Abstract
Understanding the population dynamics of benthic communities is impossible without understanding the processes related to their initial development, including recruitment. In polar areas, encrusting organisms, such as bryozoans, polychaetes, sponges and ascidians, are amongst some of the most species-rich and abundant groups of macrofaunal organisms, yet knowledge about their ecology is far from being complete. In this study, by investigating established encrusting assemblages and recruitment onto experimental substrate, we examine the level of similarity between adult populations and newly recruited assemblages in the polar realm. This study was conducted during the austral summer of 2010–2011 in Maritime Antarctica at King George Island (62°S, 58°W) at two locations contrasting in their biological and physical conditions. Despite the small distance (~3 km) between the two study sites, the local species pools (species composition, numerical abundance) differed significantly and had a great influence on the observed recruitment pattern. The species composition of new recruits overlapped with that of nearby assemblages at all examined locations. The dominance structure was also identical, with bryozoans being the major component of these assemblages. The numbers of species and individuals in the newly recruited communities and local resident assemblages were also strongly correlated. The obtained results suggest that the recruitment of encrusting fauna in the Antarctic can be very localized and occurs in close vicinity to adult populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recruitment is defined as the arrival of new individuals into the community that settle and survive after an arbitrary period of time (Connell 1985; Pineda et al. 2010). This stage of organismal life represents a crucial phase in the development of marine benthic assemblages. New arrivals settle in a given area in the form of planktonic larvae that can be transported in the water column for distances ranging from metres to kilometres (see Shanks et al. 2003; Teske 2014; Zhang et al. 2015). This has several implications for genetic exchange and survival as well as species composition and the overall structure of the given community (Todd 1998; Pineda et al. 2010). Understanding the processes controlling larval dispersal is therefore very important from an evolutionary, ecological and conservation perspective (Jablonski 1986; Cowen et al. 2006; Fogarty and Botsford 2007).
As the majority of benthic invertebrates produce pelagic larvae, we would expect that most local populations or assemblages are uncoupled from local reproduction by a dispersive larval phase, leading to a rather open system. Several studies, however, have suggested that recruitment can be localized within a site or within the proximity of parental populations and that the population and community can be viewed essentially as a closed system (Osman and Whitlatch 1998; Cowen et al. 2006). There is evidence that this process is supported by the limited dispersal of larvae or asexual recruitment (Osman and Whitlatch 1998 and references therein). Indeed, the diversity of dispersal strategies employed by sessile organisms is vast. It can range from broadcast-spawning species, which often produce a large number of larvae that have a long planktonic phase, to brooders, which produce fewer lecithotrophic larvae that have a short-lived phase in the water column or basically realize a juvenile form (Pearse and Lockhart 2004).
Extreme events and disturbance can occasionally be severe at any shallow locality, but there are geographic patterns in disturbance, and this may shape organism assemblages (Barnes and Kuklinski 2003). The intensity of disturbance typically increases towards the poles due to summer ice scour (the grounding of icebergs, scraping the sea bed), intense wind-driven wave action coupled with freezing temperatures and the occurrence of ice feet (walls or belts of ice frozen to the shore) during winter (see Gutt et al. 1996; Barnes 1999; Barnes and Conlan 2007). Some studies suggest that assemblages that experience frequent physical extremes (e.g. wave impacts) should be highly dependent on the regional input of propagules (Palmer et al. 1996). Highly disturbed polar intertidal encrusting assemblages indeed seem to mirror those formed in the nearby subtidal zone, which contrasts with the less disturbed temperate intertidal areas, where assemblages specialized to that zone occur (Kuklinski and Barnes 2008). Therefore, in shallow polar environments, which tend to be more disturbed than those at lower latitudes, we would intuitively expect recruitment to have a higher dependence on the species pool from nearby assemblages. Similarly, with decreasing depth, disturbance caused by ice and wave action increases, making shallower parts much more dynamic and unstable. Therefore, again, local assemblages from the shallows should resemble those from nearby areas.
The majority of Antarctic encrusting assemblages are dominated in terms of species number and abundance by bryozoans and polychaetes (Barnes et al. 1996; Stanwell-Smith and Barnes 1997; Bowden et al. 2006). Most of these organisms possess bulky lecithotrophic larvae that often settle to the firm substrate within hours if not minutes (Hughes et al. 2001). The duration of the larval phase has shown a strong positive correlation with the dispersal potential of many taxa, and the more time propagules spend in the water column, the farther they tend to be dispersed (Shanks et al. 2003). This would lead us to predict that, at a local scale, newly recruited Antarctic assemblages dominated by taxa with a short pelagic larval duration should mirror assemblages of nearby established populations. However, recent reviews of this issue question whether the pelagic larval duration is good predictor of population structure (Weersing and Toonen 2009). Additionally, recent genetic studies of Antarctic organisms reveal differences in population structure over the fine scale of kilometres even in broadcast-spawning marine molluscs (Hoffman et al. 2012).
As Kuklinski et al. (2014) indicated in their study of bryozoan assemblages in the Antarctic, one of the most important factors shaping the initial structure of these assemblages (e.g. species composition, abundance) is the numerical structure, including the relative abundance and dominance structure of neighbouring adult populations. In this study, we tested whether the mentioned pattern for bryozoans could also be applied to a whole encrusting assemblage. The presented study investigated the recruitment of Antarctic encrusting organisms on hard substrate as well as the composition of local adult assemblages. The experimental design enabled us to investigate the level of similarity between newly recruited and established adult assemblages over a small scale of 3 km at three shallow subtidal depths (6, 15 and 21 m). By examining local assemblages together with the zooplankton composition, we also analysed whether recruitment is coupled with larval occurrence in the water mass overlying the bottom. All these data provide insight into how the recruitment process of Antarctic encrusting fauna is linked to local adult populations.
Materials and methods
Study area
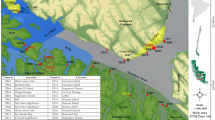
The study was conducted in Admiralty Bay, a fjord-like embayment, at King George Island (62°S, 58°W) (Fig. 1), which is the largest of the South Shetland Islands. Based on its climatic conditions, the area is considered as being part of the Maritime Antarctic region. The surface water temperature in the bay varies between −1.70 and 2.3 °C throughout the year, while the salinity ranges from 33.5 to 34.4 (Tokarczyk 1987; Rakusa-Suszczewski 1995). Both parameters are influenced by water exchange with the Bransfield Strait and are modified by fresh water from melting glaciers during the summer (Tokarczyk 1987; Rakusa-Suszczewski 1995). A large proportion of the shores of Admiralty Bay are covered by glaciers. The average tidal range is 1.4 m, with maximum tides up to 2.1 m (Catewicz and Kowalik 1984). Circulation of surface currents in the area is complex and dependant on wind and tidal forces (Pruszak 1980). Winds in Admiralty Bay prevail from three major directions SW (24.1%), W (21.8%) and N (17.0%), and the surface currents follow the same pattern (Pruszak 1980). Additionally, vertical whirlpools of water that produce upwelling currents are also recorded. Below 25 m depth, current circulation has less association with the prevailing wind directions and is driven to some extent by tidal and inertial forces generated by open sea currents (Pruszak 1980). The exchange of upper water masses (down to 100 m depth) between Admiralty Bay and the nearby Bransfield Strait is assumed to occur for one to two weeks (Pruszak 1980). The current velocities span an order of magnitude, reaching 100 cm/s; however, within the bay inlets, more common maximum values range from 20 to 40 cm/s.
For the purpose of this study, two contrasting locations in one of the branches of Admiralty Bay—Ezcurra Inlet—were selected (Fig. 1). Station U is located on the northern coast of the inlet. It is under a much stronger influence from the open sea and is less influenced by the glaciers and the factors associated with them (ice scouring, sedimentation, fresh water runoff) compared to station W, which is located on the southern coast. The sea bottom at both stations is inclined at approximately 30°–40°. It is composed of a mixture of cobbles and pebbles overlying mud, but mud predominates at station W, and some of the rocks are partly covered with it. At both locations, the sea bottom is periodically affected by icebergs.
Sampling protocol and data analysis
The study was conducted during austral summer between 18 November 2010 and 04 February 2011. Summer is the period when the highest larval settlement is observed in Antarctic waters, therefore being the most suitable period for such investigations (Absher et al. 2003; Bowden et al. 2009).
To identify the local species pool (species composition, numerical abundance) of epifauna at each station from all three investigated depths (6, 15 and 21 m), 100 rocks of various sizes were haphazardly collected. To estimate the species composition of the recruiting assemblages as well as their abundance and dominance structure at each site and depth, 50 rocks devoid of fauna were deployed. These rocks were collected from the low intertidal zone near the Polish Polar Station “Arctowski”. All rocks were of the same mineralogical composition. Each rock was examined for the presence of fauna and flora, which were removed if present. The surface area of the rocks, both those with local fauna and experimental ones, was estimated using an inelastic net marked with a grid of square centimetres. To confirm the patterns of recruitment observed on the experimental rocks, three flat Perspex settlement panels, identical in size and shape (15 × 15 × 0.3 cm), were additionally deployed at the same sites as the experimental rocks. As the surfaces of the panels are identical at all locations, this enabled the full comparability of recruitment results among sites and depths.
All organisms directly attached to the investigated substrates were identified to the lowest possible taxonomic level, typically to species. Bryozoan data were gathered from Kuklinski et al. (2014). The number of individuals was counted. In the case of colonial organisms, each colony was considered as one individual.
To determine the larval content in the water column above the experimental sites, plankton samples were collected with a net (20 μm mesh size, diameter 0.25 m) three times during the day throughout the duration of the project. The net was towed as close as possible to the sea bottom (approx. 0.5–1 m above) over a distance of 20 m, resulting in a water sample of 0.98 m3 volume.
To compare richness and the densities of individuals among depths, locations and the three different substrates (rocks with resident established local assemblages, cleaned rocks and panels with new recruits), species abundance curves (increase in the number of epibiont species with an increasing number of individuals) were plotted. The number of rocks with established fauna and that of experimental rocks was equal at each site and depth, yet the overall surface area of the rocks differed. Therefore, additional analyses were performed with the use of the species abundance curves, where equal rock surface areas were used for each site and depth. Computing 95% confidence intervals (using the formulae by Colwell et al. 2004) allows for the statistical comparison of the species richness in the datasets. The differences are not significant at p < 0.05 if the 95% confidence intervals overlap (Colwell et al. 2004). As the sample sizes or, in our approach, surface areas were the same, the comparison of the species abundance curves provided a straightforward methodology for assessing the difference in species richness and abundance of the studied assemblages.
All data for the density of individuals were standardized to the same surface area (m2), and, in addition to the species abundance curve analysis, the relative similarity among the established, experimental and panel assemblages was assessed and displayed using the PRIMER software package following a square root transformation of the data. Bray–Curtis similarity measures were calculated (Bray and Curtis 1957). The interrelationships among samples were mapped using the ordination technique non-metric multidimensional scaling (nMDS).
To measure whether the water column is stratified, the water temperature was recorded every 10 min for the duration of the whole experiment at each depth using HOBO Onset temperature loggers (model UA-002-64 Pendant Temp/Light). To explore the significant differences in temperature among depths and locations, analysis of variance (ANOVA) following log (x + 1) data transformation (to improve normality and homogeneity) was used.
All calculations were conducted using EstimateS 9 (Colwell 2013), while plots and ANOVA analysis were done using Statistica 8.
Results
On 605 rocks (total surface area of 280,096 cm2) with resident, established assemblages, 21,944 individuals belonging to 41 taxa were found (Table 1). The assemblage structure at both locations was very similar, with bryozoans being the most species-rich group (34 taxa constituting 85% of the investigated encrusting fauna). For the detailed numerical composition of the communities at the given locations, see Table 1.
Both the number of taxa and individuals in the established assemblages were higher at station U than at station W (Figs. 2, 3; Table 1). At station U, the number of taxa ranged from 26 (at 6 m depth) to 35 (at 21 m depth), while at station W, it ranged from 15 (at 21 m depth) to 17 (at 6 m depth) (Fig. 2A, B). The number of individuals at station U ranged between 5939 (at 6 m depth) and 6563 (at 15 m depth), while at station W, it ranged between 761 (at 21 m depth) and 1210 (at 6 m depth) (Fig. 2A, B). While the total number of individuals differed among depths at each station, in case of the number of taxa, as shown by the confidence intervals, which overlapped in each case, there were no significant differences among depths (Fig. 2). In general, the taxonomic composition on the rocks from station W overlapped with that at station U. Nineteen taxa were present only at station U, and two taxa were present only at station W (for details, see Table 1).
Species abundance curves for epifaunal assemblages found on rocks collected at site U (A) and site W (B), new recruits found on experimental rocks devoid of fauna at the time of deployment at site U (C) and site W (D) and new recruits found on panels at site U (E) and site W (F). In parentheses, the total area of analysed rocks or panels at a given depth is shown (E, F please note the different scales for panel results)
Summarized for all depths at a given location, species abundance curves for epifaunal assemblages found on rocks collected at site U and site W, new recruits found on experimental rocks devoid of fauna at the time of deployment at site U and site W and new recruits found on panels at site U and site W. In parentheses, the total area of analysed rocks or panels at each location
On the 370 retrieved experimental rocks (total surface area of 101 420 cm2) that were devoid of fauna at the start of the experiment, 22,663 individuals that were members of 12 taxa were recorded all together (Table 1). The patterns in the number of taxa and individuals were similar to those observed on the rocks with established assemblages (Figs. 2, 3; Table 1). Both the number of taxa and individuals were higher at station U (Fig. 3). At station U, the number of taxa ranged from eight (at 6 and 15 m depth) to nine (at 21 m depth), while at station W, the number of taxa ranged from three (at 21 m depth) to five (at 6 m depth) (Fig. 2C, D). The number of individuals at station U was between 5729 (at 21 m depth) and 8062 (at 15 m depth), while at station W, it was between 248 (at 15 m depth) and 2067 (at 21 m depth) (Fig. 2C, D). Similar to the established assemblages, the number of individuals differed among depths at each station (U and W). In terms of species number, as shown by the confidence intervals, which overlapped in each case, there was no significant difference among depths. Similar to the established assemblages on rocks, there was no clear trend in terms of an increase or decrease in the taxa richness or abundance of newly recruited organisms with depth (Fig. 2). Almost all taxa present on the rocks at station W were also present on the rocks at station U (see Table 1). The structure of the assemblages at both locations was very similar, with bryozoans being the most taxa-rich group (nine taxa constituting 81% of the investigated encrusting fauna) (Table 1).
On the 18 experimental panels (total surface area 1800 cm2), 2247 individuals of 19 taxa were recorded (Table 1). Although the overall number of individuals was higher at station U (1340) than at station W (907), the number of taxa was identical at both locations (16) (Figs. 2, 3; Table 1). There were no striking differences in the number of taxa or individuals. The richness of taxa varied between six (at 6 m depth) and 15 (at 15 m depth) at station U and between five (at 6 m depth) and 14 (at 21 m depth) at station W (Fig. 2E, F). The number of individuals ranged between 64 (at 6 m depth) and 728 (at 15 m depth) at station U and between 50 (at 6 m depth) and 789 (at 21 m depth) at station W (Fig. 2E, F). Although there was an increase in the number of taxa and individuals with depth, the confidence intervals overlapped for all depths at both sites, therefore indicating a lack of statistical significance among the investigated depths in regard to the number of taxa (Fig. 2E, F). The species composition on the panels from station W overlapped with that at station U with a few exceptions (the bryozoan Beania sp. was absent at station U, see Table 1). The assemblage structure at both locations was very similar, with bryozoans being the most species-rich group (Table 1).
The taxonomic composition of new recruits on both the experimental rocks and panels overlapped with that of the nearby established assemblages at the majority of the examined locations and depths (Table 1). The bryozoan Inversiula nutrix and spirorbid polychaetes were the most abundant species in the majority of cases, including the experimental rocks and panels as well as the resident, established assemblages (Table 1).
Overall, the species abundance curve analysis clearly indicated that the numbers of newly recruited species and individuals display strong correlations with the numbers of species and individuals recorded in the local old assemblages (Figs. 2, 3). This pattern was evident in both analyses, the first including an equal number of rocks at each site and depth but with differing overall surface areas of the rocks and the second when an additional analysis was performed (not visualized in this study) using the species abundance curves, where equal surface areas of the rocks were considered for each site and depth. In addition, quantitative differences in the species composition of the assemblages between the stations were confirmed by multivariate analysis (nMDS), showing the sites as being clustered into two distinct groups: station U and station W (Fig. 4).
Multidimensional scaling plot based on Bray–Curtis similarities using square root-transformed abundance data (standardized to densities of individuals on substrate per m2) for the recruited assemblages on local rocks (R), experimental rocks (Exp R) and panels (P) at two locations: U and W (see Fig. 1 for station locations)
In terms of the plankton samples, nine major group of organisms were recorded, including Cnidaria, Gastropoda, Polychaeta, Isopoda, Copepoda, Amphipoda, Ostracoda, Nematoda and Foraminifera (Table 2). The number of taxa was higher at station U (11) than at station W (seven) and consisted in the majority of cases of holoplanktonic or potentially mobile benthic groups. The number of individuals was higher at station W (70) than at station U (56).
At both locations (U and W), the water temperature significantly differed among depths (ANOVA, stn U: F (2, 35856) = 207.25, p < 0.001; stn W: F (2, 29673) = 670.82, p < 0.001). In addition, the average water temperature values significantly differed between the two locations (ANOVA, F (2, 65533) = 50.33, p < 0.001). However, the overall water temperatures on average (mean ± standard deviation) were within a very small range, and all the values were between 0.76 ± 0.54 °C (station A, 6 m depth) and 1.00 ± 0.56 °C (station B, 6 m depth).
Discussion
Encrusting fauna go through a pelagic phase of variable duration in their life cycle in the form of larvae (e.g. Ryland 1970). This would intuitively suggest that the larvae have the ability to disperse over large distances and colonize areas far away from their maternal colonies. However, there have been studies indicating recruitment to be both localized on a scale of metres to occurring at large distances on a scale of kilometres away from maternal populations (Booth and Brosnan 1995; McQuaid and Phillips 2000; Pineda et al. 2010). This investigation revealed a clearly higher species number and abundance within established assemblages at site U in comparison to site W. This leads to a similar pattern among new recruits, with higher numbers of species and individuals at site U. This pattern suggests that recruitment processes in encrusting fauna in the Antarctic are driven to some degree by the structure of local populations and seem to occur close to maternal organisms. Thus, these assemblages seem to form rather closed systems even though possessing the aforementioned capability of dispersal through the water column.
At both studied sites, newly recruited assemblages were impoverished in regard to species number compared to resident, established assemblages. This study was conducted during austral summer, when the larval concentration in the water column and recruitment in Antarctic waters seem to be highest; however, it is known that the larvae of some species might also be present in the water column earlier and later in the season (Bowden et al. 2009). As the experiment lasted for only a few summer months, both species richness and the composition of newly recruited assemblages undoubtedly have to differ from the existing adult assemblages.
The difference in recruitment between locations U and W was much more pronounced among experimental rocks than panels. Although the overall abundance was higher on panels at location U than W, the differences between the two locations were not as visible as in the case of experimental rocks (see Figs. 2, 3, 4). Experimental rocks fully mimic the local conditions for settlement, which in case of this study means that they were often lying on the sandy/muddy bottom (location W). This most likely prevents the colonization of the underside of the rocks, which is the preferred surface for the majority of the encrusting fauna, as they are photonegative in terms of settlement (Ryland 1960; Barnes et al. 1996). Panels with their surfaces slightly elevated above the sea bottom served as a unique, artificial substrate, which offered many shaded areas for settlement that were protected from the often detrimental effects of sedimentation. This slight difference in the structure of the substrate could also explain why more species settled on the panels than on the experimental rocks. Also worth mentioning are the different surface areas of the two substrates. Three panels at each depth with a total surface area of 300 cm2 could not be a sufficient sample size to provide the true pattern of recruitment at a given site. Therefore, we recommend the deployment of a larger number of panels for a longer period of time in future experimental investigations focusing on recruitment.
Although differences in recruitment on panels at sites U and W existed, they were at a much smaller scale than those observed on the experimental rocks (see Figs. 2, 3, 4). This might suggest that apart from the existing local population parameters that influence recruitment, this process is also modified to some extent by substrate heterogeneity. Non-uniform rocks vary in shape and surface structure more than smooth panels. It is likely that there are also localized physical conditions that were not addressed by this study that could contribute to the observed pattern.
A number of studies have shown that the distribution of larvae is governed by hydrological conditions, including currents, advections or water stratification (e.g. Pineda 1991; Pineda et al. 2010). The current patterns in the shallows at the study locations are driven by wind direction, which is from the south-west or western sector in the majority of cases (annually 45.9% in total), but northern directions have also been recorded (17.0%) (Pruszak 1980). This basically means that the water masses between stations U and W, which are just ~3 km apart, will be frequently mixed, leading to the possible exchange of propagules. The results obtained by this study are not fully explained by local water mass exchange, indicating that the impact of that factor on near-bottom recruitment needs further investigation to be fully understood.
The lack of clear pattern observed during this study in terms of species and individual number with depth between the established assemblages and those on the experimental substrates may suggest some level of exchange among the investigated depths. As there was no stratification in the water column, as shown by very small water temperature differences among depths during the time of investigation (between 0.76 and 1.00 °C), it is most likely that tidal forces, wind mixing and larval behaviour led to the observed results. Previous investigations show that when the study locations are in close vicinity, factors concerning the substrate characteristics become more influential in shaping epifaunal assemblages than large-scale processes, such as hydrology or the local species pool (Balazy and Kuklinski 2017). Here, the study area is a rather enclosed inlet, and thus larval behaviour (substrate selection), physiology (competency, energy reserves), biological interactions (predation) or local differences in substrate heterogeneity should have also a greater impact than hydrodynamic forces (e.g. currents) at the scale investigated here (e.g. Ryland 1974; Clark et al. 2017).
It is believed that the intensification of benthic organism larvae settlement occurs in areas with high adult density (Crisp 1974; Osman and Whitlatch 1995; Pineda 2000; Kent et al. 2003). In such cases, the presence of other settlers serves as a stimulus for the colonization of a given area (Crisp 1974). Therefore, in areas with great adult densities and high recruitment, we should also observe a high supply of larvae. Our study revealed no encrusting organism larvae in the plankton samples collected from just above the sea bottom at any of the localities or depths sampled three times during the project (see Table 2). We are aware of plankton patchiness and that the sampling interval may not accurately estimate the overall larval species richness and abundance at our study sites (Pineda et al. 2010). However, in general, other studies on larvae or plankton from polar seas and elsewhere have also revealed very few, if any, larvae of encrusting fauna (especially bryozoans and spirorbid polychaetes), which dominate newly recruited faunal assemblages (Bowden et al. 2009; Kuklinski et al. 2013). As many of these studies involve annual sampling or even sampling over longer periods, this suggests that the majority of encrusting fauna larvae are simply not present in the water column in the zone covered by the sampling techniques, which are usually conducted metres above the sea bottom. These larvae most likely develop and propagate in the nearest bottom habitat. This, of course, has consequences for their dispersal capabilities. Currents and water movement are stronger with increasing distance from the boundary layer of the sea bottom (Vogel 1994). Developing larvae near the sea bottom likely have little chance to propagate over large distances, as this investigation seems to indicate. Short-distance dispersal, especially for bryozoans, has also been suggested by genetic population studies. It was found that population structure corresponds with the contrasting modes of larval dispersal (Goldson et al. 2001; Porter et al. 2002; Watts and Thorpe 2006). Bryozoan populations with lecithotrophic larvae, which have limited dispersal, show genetic differentiation over distances as small as 10 m, whereas populations with planktotrophic larvae, with better dispersal abilities, display higher levels of genetic heterogeneity over much larger spatial scales (Goldson et al. 2001; Porter et al. 2002; Watts and Thorpe 2006). Lecithotrophic larvae of the temperate bryozoan Celleporella hyalina were found to be able to swim for only up to 4 h, with a preference to settle within an hour of release (Hughes et al. 2001). Even in an Antarctic broadcast spawner (the limpet Nacella concinna) that possesses free-swimming planktotrophic veliger larvae, minute genetic differences were recorded over a small scale of a few kilometres in Ryder Bay, Adelaide Island (Hoffman et al. 2012). Antarctic bryozoans constitute 85% of the established encrusting assemblages. The vast majority of them in this study possess lecithotrophic larvae (indicated by the presence of a brooding chamber; Hayward 1995) with limited dispersal capabilities. Therefore, the observed matching diversity patterns between newly recruited assemblages and nearby resident adult assemblages are not a surprise.
To conclude, our results suggest that the recruitment of encrusting fauna in the Antarctic can be very localized and occurs in close vicinity to adult populations. This pattern of recruitment has implications for the conservation and management of these fragile assemblages, which suggests that the protection of these assemblages must be very localized and targeted. This study also revealed many gaps in our knowledge and future research directions, especially those concerning factors influencing the recruitment of encrusting fauna, which includes, for example, such issues as larval behaviour, tidal forces and wind mixing.
References
Absher TM, Boehs G, Feijó AR, da Cruz AC (2003) Pelagic larvae of benthic gastropods from shallow Antarctic waters of Admiralty Bay, King George Island. Polar Biol 26:359–364
Balazy P, Kuklinski P (2017) Arctic field experiment shows differences in epifaunal assemblages between natural and artificial substrates of different heterogeneity and origin. J Exp Mar Biol Ecol 486:178–187
Barnes DKA (1999) The influence of ice on polar nearshore benthos. J Mar Biol Assoc UK 79:401–407
Barnes DKA, Conlan K (2007) Disturbance, colonization and development of Antarctic benthic communities. Philos Trans R Soc B 362:11–38
Barnes DKA, Kuklinski P (2003) High polar spatial competition: extreme hierarchies at extreme latitude. Mar Ecol Prog Ser 259:17–28
Barnes DKA, Rothery P, Clarke A (1996) Colonisation and development in encrusting communities from the Antarctic intertidal and sublittoral. J Exp Mar Biol Ecol 196:251–265
Booth DJ, Brosnan DM (1995) The role of recruitment dynamics in rocky shore and coral reef fish communities. Adv Ecol Res 26:309–385
Bowden DA, Clarke A, Peck LS, Barnes DKA (2006) Antarctic sessile marine benthos: colonisation and growth on artificial substrata over three years. Mar Ecol Prog Ser 316:1–16
Bowden DA, Clarke A, Peck LS (2009) Seasonal variation in the diversity and abundance of pelagic larvae of Antarctic marine invertebrates. Mar Biol 156:2033–2047
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Catewicz Z, Kowalik Z (1984) Harmonic analysis of tides on Admiralty Bay. Oceanologia 15:97–109
Clark GF, Stark JS, Johnston EL (2017) Tolerance rather than competition leads to spatial dominance of an Antarctic bryozoan. J Exp Mar Biol Ecol 486:222–229
Colwell RK (2013) Estimates: statistical estimation of species richness and shared species from samples. Version 9. User’s Guide and application published at: http://purl.oclc.org/estimates
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Connell JH (1985) The consequences of variation in initial settlement vs post-settlement mortality in rocky intertidal communities. J Exp Mar Biol Ecol 93:11–45
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527
Crisp DJ (1974) Factors influencing the settlement of marine invertebrate larvae. In: Grant PT, Mackie AM (eds) Chemoreception in marine organisms. Academic Press, London, pp 177–265
Fogarty MJ, Botsford LW (2007) Population connectivity and spatial management of marine fisheries. Oceanography 20:112–123
Goldson AJ, Hughes RN, Gliddon CJ (2001) Population genetic consequences of larval dispersal mode and hydrography: a case study with bryozoans. Mar Biol 138:1037–1042
Gutt J, Starmans A, Dieckmann G (1996) Impact of iceberg scouring on polar benthic habitats. Mar Ecol Prog Ser 137:311–316
Hayward PJ (1995) Antarctic cheilostomatous bryozoa. Oxford University Press, Oxford, p 355
Hoffman JI, Clarke A, Clark MS, Fretwell P, Peck LS (2012) Unexpected fine-scale population structure in a broadcast-spawning Antarctic marine mollusc. PLoS ONE 7:32415
Hughes RN, Gliddon CJ, Goldson AJ, Hughes RN (2001) Population genetic consequences of larval dispersal mode and hydrography: a case study with bryozoans. Mar Biol 138:1037–1042
Jablonski D (1986) Larval ecology and macroevolution in marine invertebrates. Bull Mar Sci 39:565–587
Kent A, Hawkins SJ, Doncaster P (2003) Population consequences of mutual attraction between settling and adult barnacles. J Anim Ecol 72:941–952
Kuklinski P, Barnes DKA (2008) Structure of intertidal and subtidal assemblages in Arctic vs temperate boulder shores. Pol Polar Res 29:203–218
Kuklinski P, Berge J, McFadden L, Dmoch K, Zajaczkowski M, NygÅrd H, Piwosz K, Tatarek A (2013) Seasonality of occurrence and recruitment of Arctic benthic marine invertebrate larvae in relation to environmental variables. Polar Biol 36:549–560
Kuklinski P, Balazy P, Nowak M, Bielecka L (2014) Factors controlling initial development of Polar bryozoan assemblages. Studi Trent Sci Nat 94:145–151
McQuaid CD, Phillips TE (2000) Limited wind-driven dispersal of intertidal mussel larvae: in situ evidence from the plankton and the spread of the invasive species Mytilus galloprovincialis in South Africa. Mar Ecol Prog Ser 201:211–220
Osman RW, Whitlatch RB (1995) The influence of resident adults on recruitment: a comparison to settlement. J Exp Mar Biol Ecol 190:169–198
Osman RW, Whitlatch RB (1998) Local control of recruitment in an epifaunal community and the consequences to colonization processes. Hydrobiologia 375(376):113–123
Palmer MA, Allan JD, Butman CA (1996) Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. Trends Ecol Evol 11:322–325
Pearse JS, Lockhart SJ (2004) Reproduction in cold water: paradigm changes in the 20th century and a role for cidaroid sea urchins. Deep-Sea Res II 51:15333–15349
Pineda J (1991) Predictable upwelling and the shoreward transport of planktonic larvae by internal tidal bores. Science 253:548–551
Pineda J (2000) Linking larval settlement to larval transport: assumptions, potentials, and pitfalls. Oceanogr East Pac 1:84–105
Pineda J, Porri F, Starczak V, Blythe J (2010) Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. J Exp Mar Biol Ecol 392:9–21
Porter JS, Ryland JS, Carvalho GR (2002) Micro- and macrogeographic genetic structure in bryozoans with different larval strategies. J Exp Mar Biol Ecol 272:119–130
Pruszak Z (1980) Currents circulation in the waters of Admiralty Bay (region of Arctowski Station on King George Island). Pol Polar Res 1:55–74
Rakusa-Suszczewski S (1995) Thy hydrograph of Admiralty Bay and its inlets, coves and lagoons (King George Island, Antarctica). Pol Polar Res 16:61–70
Ryland JS (1960) Experiments on the influence of the light on the behaviour of Polyzoa larvae. J Exp Biol 37:783–800
Ryland JS (1970) Bryozoans. Hutchinson, London, p 175
Ryland JS (1974) Behaviour, settlement and metamorphosis of bryozoan larvae: a review. Thalassia Jugoslavica 10:239–262
Shanks AL, Grantham BA, Carr MH (2003) Propagule dispersal distance and the size and spacing of marine reserves. Ecol Appl 13:159–169
Stanwell-Smith D, Barnes DKA (1997) Benthic community development in Antarctica: recruitment and growth on settlement panels at Signy Island. J Exp Mar Biol Ecol 212:61–79
Teske PR (2014) Connectivity in solitary ascidians: is a 24-h propagule duration sufficient to maintain large-scale genetic homogeneity? Mar Biol 161:2681–2687
Todd CD (1998) Larval supply and recruitment of benthic invertebrates: do larvae always disperse as much as we believe? Hydrobiologia 375(376):1–21
Tokarczyk R (1987) Classification of water masses in the Bransfield Strait and southern part of the Drake Passage using a method of statistical multi-dimensional analysis. Pol Polar Res 8:333–366
Vogel S (1994) Life in moving fluids. Princeton University Press, New Jersey, p 467
Watts PC, Thorpe JP (2006) Influence of contrasting larval developmental types upon the population-genetic structure of cheilostome bryozoans. Mar Biol 149:1093–1101
Weersing K, Toonen RJ (2009) Population genetics, larval dispersal, and connectivity in marine systems. Mar Ecol Prog Ser 393:1–12
Zhang XZ, Haidvogel D, Munroe D, Powell EN, Klinck J, Mann R, Castruccio FS (2015) Modeling larval connectivity of the Atlantic surfclams within the Middle Atlantic Bight: model development, larval dispersal and metapopulation connectivity. Estuar Coast Shelf S 153:38–53
Acknowledgements
We would like to address special thanks to Tadeusz Stryjek for help during each step of the expedition. We would like also to thank Andrzej Tatur, Tomasz Janecki and Anna Kidawa for help in organizing the expedition. Jaroslaw Roszczyk, Mateusz Bagnicki, Lech Wisniewski and the rest of the Polish Antarctic Station “Arctowski” crew, including Kazimierz Połeć, Tadeusz Markowicz, Wiesław Sienkiewicz, Kazimierz Warowicki, Włodzimierz Tkaczyk, Waldemar Kowalski, Michał Guźniczak and Piotr Horzela, are thanked for help with logistics and great company during field work. The authors would also like to thank Paul Renaud and an anonymous reviewer for valuable comments leading to an improved version of the manuscript. This study was conducted during the Polish-Norwegian Research Programme operated by the National Centre for Research and Development under the Norwegian Financial Mechanism 2009-2014 in the framework of Project Contract No. Pol-Nor/196260/81/2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kuklinski, P., Balazy, P., Krzemińska, M. et al. Species pool structure explains patterns of Antarctic rock-encrusting organism recruitment. Polar Biol 40, 2475–2487 (2017). https://doi.org/10.1007/s00300-017-2159-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2159-3