JAC collected the data, JAC and FA analyzed the data; FA, JAC, and YRC contributed to interpretation of results; JAC, FA, and YRC wrote the manuscript. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were authorized by the Terres Australes et Antarctiques Françaises, IPEV and the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation (Authorization Number: #10343–2017062316008722 v3, from the Ethic Committee 084). For all interactions with animals, the Scientific Committee on Antarctic Research (SCAR)’s Code of Conduct for the use of Animals for Scientific Purposes in Antarctica was followed (https://www.scar.org/scar-library/search/policy/codes-of-conduct/3408-code-of-conduct-for-the-use-of-animals-for-scientific-purposes-in-antarctica?format=html).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caccavo, J.A., Raclot, T., Poupart, T. et al. Anthropogenic activities are associated with shorter telomeres in chicks of Adélie penguin (Pygoscelis adeliae). Polar Biol 44, 1391–1399 (2021). https://doi.org/10.1007/s00300-021-02892-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02892-7