Abstract
The use of light-emitting diodes (LEDs) is increasingly used in fishing gears and its application is known to trigger negative or positive phototaxis (i.e., swimming away or toward the light source, respectively) for some marine species. However, our understanding of how artificial light influences behavior is poorly understood for many species and most studies can be characterized as trial and error experiments. In this study, we tested whether exposure to white LED light could initiate a phototactic response in Antarctic krill (Euphausia superba). Trawl-caught krill were used in a controlled artificial light exposure experiment conducted onboard a vessel in the Southern Ocean. The experiment was conducted in chambers with dark and light zones in which krill could move freely. Results showed that krill displayed a significant positive phototaxis. Understanding this behavioral response is relevant to development of krill fishing technology to improve scientific sampling gear, improve harvest efficiency, and reduce potential unwanted bycatch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light-emitting diodes (LEDs) are increasingly used in commercial fisheries. Artificial light in commercial fishing gear applications can increase catch rates, contribute to reduced bycatch levels, and decrease energy costs (Nguyen and Winger 2019). In their natural environment, euphausiids use ambient light to adjust their position in the water column to preferred isolume levels, and they have developed advanced bioluminescent photophores that likely trigger responses from their conspecifics (Warner et al. 1979). However, whether artificial light triggers a phototactic response in crustaceans in terms of swimming away (negative) or toward (positive) the light source is poorly understood. Knowledge about species-specific behavioral responses to artificial light varies, but depending on the particular taxon’s response pattern, such information can be highly relevant to the development of efficient and low impact fishing technology.
Antarctic krill (Euphausia superba) is abundant in the Southern Ocean and constitutes an important species for commercial exploitation and research (Nicol et al. 2012; Krafft et al. 2015). The fishery is managed by the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) with established precautionary catch limits distributed within defined sub-areas. The current fisheries are far below the sustainable allowable levels of exploitation, and impacts may be only locally restricted. But with an increasing market demand and economic interest, it is expected that the exploitation for krill will be growing. Increased ethological understanding of the species, catch technology development and data processing development will contribute to evolve a management system adapting to future consequences of particularly increased catches and a changing ecosystem.
Krill exhibit active self-movement (Marr 1962; Kanda et al. 1982; Kils 1982; Klevjer and Kaartvedt 2011). Several traditional plankton nets with small net openings and small meshes can be selective, as the catch may over represent the species or life stages that are unable to avoid them (Sameoto 1980, 1983; Daly and Macauley 1988). Such nets are typically used to calculate dry or wet biomass weight, describe demographic species composition, and produce length distribution data that, i.e., are used to quantify biomass during acoustic surveys. The extent of net avoidance depends on the size of the net, mesh size, towing speed, and environmental conditions, such as light regime, season, and tidal state. To reduce gear avoidance to obtain representative catch samples when using small scientific active gear, researchers have used high-intensity strobe or continuous lights (70–150 W) to stun or confuse krill. Such trial and error studies with possibilities for multiple sources of stimuli affecting behavior, have, however, not demonstrated any consistent pattern. The use of strong light either increased euphausiid catch rates (Sameoto et al. 1993; Wiebe et al. 2004) or no effects were detected (Clark and Pascoe 1985). Also increased swimming activity in Meganycthiphanes norvegica have been found associated with increasing light intensity (Utne-Palm et al. 2018).
Most crustacean species have developed eyes that are highly sensitivity to light (Loew 1976; Mauchline 1980; Meyer-Rochow 1981; Nilsson 1982). In particular, the eyes of deep sea-adapted species are vulnerable even to moderate amounts of natural daylight, which can damage photoreceptor membranes (Loew 1976; Meyer-Rochow 1981; Nilsson 1982). As an alternative, use of low-intensity LED lights may be less harmful than using high-intensity lights. Humborstad et al. (2018) reported that the northern krill species Thysanoessa inermis displayed positive phototaxis when LED lights were used in passive fishing gear.
The goal of this study was to determine the type of phototactic response Antarctic krill display to white LED light under controlled experimental conditions. Depending of the results of this study, the experiences can be applied to future investigations of the use of low-intensity LED light to optimize commercial fishing and scientific sampling of Antarctic krill. Furthermore, the methodology developed in this study will be adapted to the facilities available onboard a fishing vessel. By demonstrating that fishing vessels can be used as a full-fledged research platform, in this respect to conduct controlled behavioral experiments, this may increase the opportunities to run similarly intended experiments.
Materials and methods
This experiment was conducted using live krill collected onboard the Norwegian ramp trawler FV Juvel (Aker Biomarine AS) off the South Orkney Islands (60° 35′ S, 45° 30′ W) during January–February 2018. Acoustic signals from Simrad EK60 General Purpose Transceivers connected to hull mounted ES60 transducers were used to detect krill swarms. The experimental specimens were collected using a macrozooplankton trawl (Krafft et al. 2018), which was 42 m length with a 6 × 6 m (36 m2) mouth opening and an inner net of 3 mm mesh size. The trawl was towed, by targeting a small and shallow krill acoustic signal, at a speed of ~ 2 knots and subsequently hauled onboard.
The krill were quickly and gently transferred from the codend to 15 L transparent plastic aquariums that were submerged in a 500 L holding tank that was fitted with a light-tight lid to reduce external light stimulation. The aquariums were perforated with 3 mm diameter holes (320 equally distributed on the side walls and 100 in the lid) to ensure exchange of water. These aquariums also attenuated vessel-induced movements and engine vibrations when they were submerged in the 500 L holding tank. During the first 24 h after the haul was taken onboard, the krill were inspected at regular intervals for 2–3 min by removing the lid of the holding tank. Individuals with visible signs of abnormal swimming activity and physical damage as well as dead individuals were removed. Thereafter, they were inspected daily, but no mortalities were observed over the following 4 days. Similar holding facilities have been used successfully onboard other vessels to conduct live krill experiments (Krafft and Krag, 2015; Krafft et al. 2016).
The 500 L holding tank received seawater from a separate 500 L buffer tank at a rate of 440 L water/hour via a pump (Fountain Pumps, Allegro). The buffer tank system was chosen to reduce the risk of ambient oxygen oversaturation in the turbulent water delivered from the vessel’s large internal pump system. The buffer tank was supplied with surface water at a rate adjusted to make excess water just able to flow over the edge. The high level of water exchange was chosen to most closely resemble the natural temperature conditions at the sea surface.
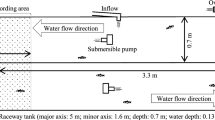
A third 500 L tank was placed inside a light-tight standard 20-foot-long steel container, that was welded to the trawl deck, to control the ambient light levels. The experimental tank (Fig. 1a) contained three equally sized chambers (~ 167 L each) with a center release chamber, a dark chamber, and a light chamber. In the light chamber, a white LED light (Osram, mod: Dot-it, 0.2 W) was placed in a perforated box on the side facing away from the entrance to the chamber to avoid direct exposure to the light source. The characteristics of this light, in terms of wavelength and intensity, were measured in air with a hyperspectral radiometer with a scalar irradiance detector between 320 and 950 nm (Ramses ASC, TriOS GmbH, Germany). An initial measurement was made in a dark-room to ensure that no confounding light source was present. The LED light was then switched on and directed directly toward the radiometer at first 0.5 m and then 1.0 m distance.
Experimental tank (500 L) with three chambers: left (dark), middle (release, in which krill were deployed), and right (light) (with LED light source). Sensors monitored water conditions continuously (oxygen, temperature, salinity), and recordings of light were made in the dark and light chambers (a). The chamber-separating walls had openings (b) that were closed between each trial in order to count the specimens in each chamber (c)
The dividing walls between the chambers were fitted with a 10 cm circular hole to enable krill to migrate freely between chambers. The holes were placed in opposite ends of the release chamber, thereby creating a light gradient when the light was turned on (Fig. 1b). An underwater photometer (Type LI-192SA; LI-COR, Lincoln, Nebraska, USA) was placed in the dark chamber and a second sensor in the light chamber to measure photosynthetically active radiation in µm/m2/s (Fig. 1a, Table 1).
The entire experimental set-up, including sensors and circulating water in all tanks (500 and 15 L), was switched on 48 h prior to the arrival of krill to ensure that all components were functioning properly, and that temperature, salinity, and oxygen content were stable and matching the ocean conditions. Before each experimental trial was initiated, the water supply in the experimental tank was closed to terminate any circulation movement. The LED light was switched on inside the light chamber and fresh krill were collected from the holding tanks and moved to the experimental tank in a 15 L black light-tight plastic transport aquarium. The number of krill used in each experiment varied to allow for analysis of density dependent impacts on the results. The transport aquarium was submerged into the release chamber for 1 h to acclimate the krill after they had experienced potential stress during the short transport from the holding tank. The krill then were released gently into the release chamber and left undisturbed in the experimental container for 10 min. The experiment was terminated by closing the holes in the chamber walls (Fig. 1c), and the number of individual krill in each chamber was counted. In total, 10 trials were performed using this approach. For control purposes, the same process was repeated every other time for the first six experiments without any light in the light chamber. None of the trials were repeated using the same experimental animals. Between trials, the tank was supplied with seawater from the buffer tank located outside the steel container via the pump and a hose that ran through the steel wall, and the excess water was released from the experiment tank through a second hose inserted through the steel side wall. Hydrological conditions were monitored continuously using oxygen sensors (Oxyguard Handy Polaris 2) and mini CTDs (Star–Oddi), which recorded temperature and salinity every 10 s (Table 1). The trials performed in the experimental tank lasted over a period of 3 days at times representing the entire 24-h cycle.
Analysis of variance (General Linear Model) was applied to test for statistical differences among the numbers of krill in the experimental chambers (SAS Institute, Cary, NC, USA). Potential effects caused by time of day and number of individuals included in the trials were tested using regression analyses (Proc Reg).
Results
The measurements of the LED light in air was characterized by a wavelength ranging between 400 and 800 nm with two distinct intensity maximum peaks (Fig. 2). The first intensity max was at 450 nm (measuring distance 0.5 m = 7.5 mW/(m2 nm) and measuring distance 1.0 m = 4.51 mW/(m2 nm),) and at 564 nm (0.5 m = 2.8 mW/(m2 nm), 1.0 m = 1.34 mW/(m2 nm)).
The 10 trials executed using the LED light included 384 krill, and the average group size was 38 ± 13 standard deviation (SD) individuals (range: 10–58 individuals). The six control trials (absence of light) included 221 krill, and the average group size was 37 ± 10 SD individuals (range: 25–52 individuals).
An overall significant difference was detected among the number of individuals in the three different chambers (GLM F = 11.40, p = 0.003). More krill were in the light chamber versus the release chamber (GLM F = 7.95, p = 0.01) and in the light chamber versus the dark chamber (GLM F = 22.91, p < 0.001). However, no statistical difference in krill number was found between the release chamber and the dark chamber (GLM F = 2.77, p = 0.11). The average proportional distribution of individuals between the chambers was 19 ± 6% in the dark chamber, 29 ± 10% in the center release chamber, and 54 ± 16% in the light chamber (Fig. 3).
The control trials showed that krill without any artificial light stimulus actively swam around in the experimental tank but ended up being equally distributed among the three experimental chambers. Thus, the number of individuals located in the three chambers did not differ significantly (GLM F = 0.08, p = 0.92). The average proportional distribution of individuals was 35 ± 6 SD% in the dark chamber, 32 ± 6 SD% in the center release chamber, and 34 ± 10 SD% in the light chamber (Fig. 3).
No effect of time of day was found (Reg F = 0.00, p = 0.96), and no effect of the number of individuals included in the trials was detected (Reg F = 1.68, p = 0.23).
Discussion
We found that krill displayed a positive phototactic response to LED light by actively swimming toward the light source under controlled conditions. This finding suggests that there is potential to increase the proportion of Antarctic krill that otherwise can avoid/escape sampling gear. This approach may be less harmful to krill than the use of high-intensity strobe lights, which stun krill and potentially cause permanent damage to their photoreceptor membranes (e.g., Wiebe et al. 2004), and may cause negative consequences for animals not caught by the net. However, to detect this extent, it will be necessary to perform systematic physiological examinations of their photoreceptor membranes under different types of light exposure.
The results of this study may contribute to a reduction in the environmental impact of the commercial fishery for krill in terms of fuel consumption per catch unit by increasing the catch efficiency of the gear. The use of lights also has the potential to change gear design to reduce drag, thereby reducing fuel consumption even more. Using positive phototaxis of krill toward low-intensity LED lights in towed commercial fishing gear requires that the krill have time to react to the light source and swim into the trawl path in order to be caught. After their release into the experimental tank, the krill used in the current study were given 10 min to distribute themselves among the dark, release, and light chambers before inspection. We tried to monitor the krill during those 10 min using night vision gear (Pulsar, Challenger, monocular), but we were unable to follow the swimming individuals or to quantify their speed in the dark experimental conditions using such technology. Free-ranging krill have been observed to swim up to 10–15 ms–1 (Kanda et al. 1982; Zhou and Dorland 2004), but this is probably also under the influence of water current (Zhou and Dorland 2004), which means that the catch efficiency of a trawl probably would be dependent on the towing direction relative to the water current. Light can potentially be used to attract krill to the area swept by the trawl or sampling net, and light can further attract krill toward the center of the mouth. Unlike many pelagic fish species, krill likely encounter the side of the trawl in front of the codend multiple times from the mouth area toward the codend (Herrmann et al. 2018; Krag et al. 2018). By also using light to achieve net-wall avoidance, gear with larger mesh sizes design can be used, which will reduce drag and the subsequent CO2 emissions from the fishery.
Future experiments should investigate the short-term spatial behavior of krill in relation to an LED light source, either experimentally or during commercial application with light-equipped trawls (e.g., using high-frequency broadband echo sounders that can detect and track the movements of individual krill). The conditions under which we observed the positive phototactic response should be viewed as baseline conditions. Future in situ studies should be conducted to determine whether the positive phototactic response observed in this study is applicable in a net or trawling situation in which krill behavior is further stimulated by audio–visual factors such as moving gear parts or hydrodynamic factors caused by the moving gear parts (e.g., the netting). Likewise, further experiments on sex and maturity stages or whether body size and swimming capacity are important, will be decisive for the design of scientific gears that include lights to prevent a size-dependent increase in the catch, or unrepresentative samples in a manner similar to that when krill avoid the gear.
Bycatch species also are likely to react to LED lights, and this potential unintended effect should be considered in future studies.
Results of this study demonstrated that facilities onboard a fishing vessel could provide the conditions needed to conduct behavioral experiments on Antarctic krill with a high degree of precision and stability of the measurements. In previous studies, use of a fishing vessel as a research platform for conducting live experiments of krill physiology has also produced high quality results (e.g., Krafft and Krag 2015; Krafft et al. 2016). Further systematic testing of the behavioral responses of krill to different light characteristics, such as intensity, wavelength, and strobing, should be conducted, and possible diurnal/seasonal patterns, influence of body size, sex or reproductive status, and effects of introduced environmental factors similar to those found in their natural habitat should be investigated. Such knowledge will be essential for successfully implementing the use of artificial light in passive or active fishing gear for scientific sampling or commercial exploitation of this species.
References
Clark MR, Pascoe PL (1985) The influence of an electric light on the capture of deep-sea animals by a midwater trawl. J Mar Biol Ass UK 65:373–393
Daly KL, Macaulay MC (1988) Abundance and distribution of krill in the ice edge zone of the Weddell Sea, austral springs 1983. Deep-Sea Res 35:1564–1576
Herrmann B, Krag LA, Krafft BA (2018) Size selection of Antarctic krill (Euphausia superba) in a commercial codend and trawl body. Fish Res 207:49–54
Humborstad O-B, Utne-Palm AC, Breen M, Løkkeborg S (2018) Artificial light in baited pots substantially increases the catch of cod (Gadus morhua) by attracting active bait, krill (Thysanoessa inermis). ICES J Mar Sci 76:2257–2264. https://doi.org/10.1093/icesjms/fsy099
Kanda K, Takagi K, Seki Y (1982) Movement of larger swarms of Antarctic krill Euphausia superba off Enderby Land during 1976–1977 season. J Tokyo Univ Fish 68:24–42
Kils U (1982) Swimming behavior, swimming performance and energy balance of Antarctic krill Euphausia superba. Biomass Sci Ser 3
Klevjer TA, Kaartvedt S (2011) Krill (Meganyctiphanes norvegica) swim faster at night. Limnol Oceanogr 56:765–774
Krafft BA, Krag LA (2015) Assessment of mortality of Antarctic krill (Euphausia superba) escaping from a trawl. Fish Res 170:102–105
Krafft BA, Krag LA, Engås A, Nordrum S, Bruheim I, Herrmann B (2016) Quantifying the escape mortality of trawl caught Antarctic krill (Euphausia superba). PLoS ONE 11(9):e0162311. https://doi.org/10.1371/journal.pone.0162311
Krafft BA, Krag LA, Knutsen T, Skaret G, Jensen KHM, Krakstad JO, Larsen SH, Melle W, Iversen SA, Godø OR (2018) Summer distribution and demography of Antarctic krill (Euphausia superba) (Dana, 1852) (Euphausiacea) at the South Orkney Islands, 2011–2015. J Crust Biol. https://doi.org/10.1093/jcbiol/ruy061
Krafft BA, Skaret G, Knutsen T (2015) An Antarctic krill (Euphausia superba) hotspot - population characteristics, abundance and vertical structure explored from a krill fishing vessel. Pol Biol 38:1687–1700. https://doi.org/10.1007/s00300-015-1735-7
Krag LA, Krafft BA, Engås A, Herrmann B (2018) Collecting size-selectivity data for Antarctic krill (Euphausia superba) with a trawl independent towing rig. PLoS ONE. https://doi.org/10.1371/journal.pone.0202027
Loew ER (1976) Light, and photoreceptor degeneration in the Norway lobster, Nephrops norvegicus (L.). Proc R Soc Land B 193:31–44
Marr J (1962) The natural history and geography of the Antarctic krill Euphausia superba. Discov Rep 32:33–464
Mauchline J (1980) The biology of Mysids and Euphausiids. In: Blaxter JHS, Russell FS, Yonge M (eds) Adv Mar Biol, vol 18. Academic Press, London, UK, p 681
Meyer-Rochow VB (1981) The eye of Orchomene sp. cf. O. rossi, an amphipod living under the Ross Ice Shelf (Antarctica). Proc R Soc Lond B 21:93–111
Nguyen KQ, Winger PD (2019) Artificial light in commercial industrialized fishing applications: a review. Rev Fish Sci Aqua 27(1):106–126. https://doi.org/10.1080/23308249.2018.1496065
Nicol S, Foster J, Kawaguchi S (2012) The fishery for Antarctic krill–recent developments. Fish (Oxf) 13:30–40
Nilsson HL (1982) Rhabdom breakdown in the eye of Cirolana borealis (Crustacea) caused by exposure to daylight. Cell Tiss Res 227:633–639
Sameoto D (1980) Qualitative measurements of euphausiids using a 120 kHz sounder and their in situ orientation. Can J Fish Aquat Sci 37:693–702
Sameoto D (1983) Euphausiid distribution in acoustic scattering layers and its significance to surface swarms. J Plankt Res 5:129–143
Sameoto D, Cochrane N, Heman A (1993) Convergence of acoustic, optical, and net-catch estimates of euphausiid abundance: use of artificial light to reduce net avoidance. Can J Fish Aquat Sci 50:334–346
Utne-Palm AC, Breen M, Løkkeborg S, Humborstad OB (2018) Behavioural responses of krill and cod to artificial light in laboratory experiments. PLoS ONE 13:e0190918
Warner JA, Latz MI, Case JF (1979) Cryptic bioluminescence in a midwater shrimp. Science (Wash, DC) 203:1109–1110
Wiebe PH, Ashjian CJ, Gallager SM, Davis CS, Lawson GL, Copley NJ (2004) Using a high-powered strobe light to increase the catch of Antarctic krill. Mar Biol 144:493–502
Zhou M, Dorland RD (2004) Aggregation and vertical migration behavior of Euphausia superba. Deep-Sea Res II 51:2119–2137. https://doi.org/10.1016/j.dsr2.2004.07.009
Acknowledgements
This study is part of the project KRILL, which is supported by the Royal Norwegian Ministry of Fisheries and Coastal Affairs and the Institute of Marine Research in Norway. We would like to extend our gratitude to Aker BioMarine ASA for providing their vessel and crew free of charge and allowing us to collect the material and conduct the experiment. We thank Dr JD Karlsen (DTU Aqua) for valuable comments about the work. We also appreciate the time and effort by editor and reviewers (1 anonymous, Drs I Everson and GA Tarling) to improve the manuscript.
Funding
Open access funding provided by Institute of Marine Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. No special permit was required to conduct the study on invertebrates.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krafft, B.A., Krag, L.A. Antarctic krill (Euphausia superba) exhibit positive phototaxis to white LED light. Polar Biol 44, 483–489 (2021). https://doi.org/10.1007/s00300-021-02814-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02814-7