Abstract
Amphipoda belong to enormously diverse benthic invertebrate groups in the Southern Ocean, playing a very important role in the Antarctic communities. However, in the majority of ecological studies they are identified only to the order level. Rare ecological studies, where amphipods were identified to the species level, concern mainly shallow sublittoral. There is also poor knowledge about the environmental features that are important in the habitat selection by these crustaceans. The aims of this study were to recognize and describe the soft bottom sublittoral amphipod assemblages of the Antarctic fjord and observe the habitat preferences of dominant species. Additionally, we investigated the usefulness of surrogacy methods in the description of amphipod assemblages. The series of 101 quantitative samples from wide depth range (25–502 m) from Admiralty Bay were studied. The cluster analysis allowed to distinguish four assemblages representing: shallow sublittoral (A), middle sublittoral (B) and deep sublittoral (C) of central basin of the bay and Ezcurra Inlet (D) fauna. The study of habitat preferences led to the recognition of the species whose distribution depended on the depth and type of the sediment. The first group encompassed shallow water species characteristic of assemblage (A). The second set of species was related to the presence of colonial organisms identifying the assemblage (B). Third group of deep water species represented assemblage C, while the group of species preferring fine-grained sediments was found in assemblage D. The surrogacy analysis demonstrated low usefulness of this method in the description of Antarctic amphipod assemblages in non-disturbed areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphipod crustaceans belong to enormously diverse benthic invertebrate groups in the Southern Ocean. There are more than 500 species of Amphipoda recorded in the Antarctic and since the 1970s of twentieth century this number grows linearly (De Broyer et al. 2007; De Broyer and Danis 2011; De Broyer and Jażdżewska 2014). The majority of species of this crustacean group are benthic, some are also pelagic. Order Amphipoda includes animals from different trophic groups: herbivorous, predators, omnivorous, as well as scavengers (Dauby et al. 2001a, b). On the other hand, these crustaceans constitute a food source for several other invertebrates, as well as vertebrates—fishes, birds and mammals (Dauby et al. 2003). Even though Amphipoda play a very important role in the Southern Ocean benthic communities, in the majority of ecological studies they are identified only to the order level (e.g. Sáiz-Salinas et al. 1997; Gambi and Bussotti 1999; Barnes et al. 2006; Rehm et al. 2006, 2011; Glover et al. 2008; Saiz et al. 2008; Cummings et al. 2010). Taxonomical studies are time consuming and difficult, which led several authors to search for alternative ways of describing the benthic communities, one of them being the use of surrogacy measures that was successfully implemented when both disturbed and undisturbed areas were studied (e.g. Włodarska-Kowalczuk and Kędra 2007; Tataranni et al. 2009). There are no similar analyses proving the usefulness of these methods with respect to the Antarctic benthos; however, the majority of papers describing the Southern Ocean communities are based on higher level identification or identification of only the most dominant taxa. Rare ecological studies, where amphipods were identified to the species level, concern mainly shallow sublittoral assemblages (Lowry 1975; Richardson and Hedgpeth 1977; Jażdżewski et al. 1991a, b; Rauschert 1991; Gambi et al. 1994; Cattaneo-Vietti et al. 2000; Arntz et al. 2006; Aumack et al. 2011; Siciński et al. 2012; Martín et al. 2016). Last years have brought new investigations where the bathyal and abyssal Antarctic fauna was studied, confirming high abundance and diversity of amphipod crustaceans and consequently their importance also in deep-sea communities (e.g. De Broyer et al. 2004; Brandt et al. 2007; Brökeland et al. 2007). However, the amphipod fauna of middle and deep sublittoral of the Antarctic region is still poorly recognized.
The importance of environmental factors in shaping benthic assemblages was often reported; however, Gutt (2007) summarizing the knowledge of the Antarctic communities stressed: “Statistical analyses of the relationships between sediments among other environmental gradients and the macrobenthos are almost missing”. Along with the lack of studies of amphipod assemblages, there is only poor knowledge about the environmental features that are important in the habitat selection by these crustaceans. Studies by Baird and Stark (2013) proved that particular species of Amphipoda (in this case infaunal Orchomenella franklini) can have well-defined preferences for sediment grain size, which influence its distribution in a small scale. Taking into account very high species richness, ecological diversity of Amphipoda and their importance in the Antarctic communities it is crucial to study also their habitat preferences because changes of the environmental conditions may influence their distribution and can cause local loss of diversity.
Admiralty Bay is one of the best studied regions in the Antarctic in terms of biodiversity. The recognition of its particular values has resulted first in the designation of the Antarctic Specially Managed Area No. 1 (ATCM document 2005) in this bay and later in the proposal of establishment of the CAML Legacy Site (Siciński et al. 2011). Admiralty Bay is situated in the West Antarctic Peninsula area (South Shetlands) where rapid climate change has been observed (Clarke et al. 2007). Among invertebrates, Amphipoda and Polychaeta are the most species-rich groups in the Admiralty Bay, represented by 172 and 162 species, respectively (Siciński et al. 2011). The shallow sublittoral is characterized by high macrozoobenthic densities with amphipods as a main group. Their densities decrease with depth and below 100 m stay at low and more or less constant level. Due to relatively small size of these peracarid crustaceans their proportion in the biomass, particularly in the deeper areas, dominated by large ascidians, bryozoans or polychaetes, is small (Jażdżewski et al. 1986, 1991b, 2001; Jażdżewska 2011; Pabis et al. 2011). Assemblages of various macrobenthic groups of Admiralty Bay, including Polychaeta, Tanaidacea, Cumacea, Isopoda and Bryozoa, were already described (Pabis and Siciński 2010a, b, 2012; Pabis and Błażewicz-Paszkowycz 2011; Siciński et al. 2011; Pabis et al. 2014; Zemko et al. in press and references therein). Also amphipods of this fjord were already the subject of studies, especially in the subtidal and in shallow sublittoral (Jażdżewski et al. 1991a, 2001) as well as in shallow glacial coves (Siciński et al. 2012). Some data about distribution of these crustaceans in deeper areas of Admiralty Bay were presented (Jażdżewski et al. 1991b; Jażdżewska 2011), but the detailed study of their assemblages in the whole depth range, as well as the influence of the environmental factors on the species distribution, has not been performed.
The aims of this study are to recognize and describe the soft bottom sublittoral amphipod assemblages of the Antarctic fjord. Owing to the availability of environmental data the habitat preferences of dominant benthic species are also studied. Additionally, the species data matrix allows to investigate the usefulness of surrogacy measures in the description of amphipod crustaceans assemblages.
Materials and methods
Study area
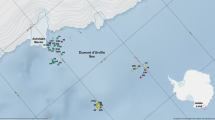
Admiralty Bay (Fig. 1a) is the largest fjord (122 km2) of King George Island situated in South Shetland Islands archipelago (West Antarctic). It consists of central basin and three inlets: Ezcurra, Mackellar and Martel. The central basin reaches 550 m depth and has wide opening (8.25 km width) to the Bransfield Strait (Kruszewski 2002). Glaciers and ice-falls cover approximately half the length of the Admiralty Bay coast (Braun and Grossmann 2002). Ezcurra Inlet, the semi-closed fjord of Admiralty Bay, is directed to the West and is divided into two (eastern and western) parts by a transverse sill. Eastern part is older and is constituted by a deep trough, while younger, western part, is shallower with intricate bottom configuration (Marsz 1983). The hydrology of Admiralty Bay is influenced by water masses coming from Bransfield Strait as well as by waters coming from melting glaciers and icebergs (Szafrański and Lipski 1982). The water currents within Admiralty Bay originate from Bransfield Strait and flow along the long axis of the bay to the NE coast, where they return and flow back along both coasts of the central basin. The currents within the inlets (especially Ezcurra Inlet) are diffused. The currents have low energy so it is suggested that the water turbulence depends mainly on the wind derived waves (Campos et al. 2013). There are no significant differences in salinity of water in particular areas of Admiralty Bay. Local decrease of salinity can only be observed in its shallowest parts in the vicinity of glaciers. Admiralty Bay waters are characterized by high mineral suspended matter content originating mostly from land deposits transported to the bay by subglacial streams. Its other sources include melting icebergs and wind-transported dust (Jonasz 1983). The highest values of mineral suspended matter content were observed in summer months in front of glaciers with clear gradient from the western-most parts of Ezcurra Inlet (where it reaches > 100 mg/l in the surface layers and ca. 60 mg/l at the depth 60 m) to the central basin (<10 mg/l both in surface waters and in the deeper parts of the bay) (Pęcherzewski 1980).
Map of Admiralty Bay with sampling sites. Grey colour signifies land areas free of glacier. The station codes are the same as in the dendrogram of cluster analysis but without depth. Samples are specified by signs representing the four groups distinguished in cluster analysis (Fig. 4)
Bottom sediments of central basin are constituted by boulders, pebbles and gravel in the intertidal, gravel and sand in the shallow sublittoral and mud (silty-clay sand and sandy-clay silt) in the deeper areas of the bay. The sediments of Ezcurra Inlet are characterized by high amount of clay fraction (Rudowski and Marsz 1996; Siciński 2004; Siciński et al. 2011; Campos et al. 2013). In the shallow sublittoral of central basin the sediments character changes significantly together with depth; however, below 150–200 m, they become homogenic. The deposits are poorly or very poorly sorted and cover the entire spectrum of grain size expressed by Φ scale (from medium sand to very fine silt). The studies of physico-chemical and hydrographical conditions of Admiralty Bay are summarized in Siciński et al. (2011) and Campos et al. (2013).
Field sampling and sediment analysis
The material was collected between 17th of December 1984 and 5th of January 1986 during 9th Polish Antarctic Expedition. In total 101 quantitative samples were taken using Van Veen Grab (0.1 m2) in central basin (C) of Admiralty Bay (73 samples) and in Ezcurra Inlet (E) (28 samples) (Fig. 1a, b). The collecting depth range in central basin was 37 to 502 m, while in Ezcurra Inlet it ranged from 20 m to the maximal depth of the inlet, which is 150 m. Samples were sieved on the 0.5-mm sieve, fixed in 4% buffered formalin solution and, after sorting, preserved in 75% ethyl alcohol.
The analysis of grain size was performed for 57 samples following the method presented in the paper by Siciński (2004).
Data analysis
To estimate the total species richness of the area the Chao 2 estimator was used. This estimator uses the information about rare and uncommon species to predict the number of species that are missing in samples (Chao 2004). The Michaelis–Menten (MM) estimator was used to generate the species accumulation curve. Both estimators were calculated using EstimateS software (Colwell 2013).
The similarities between samples were calculated using the Bray–Curtis similarity formula on species density (number of individuals per 0.1 m2). One family, Photidae, even though relatively well represented in the samples, was excluded from the analysis. The species belonging to this family are very fragile and, while sample collection and washing, taxonomically important appendages often become broken, making species identification impossible. Due to the fact that the difference between the most and the least abundant species was more than 20 times, and to increase the importance of rare species (singletons and doubletons) which constituted 1/3 of all taxa, fourth root transformation of data was used. Hierarchical agglomerative clustering was performed using the group average method. The formation of assemblages was tested using similarity profile analysis (SIMPROF) permutation tests. The cluster analysis was combined with non-metric multidimensional scaling (nMDS) for sample ordination. To check the usefulness of surrogate measures in recognizing invertebrate assemblages, similar cluster analyses on the basis of both genera and family were performed. Each assemblage discriminated was characterized using species richness (number of species), diversity (Shannon index) and evenness (Pielou index) indices (Magurran 2004). Similarity of percentage (SIMPER) analysis was used to reveal the percentage contributions of species which accounted for ≥ 90% of the similarity within, and dissimilarity between, cluster groups. Cluster, SIMPROF and SIMPER analysis were performed using PRIMER package v.6. Mean with standard errors and standard deviations (SD) were calculated for density values and all richness and diversity indices in each assemblage. The homogeneity of variance was checked using Levene’s test. Depending on its results the differences of the indices calculated were tested using non-metric Kruskal–Wallis test with Dunn test post hoc or one-way ANOVA with Tukey’s test in STATISTICA 12 package. For each species the association index DAI (the percentage of individuals of given species recorded in each recognized assemblage to the sum of individuals of this species in the whole material) was calculated (Salzwedel et al. 1985).
To investigate the habitat preferences of benthic amphipods Canonical Correspondence Analysis (CCA) was conducted. The study was performed on the basis of 57 samples for which grain size analysis was done. These samples were randomly chosen taking into account, however, to cover both areas (central basin and Ezcurra Inlet) and the whole depth range of the study. The following environmental factors were taken into account: percentage of skeletal fraction (mineral particles with diameter larger than 1 mm), percent share of sand, silt and clay, sorting coefficient (So), depth, presence of biogenic structures expressed by the biomass of ascidians and bryozoans. The biomass of two other groups that are responsible for biogenic structures formation (Porifera and Hydrozoa) in the chosen samples was very low, so it was not taken into consideration. The total density of all amphipods in the chosen 57 samples was calculated and later only the species that exceeded 0.5% of dominance were included in the analysis (33 spp.). The density matrix was square-root transformed. To check the statistical significance of the results Monte Carlo test with 499 permutations was done. The analysis was performed in CANOCO for Windows 4.5. The differences between groups of species correlated with chosen factors were tested using ANOSIM in Primer v. 6.
Results
In the whole material studied (2886 individuals) 120 amphipod species from 77 genera and 30 families were identified. Stenothoidae (18 spp.), Lysianassidae (15 spp.) and Pontogeneiidae (10 spp.) proved to be the most speciose families (Fig. 2). The species accumulation curve based on Michaelis–Menten estimator did not reach the asymptote (Fig. 3), while 178 species were estimated by Chao2 as total number of amphipod species in the area.
Cluster analysis
On the basis of species composition and their density, the cluster analysis allowed to distinguish four main amphipod assemblages (Fig. 4). The SIMPROF test confirmed the significance of the grouping (Π= 2.705, p = 0.001). Ordination of samples showed that the distribution of stations is generally following the depth gradient from left to right. Additionally the samples coming from Ezcurra Inlet grouped themselves together. Superimposing the grouping obtained from cluster analysis (at 25% similarity level) proved the existence of the recognized assemblages (Fig. 5).
Dendrogram of samples (Bray–Curtis similarity of fourth-root-transformed species density values, group-average grouping method). C—central basin, E—Ezcurra Inlet, numerical code designate sequential number of sample and collection depth. Asterisk (*) indicates stations with sediment data used for CCA analysis. Dashed lines indicate the samples which are randomly positioned in the dendrogram
nMDS plot (Bray–Curtis similarity of fourth-root-transformed density values) with superimposed clusters from Fig. 4 at similarity level of 25%. C—central basin, E—Ezcurra Inlet, numerical code designate sequential number of sample. The sign attributed to each sample indicates to which of the groups separated by cluster analysis (Fig. 4) the sample belongs
Assemblage A (shallow sublittoral of central basin of Admiralty Bay)
This cluster grouped six shallow water stations situated in the central basin of Admiralty Bay at the depth range of 37–46 m (Fig. 1b). The assemblage is characterized by the highest mean number of individuals per sample (132 ± 126.1 ind./0.1 m2) and is constituted by 35 species with Schraderia gracilis (45.5 ± 69.4 ind./0.1 m2) and Hippomedon kergueleni (17.8 ± 21.1 ind./0.1 m2) as the most abundant ones (Figs. 6, 7, Online Resource 1). The second one and Prostebbingia brevicornis were also the most frequent species in this assemblage, being present in all samples. Ten species were found only in this assemblage (DAI = 100%) (Online Resource 1).
Assemblage B (middle sublittoral of central basin of Admiralty Bay)
Eleven samples from middle sublittoral of central basin of Admiralty Bay were grouped into cluster B (Fig. 1b). Generally, the depth of samples was between 50 and 72 m, with two stations from below 100 m (120 and 156 m). There were 62 species recorded in this assemblage. Species richness (the mean number of species per sample) and diversity expressed by Shannon index were the highest there (Fig. 6). The most abundant and frequent species were Schraderia gracilis (10.9 ± 18.3 ind./0.1 m2, F = 82%) and Heterophoxus videns (9.0 ± 7.8 ind./0.1 m2, F = 91%). Other numerically important species included Kuphocheira setimana (3.6 ± 3.8 ind./0.1 m2, F = 64%), Atylopsis fragilis (3.0 ± 4.5 ind./0.1 m2, F = 73%) and Orchomenella denticulata 4.6 ± 14.4 ind./0.1 m2, F = 18%) (Fig. 7, Online Resource 1). However, it is worth noticing that the last species, in contrast to the first two, had a low frequency. Eleven species were recorded only in this assemblage (Online Resource 1).
Assemblage C (deep sublittoral of central basin of Admiralty Bay)
This cluster grouped 42 samples from the depths below 80 m of central basin of Admiralty Bay (and three additional stations from Ezcurra Inlet from the depths 45–78 m) (Fig. 1a, b). Eighty-six species contributed to this assemblage, 28 of which were unique for this group (Online Resource 1). The highest evenness (Pielou index) was observed there (Fig. 6). The most abundant species was Heterophoxus videns (4.3 ± 3.8 ind./0.1 m2) and it was followed by Aeginoides gaussi (3.0 ± 18.0 ind./0.1 m2), Urothoe sp. 1 (2.0 ± 4.1 ind./0.1 m2), Heterophoxus trichosus (1.5 ± 2.0 ind./0.1 m2), Waldeckia obesa (1.4 ± 1.5 ind./0.1 m2), Cephalophoxoides kergueleni (1.3 ± 2.1 ind./0.1 m2) and Ampelisca anversensis (0.6 ± 0.7 ind./0.1 m2) (Fig. 7). Both Heterophoxus species, as well as W. obesa and Ampelisca anversensis, were present in more than half of the samples of this group of stations (Online Resource 1).
Assemblage D (inner fjord influenced by glacial phenomena)
Twenty-eight species from 23 samples distributed mainly in Ezcurra Inlet in its whole studied depth range (20–146 m) constituted this assemblage (Fig. 1a, b). Only three deep-water samples (290, 333 and 405 m) from central basin belonged to this group. This assemblage was characterized by the lowest values of density as well as species richness and Shannon index (Fig. 6). Heterophoxus trichosus (4.7 ± 3.8 ind./0.1 m2) was the most abundant and frequent species. It was also the only species present in all samples of this assemblage. The other common and abundant species was Heterophoxus videns (2.3 ± 5.8 ind./0.1 m2, F = 43.5%) (Fig. 7). Barely two species were found only in this group (Online Resource 1).
Sixteen samples from various depths and localities are randomly positioned on the dendrogram. These samples contain the species which were found also in the samples forming groups A–D; however, the species density and richness is distinctly lower. It seems that the depauperation of the samples is accidental and unrelated to the formation of specific groups.
Statistically significant differences in the species richness and diversity measures were noted. Kruskal–Wallis test with Dunn’s test demonstrated statistically significant differences in the mean number of species and mean density between assemblages A vs. D, B vs. D and C vs. D. There were significant differences between groups A vs. B, A vs. D, B vs. D and C vs. D with respect to Shannon index (one-way ANOVA with Tukey’s test, P < 0.05). No statistically significant differences were observed with respect to Pielou index.
The average within-group similarities ranged from 30% (assemblage C—29.91%) to ca. 40% (assemblage D—44.44%) (Table 1). One species (Heterophoxus trichosus) contributed most to the within-group similarity for assemblage D. Its contribution was more than 85%. The same species, together with W. obesa and Heterophoxus videns were responsible for more than 50% similarity within group C. Only two species (Heterophoxus videns and Schraderia gracilis) exceeded 10% of contribution to the similarity within group B, whereas in the case of assemblage A they included: Hippomedon kergueleni, Prostebbingia brevicornis and Monoculodes scabriculosus. The average dissimilarity between the groups recognized was between 80 and 93%. The highest value (93.05%) was observed between groups A and D, followed by dissimilarity between assemblages A and C (92.75%). The lowest dissimilarity occurred between groups B and C (80.46%). The species which contributed most to the dissimilarity between assemblage A and all the rest of groups was Hippomedon kergueleni (6.79% with group B, 8.5% with group C and 13.37% with group D). Hetreophoxus videns and Schraderia gracilis were the other species that considerably differentiated groups A and B. In the case of differences between assemblages A and C it was Prostebbingia brevicornis and Heterophoxus videns, whereas between groups A and D it was Prostebbingia brevicornis, Heterophoxus trichosus, H. videns, Monoculodes scabriculosus, and Schraderia gracilis. All the species listed above contributed > 5% to the differences between these groups. The only species that contributed almost 5% to the average dissimilarity between assemblages B and C was Schraderia gracilis (4.94%). The species which were the most important in differentiation of the groups B and D were Heterophoxus trichosus, H. videns and Schraderia gracilis, while in the case of assemblages C and D they included the two species from the genus Heterophoxus followed by W. obesa.
Distribution of Amphipoda according to environmental factors
Canonical Correspondence Analysis, conducted using all the factors listed in the Material and methods, showed that the percent share of sand in the sediments should be removed from the analysis as it has very high correlation with other factors. The shallowest sandy bottom stations in central basin were characterized by the presence of skeletal fraction in the sediments and higher biomass of Ascidiacea and Bryozoa (Fig. 8). Studying the distribution of samples (Fig. 4) one can notice that they constituted assemblages A and B. The stations that were situated deeper in the central basin of Admiralty Bay were positively correlated with the presence of smaller fraction of sediment, mostly silt. The samples from greater depths and characterized by finer sediments are grouped into the assemblage C. Stations contributing to assemblage D, situated in Ezcurra Inlet, showed positive correlation with clay fraction in sediments.
Distribution of stations according to environmental factors. The station codes are the same as in the dendrogram of cluster analysis but without depth. So—sorting coefficient, As. bm—ascidian biomass, Br. bm—bryozoan biomass, s.f.—skeletal fraction (> 1 mm Ø). Samples are specified by signs representing the groups distinguished in cluster analysis (Fig. 4)
The results of analysis of amphipod species distribution according to the environmental parameters are presented in Fig. 9. The first two axes explain 53.6% of variance (Table 2). The most important factors that are correlated with amphipod species distribution include: amount of silt in the sediments, depth, and presence of biogenic structures, particularly bryozoans. Subsequently, sorting coefficient, ascidian biomass, and skeletal fraction are correlated with species occurrence (Table 3). The chosen factors together explain 32% of the total variation. It is possible to recognize five groups of species which have different habitat preferences. The first one assembling shallow-water species, having also preferences to coarse sediments, is constituted by Paramoera hurleyi, Prostebbingia brevicornis, Hippomedon kergueleni, and Schraderia gracilis. The second group, including also species with shallow-water and coarse sediments preferences, but being at the same time somehow correlated with higher abundance of biogenic structures, consists of: Haplocheira plumosa, K. setimana, Orchomenella acanthurus, Monoculodes scabriculosus, Oediceroides macrodactyla, Atylopsis fragilis, Parhalimedon turqueti, Schraderia acuticauda, and Ampelisca richardsoni. Liljeborgia polydeuces, Harpiniopsis aciculum, Ampelisca anversensis, C. kergueleni, Urothoe sp. 1, Aeginoides gaussi, Figorella sp. 1 belong to the species that have clear deep-water and finer sediments preferences. The group of species preferring very fine sediments (clay) is composed of three species: Heterophoxus trichosus, Monoculodes sp. 1, and Paroediceroides sinuata. It was possible to observe also the group of ubiquitous species including Heterophoxus videns, W. obesa, Liljeborgia georgiana, and Scaphodactylus dentimanus. The differences between almost all the groups listed above were statistically significant (ANOSIM, p < 0.05) (Table 4). The group of species correlated with clay in sediments and the ubiquitous species group are the only two groups which do not show statistically significant differences.
Distribution of 33 species according to environmental factors. Vector codes same as in Fig. 5. Species codes: A.ga Aeginoides gaussi, A.an. Ampelisca anversensis, A.ri. A. richardsoni, A.fr. Atylopsis fragilis, C.ke. Cephalophoxoides kergueleni, F.sp.1 Figorella sp. 1, G.in. Gnathiphimedia incerta, H.pa Halicella parasitica, H.pl. Haplocheira plumosa, H.ac. Harpiniopsis aciculum, H.tr. Heterophoxus trichosus, H.vi. H. videns, H.ke. Hippomedon kergueleni, K.se. Kuphocheira setimana, L.ge. Liljeborgia georgiana, L.po. L. polydeuces, M.la. Metopoides latus, M.sc. Monoculodes scabriculosus, M.sp.1. Monoculodes sp. 1, O.ma. Oediceroides macrodactylus, O. ac. Orchomenella acanthura, O.wa. Oradarea walkeri, P.od. Paralysianopsis odhneri, P.hu. Paramoera hurleyi, P.tu. Parhalimedon turqueti, P.si. Paroediceroides sinuata, P.br. Prostebbingia brevicornis, S.de. Scaphodactylus dentimanus, S.ac. Schraderia acuticauda, S.gr. Schraderia gracilis, S.jo. Stegopanoploea joubini, U.sp.1 Urothoe sp. 1, W.ob. Waldeckia obesa
Surrogacy analysis
The distribution of samples in the cluster analysis of genera and families did not present any clear pattern. The general similarity of samples was growing together with moving to higher level of identification. On the other hand, the samples which constituted particular groups in the species-based analysis became mixed (Figs. 10, 11). Only the samples coming from Ezcurra Inlet grouped together, regardless of the taxonomic level studied.
Dendrogram of samples (Bray–Curtis similarity of fourth-root-transformed genera density values, group-average grouping method). C—central basin, E—Ezcurra Inlet, numerical code designate sequential number of sample and collection depth. The sign attributed to each sample indicates to which of the groups separated by cluster analysis (Fig. 4) the sample belongs
Dendrogram of samples (Bray–Curtis similarity of fourth-root-transformed families density values, group-average grouping method). C—central basin, E—Ezcurra Inlet, numerical code designate sequential number of sample and collection depth. The sign attributed to each sample indicates to which of the groups separated by cluster analysis (Fig. 4) the sample belongs
Discussion
The species composition of Admiralty Bay reflects the pattern observed in the whole Southern Ocean (De Broyer and Jażdżewska 2014). Slight differences in the sequence of the most speciose groups (Fig. 2) come from the fact that in the cited paper the representatives of all lysianassoid as well as all eusiroid families were treated together. If the species from those families found in the present study were combined, the order would be the same. The species accumulation curve calculated using Michaelis–Menten estimator did not reach the asymptote (Fig. 3), while the Chao 2 estimator indicated that the total number of species in Admiralty Bay could be 178. This number is very similar to the number of benthic amphipod species already recorded from this area, which is 172 (Siciński et al. 2011). If the pelagic Amphipoda are taken into account, this number grows to 177 species (Jażdżewska 2011). This confirms that the area is one of the best studied regions in the Antarctic with respect to amphipod fauna. This is a result of long-term thorough research undertaken there since the establishment of Polish and Brazilian scientific stations (Siciński et al. 2011). One has to take into account, however, that all the indices which provide the information about the estimated species richness underestimate this value (Gotelli and Colwell 2011). In consequence, a slight increase of the number of amphipod species can still be expected, especially when poorly studied habitats, such as hard bottom or macroalgae holdfasts, are included. On the other hand, De Broyer and Danis (2011) proved that in the whole Southern Ocean the amphipod species accumulation curve is far from reaching the asymptote. These contrasting results can come from relatively small area of Admiralty Bay which at the same time was very well sampled during several years. Additionally, the sampling covered the whole depth range of the bay, whereas large areas of deep-sea bottom of the Southern Ocean still remain “untouched”. It has been relatively short time since more intense deep-sea sampling started and the results showed the existence of very diverse bathyal and abyssal crustacean fauna (e.g. Brandt et al. 2007).
Habitat preferences of key amphipod species forming the assemblages distinguished
Three assemblages of the soft bottom of central basin of Admiralty Bay and one in Ezcurra Inlet were distinguished. The samples grouped according to the depth and the environmental conditions of the stations.
Shallow sublittoral sandy bottom assemblage (A) was characterized by moderate species richness and high density values (Fig. 6). All species contributing most to this assemblage were commonly found in Antarctic shallow sublittoral with Schraderia gracilis, Paramoera hurleyi and Prostebbingia brevicornis reported from coarse sediments where macroalgae were present (Bregazzi 1972a, b, 1973; Arnaud 1974; Thurston 1974; De Broyer 1983; Presler 1986; Jażdżewski et al. 1991a, b; Rauschert 1991; Munn et al. 1999; Takeuchi and Watanabe 2002; Huang et al. 2006, 2007). They are considered as herbivorous or herbivorous and detritivorous species. Also Monoculodes scabriculosus is believed to be associated with macroalgae; in its gut content the detritus remains and diatoms were observed (Bregazzi 1973; Oliver et al. 1982; De Broyer 1983; Rauschert 1991). Orchomenella acanthurus is recognized as mobile surface necorphagous or detritiphagous species (De Broyer et al. 2001, 2003), whereas Hippomeon kergueleni is shallow-water scavenger, which during the day burrows in sediments and after sunset actively swims to search for food (Bregazzi 1973; Presler 1986; Jażdżewski et al. 1991a, b). Another abundant species in this assemblage is also Prostebbingia gracilis which is a herbivorous species found in high densities on Desmarestia sp. (Huang et al. 2006, 2007). The comparison of the present results with data from Jażdżewski et al. (1991a) indicates that also areas shallower than 37 m are inhabited by similar amphipod fauna when species richness, diversity and composition are taken into account. This leads to the assumption that the shallow sublittoral assemblage in this Antarctic fjord covers the depths from 5 to ca. 50 m.
There is almost no information about the habitat and food preferences of the species which played important role in the amphipod assemblage B from middle sublittoral of central basin of Admiralty Bay. Schraderia acuticauda was previously reported from Kerguelen Islands as well as in Fildes Strait at King George Island. It was collected from the stations where the bottom was constituted by pebbles and where macroalgae, bryozoans as well as ascidians were present (Arnaud 1974; Bellan-Santini and Ledoyer 1974; Rauschert 1991). Atylopsis fragilis was reported from coarse sediments of Maxwell Bay (King George Island), where bryozoan colonies were observed. Additionally the bottom areas where this species was noted were under the influence of strong tidal currents (Rauschert 1989). Haplocheira plumosa and K. setimana belong to the subfamily Corophiinae, whose representatives are known as suspension feeders (Myers and Lowry 2003). The first species was found in the bottom of various character (mud, stones) often in association with macroalgae and colonial organisms—bryozoans, hydrozoans, sponges (Stebbing 1888; Bellan-Santini 1972; Arnaud 1974; Rauschert 1991; Takeuchi and Watanabe 2002). Thurston (1974) assumed that algae and encrusting organisms can be the ground for construction of tubes in which Haplocheira plumosa lives. The only information about the habitat type for the second species—K. setimana—comes from Signy Island, where it was reported from muddy bottoms of shallow waters (Thurston 1974). Also Ampelisca richardsoni is known as filter-feeder which consumes mainly phytoplankton (Nyssen et al. 2005). This species leads rather sedentary life, which is a common feature of representatives of the family Ampeliscidae. They live in tubes partly dug in the sediment, from which they expose antennae and setose gnathopods to collect food particles (Mills 1967). The last species in this group, Parhalimedon turqueti, was recorded from a large spectrum of sediment types, from clay through fine to coarse sand to stations with pebbles. The samples where it was found, very often contained different species of macroalgae (Schellenberg 1931; Thurston 1974; Rauschert 1991). The species was regarded as shallow-water one with records between 5 and 50 m (De Broyer et al. 2007); however, the present analysis allowed to widen its depth range as the deepest station with this species in studied fjord was situated at 206 m.
The third amphipod assemblage, characterizing deeper areas of the central basin of Admiralty Bay, was dominated by two ubiquitous species (Heterophoxus videns and W. obesa). Other important species are Heterophoxus trichosus and species clearly preferring deep water areas (Fig. 9). The species living here also seemed to have preferences for finer sediments, mostly silt, that occur in deeper parts of Admiralty Bay. The representatives of the genus Ampelisca are recognized as suspension feeders which live in tubes partly burrowed in the sediment. It is noteworthy that the two species of Ampelisca which were studied here seem to have different habitat preferences: Ampelisca richardsoni preferring shallower water and sediments of larger grain size, while Ampelisca anversensis lives deeper in finer sediments. This kind of separation was observed between some other representatives of Ampeliscidae (Mills 1967) and might also be associated with different food preferences. Ampelisca richardsoni, living in shallower water, is known to feed on phytoplankton (Nyssen et al. 2005) that could be less available in deeper parts of the shelf, so one can expect that Ampelisca anversensis uses also some other type of food. However, no stomach content studies are available yet. C. kergueleni and Harpiniopsis aciculum belong to the family Phoxocephalidae that is considered to group species that are permanent burrowers (De Broyer et al. 2003). This can explain their preferences for the type of sediments in which it is easier to dig. However, some authors have reported these species not only from muddy bottoms, but also sandy sediments, so it is possible that in the case of these species it is rather the depth that influences their distribution (Ren and Huang 1991; De Broyer et al. 2007; Alonso de Pina et al. 2008). Aeginoides gaussi belongs to the family Caprellidae that is known to be species rich in shallow waters in low latitudes. In the polar regions, its diversity decreases; additionally Antarctic caprellid species prefer rather deeper areas (Thiel et al. 2003). Aeginoides gaussi was recorded in the Southern Ocean from relatively wide depth range (20-1501 m); however, the shallow water findings (above 80 m) were rare (De Broyer et al. 2007). This confirms rather deep-sea preferences of this species in the Antarctic. Aeginoides gaussi was reported from a wide variety of habitats, but the presence of large colonial organisms like hydrozoans, bryozoans or ascidians was their common feature (De Broyer et al. 2007). Other important taxa constituting deep-water amphipod assemblage include the species only recently described (L. polydeuces) (d’Udekem d’Acoz 2008) or possibly new to science (Urothoe sp. 1, Figorella sp. 1). The former was reported from similar depth range (137–602 m), but habitat and food preferences of all of them remain unknown.
The assemblage of Ezcurra Inlet is dominated by Heterophoxus trichosus; the other species that were common are Paroediceroides sinuata and Monoculodes sp. 1. They belong to two families: Phoxocephalidae and Oedicerotidae, whose representatives are regarded as permanent burrowers (De Broyer et al. 2003) but their feeding preferences are yet to be discovered. In other Antarctic and sub-Antarctic localities, the two first species were found in a wide variety of bottom types (Schellenberg 1926; Alonso de Pina et al. 2008). The character of bottom areas inhabited by amphipod assemblage D points out that it is influenced by adverse environmental conditions. The factor responsible for its formation is the intensive sedimentation caused by strong input of mineral suspension coming from melting glaciers and mostly subglacial streams, active in this part of Admiralty Bay. It is worth noticing that the species which were present here were also found in other assemblages (middle and deep sublittoral of central basin). Thus, it can be assumed that they do not prefer the glacially influenced areas but they are less sensitive and can survive in unfavourable conditions.
A group of ubiquitous species also had an important input in the distinguished assemblages (Fig. 9). They all are common Antarctic species reported from wide variety of habitats and depth ranges. Heterophoxus videns was reported from a depth range covering the whole Antarctic shelf and extending even deeper (2-1031 m) (De Broyer et al. 2007). This small, endobenthic species actively burrowing in the sediments is treated as predator or opportunistic scavenger. It feeds on a wide variety of food, which suggests feeding in the upper layer of sediments. In its gut content, nematods, copepods and diatoms as well as the juveniles of Polychaeta were observed, and that confirms the predatory way of feeding (Oliver et al. 1982; Oliver and Slattery 1985). Also fragments of larger animals were found indicating the necrophagous habit of H. videns (Dauby et al. 2001a, b). The wide feeding spectrum allows this species to survive in different habitats. Another species in this group, W. obesa, is scavenger which has large swimming abilities (Arnaud 1970; Arnaud et al. 1986; Presler 1986; Dauby et al. 2001a, b; De Broyer et al. 2001). Laboratory analyses have proved that it is very sensitive for food chemical signals and it moves immediately in the direction of the scent stimulus (Dauby et al. 2001a, b). Taking this into account it seems that the character of sediments and other environmental conditions do not influence this species so much. L. georgiana is regarded as detritivorous or predator species. In its gut content also fragments of larger animals were found, so occasionally it can feed also as a scavenger (Dauby et al. 2001a, b). It is rather a large species (reaching 27 mm) (d’Udekem d’Acoz 2008) which can pass over larger distance in search for food. Consequently, its presence in a wide variety of habitats can be explained more by the local food availability than the depth or the sediment conditions. Little is known about the last species in the ubiquitous species group—Scaphodactylus dentimanus. This taxon was reported from a very wide depth range (0–1000 m) (De Broyer et al. 2007; Jażdżewska and Krapp-Schickel 2011), but until 2011 it was known from less than 20 individuals. Bellan-Santini (1972) reported it from the stations with stones and hydrozoans, bryozoans and sponges, whereas Ren and Huang (1991) found it on sandy and muddy bottoms. The largest collection of this species is reported by Jażdżewska and Krapp-Schickel (2011); however, apart from depth, no other ecological data for the stations studied were available.
Summarizing the data on the structure of assemblages and habitat preferences of the dominant amphipod species of Admiralty Bay it should be underlined that the chosen environmental features explained only 1/3 of the total species variation observed. Taking into account constant temperature, salinity and oxygen availability in this Antarctic fjord, it seems that other factors, potentially food availability or intra- and inter-specific competition, including also other groups of invertebrates (e.g. Polychaeta), shape the benthic communities there.
Distribution and character of amphipod assemblages
Analysing the depth ranges and species composition of the amphipod assemblages distinguished it can be assumed that the sublittoral of the Antarctic fjords can be divided as follows. The shallowest subtidal zone is inhabited by a small group of very abundant species (Gambi et al. 1994; Cattaneo-Vietti et al. 2000; Jazdzewski et al. 2001; Siciński et al. 2011). Below this zone shallow sublittoral (5–50 m) assemblage is present—the diversity increases but the abundance diminishes. This assemblage is constituted by species preferring coarse sediments but it seems that it is also the presence of macroalgae that influences the composition of this assemblage. Richardson and Hedgpeth (1977), who studied macrobenthos assemblages in Arthur Harbour (Anvers Island), noticed a large number of Schraderia gracilis and Prostebbingia gracilis at two stations from the depths of 18–23 m, with large amount of decaying algae. Also other species, characteristic of the shallow sublittoral assemblage, were regularly reported from the areas rich in macroalgae. No remarks about the algal presence in currently studied samples were made; however, it is known that the phytal zone covers ca. 30% of Admiralty Bay bottom surface and the most dense macroalgal populations occur in the central basin (Zieliński 1990). Between 50 and 80 m the middle sublittoral assemblage can be distinguished which covers the transitional zone between the shallow water regions affected by the severe Antarctic conditions (e.g. grounded ice, tidal currents or currents caused by storms) (Gutt 2001; Campos et al. 2013) and deeper water community. More stable conditions allow sessile organisms, like ascidians or bryozoans, to grow, which in consequence forms a suitable habitat for epifaunal species (Pabis et al. 2011). Together with depth also the food availability and its sources change. Although the algal belt in Admiralty Bay reaches 90 m depth (Zieliński 1990), the species regarded as herbivorous are not so abundant here, which suggests that the importance of fresh plant material as a food source is lower than above 50 m. On the other hand, the species which benefit from the suspended organic matter become more numerous. The mixture of diverse environmental conditions and a wide variety of food available can be the reasons for the highest diversity observed in this assemblage. A similar pattern was observed for Polychaeta of Admiralty Bay, which were more diverse in the areas characterized by higher habitat complexity (Pabis and Siciński 2012). Deeper in the sublittoral (below 80–90 m) the fresh plant matter is scarcely available, so herbivorous species disappear and their place is taken by suspension-feeders and particularly deposit-feeders. Additionally, the character of sediments changes with depth. Initially, with growing depth, the grain size diminishes considerably; however, below 150–200 m depth the sediments are characterized by fine particles and become well sorted, which promotes the presence of infaunal species.
It is important to notice that the assemblages of central basin of Admiralty Bay are gradually exchanged—there is no drastic change between the groups distinguished. Stability of the environment and food availability which both change with depth are the explanations for the development of the above-mentioned assemblages.
The analysis distinguished also the assemblage of Ezcurra Inlet—the area influenced by the intensive sedimentation which derives from glaciers. The high amount of suspended matter, and in consequence high sedimentation rate, with domination of fine sediment, was the reason for the formation of very poor assemblage represented by species that can survive in these severe conditions.
Comparison of amphipod assemblages with other macrofaunal assemblages
There are not many quantitative studies in the Antarctic which covered similar depth range. The above-presented division of assemblages on the basis of Amphipoda is similar to the results of studies of other benthic groups in Admiralty Bay. The analysis of zoobenthic biomass distribution (Pabis et al. 2011), as well as the study of Polychaeta, Cumacea and Tanaidacea (Błażewicz-Paszkowycz and Jażdżewski 2000; Siciński 2004; Pabis and Błażewicz-Paszkowycz 2011; Siciński et al. 2011) allow to recognize five macrobenthos “assemblages” in this Antarctic fjord. The shallowest subtidal/sublittoral is characterized by a very high biomass of Amphipoda and Isopoda with relatively low species richness. Middle sublittoral of central basin is inhabited by diverse assemblage of high biomass composed mainly by colonial organisms and large Polychaeta and Echinodermata. The zoobenthos of the deepest areas of Admiralty Bay can also be distinguished as a separate, peculiar assemblage. Additionally very poor assemblage of middle and deep sublittoral of Ezcurra Inlet was recorded in all cited analyses. The general pattern of amphipod distribution is similar. However, there are some differences in the depth ranges of particular assemblages in central basin. In the case of the whole macrozoobenthos the middle sublittoral assemblage extends between 50 and 270 m, while the deepest parts of the bay (400–500 m) constitute additional group. Moreover, Pabis and Siciński (2012) observed that polychaete soft bottom assemblages of deeper sublittoral (200–300 m) are different in the areas with dropstones inhabited by bryozoans and ascidians from the areas without biogenic structures. The present analysis included the same samples that were studied by Pabis and Siciński (2012) but no differences in species composition between these two groups of samples were observed. In the case of Amphipoda the importance of Bryozoa and Ascidiacea seems to be more pronounced in shallower areas (above 100 m). The phenomenon can be explained by the differences in ecology of these two groups. Most amphipod species are mobile with relatively high swimming abilities, so they can freely move between the areas rich in food. In consequence in the deeper parts of the shelf, where the food availability is lower, they just choose more suitable place. On the other hand, in the middle sublittoral there exists a group of suspension feeding, possibly epifaunal Amphipoda, which benefits from the suspension present at these depths. The separation of the assemblage inhabiting only the deepest parts (400–500 m) of Admiralty Bay is not observed in the presently studied amphipod collection. However, it is important to note that there were only six samples which were collected at these depths. The densities of Amphipoda in these samples were very low, which resulted in spreading the samples in different branches of cluster analysis.
In the glacially influenced area of Ezcurra Inlet the amphipod assemblage was characterized by low densities and low species richness. In the case of Tanaidacea, low diversity, but high densities in glacial fjord were observed, which can be explained by the avoidance of coarser sediments by these crustaceans (Błażewicz-Paszkowycz and Jażdżewski 2000). On the other hand, Isopoda, another peracarid group which was recently thoroughly studied in Admiralty Bay, was almost absent in Ezcurra Inlet (Zemko et al. in press). It derives mostly from differences in the ecology of these groups. Tanaids which live in the tubes dug in the sediments can be found in the areas with finer and better sorted sediments, isopods seem to be sensitive to high suspension and avoid areas affected by glacier activity, whereas some amphipod species can survive adverse environmental conditions so they can be found in such places. However, it is not their preferable habitat so they are not abundant and are represented only by the most resistant species. Similar pattern of macrozoobenthos assemblages in the disturbed areas was also observed in the Antarctic by Richardson and Hedgpeth (1977) in Arthur Harbour as well as in the Arctic (Włodarska et al. 1996; Wlodarska-Kowalczuk and Pearson 2004; Wlodarska-Kowalczuk et al. 2005; Laudien et al. 2007). All these authors reported low diversity values in glacially influenced areas. They also noticed that in the vicinity of glaciers small, motile and discreetly motile detritophagous as well as opportunistic species dominate. The longer the distance from the glacier the larger the share of other functional groups, especially suspension-feeders. This can be explained by the instability of sediments and a very high sedimentation rate close to the glacier. The high input of suspended matter swamp benthic organisms and clog the filtering apparatus (Moore 1977).
Gutt (2007) analysed the general community structure of the Antarctic and proposed the concept of several community groups which in later publications were termed assemblages (Gutt et al. 2014). His division is based on the whole benthic macrofauna and presents large-scale pattern; however, it is possible to find some similarities between his results and the present study conclusions. In Admiralty Bay it is possible to observe the two core assemblages discriminated by Gutt (2007). The first one, suspension-feeder assemblage (SSFA), resembles the amphipod assemblage from middle sublittoral (B) which combines the species associated with sessile, colonial organisms. One of the variations of SSFA assemblage is the one dominated by organisms “other than sponges” (OTH) (Gutt 2007, Fig. 2; Gutt et al. 2014) and it is the case of middle sublittoral assemblage of Admiralty Bay recognized in this study because the presence of Porifera, expressed by their biomass in this assemblage, is small. The SSFA assemblage is regarded as the one of the highest diversities, which is also the case of presently discriminated assemblage B. Since the gear used to obtain samples for the present study is not very effective in collecting sponges it is possible that there are areas in Admiralty Bay where the assemblage dominated by sponges (SPO) could be observed as well. Deeper in the bay another core assemblage can be detected. It is motile detritus feeders and infaunal assemblage (MOIN), which corresponds to the deep sublittoral assemblage (C). Studying the amphipod species composition the infauna-dominated (INF) variety of MOIN assemblage is present in the studied area. It is noteworthy that taking into account the presence of Ascidiacea and Bryozoa in the studied samples, also the mixed assemblage (MIX) can be observed in Admiralty Bay. The separation of the two varieties of the MOIN assemblage was observed by Pabis and Siciński (2012), who studied the polychaete fauna. However, similar trend is not observed in the case of Amphipoda. These contrasting observations can be explained on the one hand by the higher number of infaunal amphipod species which do not benefit so much from the sessile organisms. On the other hand, higher mobility of epifaunal Amphipoda (than Polychaeta), which can actively swim and move between suitable places, causes the uniformity of the deeper shelf assemblage. Gutt (2007) also separated physically controlled (PHYCO) assemblage which can be found in Admiralty Bay as well. One of them is shallow sublittoral assemblage A. It should be pointed out that Gutt (2007) has underlined the importance of physical conditions (ice scouring, grounded ice, wind, storms) in shaping PHYCO assemblage. However, analysing the species composition of Admiralty Bay assemblage A and the habits of component species reported in the literature (e.g. Thurston 1974; Huang et al. 2006, 2007) it seems that it is not only the physical conditions which influence the shallow-water assemblage but also the presence of macroalgae, as they do not only provide food but also the shelter for several species (Pabis and Siciński 2010b). Another physically controlled assemblage which can be observed in Admiralty Bay is the one characteristic of Ezcurra Inlet. In this case, the driving factor for its development is the intensive sedimentation of the inorganic matter transported to the bottom from melting glaciers. It should be mentioned that these two PHYCO assemblages considerably differ in terms of amphipod densities as well as taxon composition. Summarizing, in the studied Antarctic fjord the coexistence of different core assemblages can be observed, which shows high patchiness of the macrobenthos fauna in the Southern Ocean. This phenomenon of a large variety of assemblages in the area of West Antarctic Peninsula was already observed while studying the biogeographic distribution of the macrobenthos assemblages in the Antarctic (Gutt et al. 2013).
From species to families—usefulness of surrogacy measures
In the recent years, several authors underlined the usefulness of surrogacy measures in studying invertebrate communities. They were successfully used in studying human-impacted areas (Bertasi et al. 2009), naturally disturbed regions (Włodarska-Kowalczuk and Kędra 2007) but also non-disturbed areas (Tataranni et al. 2009). Terlizzi et al. (2009) also found that replacing species by genera or families do not cause much information loss in case of beta-diversity of mollusks in four marine localities in Norway, Italy, New Zealand and the Arctic. There were, however, some publications which showed that the results obtained from surrogates do not always reflect the ones got from more detailed analyses and can also depend on the statistical method used (Mellin et al. 2011). The results of the presently studied material showed that the use of higher taxonomic level (genera and particularly families) does not allow for the recognition similar assemblages in non-disturbed areas as were found on the basis of species study (Figs. 4, 10, 11). When analysing the cluster grouping one can notice that the samples from the disturbed area (assemblage D) group themselves together, regardless of the level of taxa identification. On the other hand, the groups A, B and C could not be recognized anymore. This situation can be explained by the replacement of taxa belonging to the same genus or family in different assemblages. One has to take into account that due to very high taxonomic richness of Antarctic Amphipoda, two species of different habitat and food preferences that have an important input in separate assemblages can belong to the same genus or family. Additionally, the environmental spectrum of species from particular Antarctic amphipod family can be very wide (Barnard and Karaman 1991). As a result, using the higher taxonomic level in assemblage analyses can generate false impression of uniformity of the community structure, especially in smaller spatial scale. The only assemblage that remains separated in species, genera and families analyses is the one from Ezcurra Inlet. The very low densities of Amphipoda and the presence of one dominant taxon are probably responsible for that result. This was already observed in the study of macrobenthos fauna of Kongsfjord, the Arctic fjord influenced by intensive suspended matter inflow from glaciated area (Włodarska-Kowalczuk and Kędra 2007). These authors proved that the family level was sufficient to observe the pattern of soft-bottom fauna distribution in disturbed sites.
Due to high importance of Amphipoda in shaping the Antarctic communities it is important to underline low efficacy of the higher level identification of this group in the assemblages recognition and the need for more detailed taxonomical studies of this group before analysing distribution patterns of macrozoobenthos in the Southern Ocean.
References
Alonso de Pina GA, Rauschert M, De Broyer C (2008) A catalogue of the Antarctic and sub-Antarctic Phoxocephalidae (Crustacea: Amphipoda: Gammaridea) with taxonomic, distribution and ecological data. Zootaxa 1752:1–40
Arnaud PM (1970) Frequency and ecological significance of necrophagy among the benthic species of Antarctic coastal waters. In: Holdgate MW (ed) Antarctic ecology. Academic Press, London and New York, pp 259–266
Arnaud PM (1974) Contribution á la bionomie marine benthique des régions antarctiques et subantarctiques. Téthys 6:467–653
Arnaud PM, Jażdżewski K, Presler P, Siciński J (1986) Preliminary survey of benthic invertebrates collected by Polish Antarctic Expeditions in Admiralty Bay (King George Island, South Shetland Islands, Antarctica). Pol Polar Res 7:7–24
Arntz WE, Thatje S, Linse K, Avita C, Ballesteros M, Barnes DKA, Cope T, Cristobo FJ, De Broyer C, Gutt J, Isla E, López-González P, Montiel A, Munilla T, Ramos Esplá AA, Raupach M, Rauschert M, Rodríguez E, Texidó N (2006) Missing link in the Southern Ocean: sampling the marine benthic fauna of remote Bouvet Island. Polar Biol 29:83–89. doi:10.1007/s00300-005-0047-8
ATCM XXVIII (2005) Review of the Admiralty Bay Antarctic Specially Managed Area management plan (ASMA No 1). ATCM XXVIII, Stockholm, pp 1–31
Aumack CF, Amsler CD, McClintock JB, Baker BJ (2011) Changes in amphipod densities among macroalgal habitats in day versus night collections along the Western Antarctic Peninsula. Mar Biol 158:1879–1885. doi:10.1007/s00227-011-1700-0
Baird HP, Stark JS (2013) Population dynamics of the ubiquitous Antarctic benthic amphipod Orchomenella franklini and its vulnerability to environmental change. Polar Biol 36:155–167. doi:10.1007/s00300-012-1246-8
Barnard JL, Karaman GS (1991) The families and genera of marine Gammaridean Amphipoda (Except Marine Gammaroidea). Rec Aust Mus (Suppl) 13:1–866
Barnes DKA, Linse K, Waller C, Morely S, Enderlein P, Fraser KPP, Brown M (2006) Shallow benthic fauna communities of South Georgia Island. Polar Biol 29:223–228. doi:10.1007/s00300-005-0042-0
Bellan-Santini D (1972) Invertébrés marins des XIIème et XVème Expéditions Antarctiques Françaises en Terre Adélie. 10. Amphipodes Gammariens. Téthys (Suppl.) 4:157–238.
Bellan-Santini D, Ledoyer M (1973, 1974) Gammariens (Crustacea–Amphipoda) des Iles Kerguelen et Crozet. Téthys 5:635–708
Bertasi F, Colangelo MA, Colosio F, Gregorio G, Abbiati M, Ceccherelli VU (2009) Comparing efficacy of different taxonomic resolutions and surrogates in detecting changes in soft bottom assemblages due to coastal defence structures. Mar Pollut Bull 58:686–694. doi:10.1016/j.marpolbul.2009.01.003
Błażewicz-Paszkowycz M, Jażdżewski K (2000) Quantitaive data on Tanaidacea of Admiralty Bay (King George Island, South Shetland Islands, Antarctica). Pol. Polar Res 21:171–180
Brandt A, De Broyer C, De Mesel I, Ellingsen KE, Gooday AJ, Hilbig B, Linse K, Thomson MRA, Tyler PA (2007) The biodiversity of the deep Southern Ocean benthos. Philos T Roy Soc B 362 39–66. doi:10.1098/rstb.2006.1952
Braun M, Grossmann H (2002) Glacial changes in the areas of Admiralty Bay and Potter Cove, King George Island, maritime Antarctica. In: Beyer L, Bolter M (eds) Geoecology of the Antarctic ice–free coastal landscapes. Springer, Berlin, pp 75–90
Bregazzi PK (1972a) Life cycles and seasonal movements of Cheirimedon femoratus (Pfeffer) and Tryphosella kergueleni (Miers) (Crustacea: Amphipoda). Brit Antarct Surv B 30:1–34
Bregazzi PK (1972b) Habitat selection by Cheirimedon femoratus (Pfeffer) and Tryphosella kergueleni (Miers) (Crustacea: Amphipoda). Brit Antarct Surv B 31:21–31
Bregazzi PK (1973) Locomotor activity rhythms in Tryphosella kergueleni (Miers) and Cheirimedon femoratus (Pfeffer) (Crustacea, Amphipoda). Brit Antarct Surv B 33–34:17–32
Brökeland W, Choudhury M, Brandt A (2007) Composition, abundance and distribution of Peracarida from the Southern Ocean deep sea. Deep-Sea Res Pt II 54:1752–1759. doi:10.1016/j.dsr2.2007.07.014
Campos LS, Barboza CAM, Bassoi M, Bernardes M, Bromberg S, Corbisier TN, Fontes RFC, Gheller PF, Hajdu E, Kawall HG, Lange PK, Lanna AM, Lavrado HP, Monteiro GSC, Montone RC, Morales T, Moura RB, Nakayama CR, Oackes T, Paranhos R, Passos FD, Petti MAV, Pellizari VH, Rezede CE, Rodrigues M, Rosa LH, Sechi E, Tenenbaum DR, Yoneshigue-Valentin Y (2013) Environmental processes, biodiversity and changes in Admiralty Bay, King George Island, Antarctica. In: Verde C, di Prisco G (eds) Adaptation and evolution in marine environments, volume 2, the impacts of global change on biodiversity. Springer, Heidelberg, New York, Dordrecht, London, pp 127–156. doi:10.1007/978-3-642-27349-0
Cattaneo-Vietti R, Chiantore M, Gambi MC, Albertelli G, Cormaci M, Di Geronimo I (2000) Spatial and vertical distribution of benthic littoral communities in Terra Nova Bay. In: Faranda FM, Guglielmo L, Ianora A (eds) Ross Sea ecology. Springer, Berlin Heidelberg, pp 503–514
Chao A (2004) Species richness estimation. In: Balakrishnan N, Read CB, Vidakovic B (eds) Encyclopedia of statistical sciences. Wiley, New York, pp 1–23
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DK, Smith RC (2007) Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos T Roy Soc B 362:149–166. doi:10.1098/rstb.2006.1958
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. Persistent. http://purl.oclc.org/estimates
Cummings VJ, Thrush SF, Chiantore M, Hewitt JE, Cattaneo-Vietti R (2010) Macrobenthic communities of the north-western Ross Sea shelf: links to depth, sediment characteristics and latitude. Antarct Sci 22:793–804. doi:10.1017/S0954102010000489
d’Udekem d’Acoz C (2008) Shelf and abyssal Liljeborgia BATE, 1861 of the Southern Ocean (Crustacea, Amphipoda, Liljeborgiidae). Bull Inst R Sc N B-B 78:45–286
Dauby P, Scailteur Y, Chapelle G, De Broyer C (2001a) Potential impact of the main benthic amphipods on the eastern Weddell Sea shelf ecosystem (Antarctica). Polar Biol 24:657–662. doi:10.1007/s003000100265
Dauby P, Scailteur Y, De Broyer C (2001b) Trophic diversity within the eastern Weddell Sea community. Hydrobiologia 443:69–86. doi:10.1023/A:1017596120422
Dauby P, Nyssen F, De Broyer C (2003) Amphipods as food sources for higher trophic levels in the Southern Ocean: a synthesis. W: Huiskes AHL. In: Gieskes WWC, Rozema J, Schorno RML, van der Vies SM, Wolff WJ (eds) Antarctic biology in a global context. Backhuys, Leiden, pp 129–134
De Broyer C (1983) Recherches sur la systématique et l’évolution des crustacés amphipodes gammarides antarctiques et subantarctiques. Dissertation, University Louvain-La-Neuve, 468 pp
De Broyer C, Danis B (2011) How many species in the Southern Ocean? Towards a dynamic inventory of the Antarctic marine species. Deep-Sea Res Pt II 58:5–17. doi:10.1016/j.dsr2.2010.10.007
De Broyer C, Jażdżewska A (2014) Biogeographic patterns of Southern Ocean benthic amphipods. In: De Broyer C, Koubbi P et al (eds) Biogeographic atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 155–165
De Broyer C, Scailteur Y, Chapelle G, Rauschert M (2001) Diversity of epibenthic habitats of gammaridean amphipods in the eastern Weddell Sea. Polar Biol 24:744–754. doi:10.1007/s003000100276
De Broyer C, Chapelle G, Duchesne PA, Munn R, Nyssen F, Scailteur Y, van Roozendael F, Dauby P (2003). Structural and ecofunctional biodiversity of the amphipod crustacean benthic taxocenoses in the Southern Ocean. In: Marine Biota and Global Change, Belgian Scientific Research Programme on the Antarctic, Scientific results, 58 pp
De Broyer C, Nyssen F, Dauby P (2004) The crustacean scavenger guild in Antarctic shelf, bathyal and abyssal communities. Deep-Sea Res Pt II 51:1733–1752. doi:10.1016/j.dsr2.2004.06.032
De Broyer C, Lowry JK, Jazdzewski K, Robert H (2007) Catalogue of the Gammaridean and Corophiidean Amphipoda (Crustacea) of the Southern Ocean with distribution and ecological data. Synopsis of the Amphipoda of the Southern Ocean 1:1–325
Gambi MC, Bussotti S (1999) Composition, abundance and stratification of soft-bottom macrobenthos from selected areas of the Ross Sea shelf (Antarctica). Polar Biol 21:347–354
Gambi MC, Lorenti M, Russo GF, Scipione MB (1994) Benthic associations of the shallow hard bottoms off Terra Nova Bay, Ross Sea: zonation, biomass and population structure. Antarct Sci 6:449–462
Glover AG, Smith CR, Mincks SL, Sumida PY, Thurber AR (2008) Macrofaunal abundance and composition on the West Antarctic Peninsula continental shelf: Evidence for a sediment ‘food bank’ and similarities to deep-sea habitats. Deep-Sea Res Pt II 55:2491–2501. doi:10.1016/j.dsr2.2008.06.008
Gotelli NJ, Colwell RK (2011) Estimating species richness. In: Magurran AE, McGill BJ (eds) Biological diversity: frontiers in measurement and assessment. Oxford University Press, New York, pp 39–54
Gutt J (2001) On the direct impact of ice on marine benthic communities, a review. Polar Biol 24:553–564. doi:10.1007/978-3-642-59419-9_21
Gutt J (2007) Antarctic macro-zoobenthic communities: a review and an ecological classification. Antarct Sci 19:165–182. doi:10.1017/S0954102007000247
Gutt J, Griffiths H J, Jones CD (2013) Circumpolar overview and spatial heterogeneity of Antarctic macrobenthic communities. Mar Biodivers 43:481–487. doi:10.1007/s12526-013-0152-9
Gutt J, Barnes D, Lockhart SJ (2014) Classification and spatially explicit illustration of Antarctic macrobenthic assemblages: a feasibility study. In: De Broyer C, Koubbi P et al (eds) Biogeographic atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 229–232
Huang YM, McClintock JB, Amsler CD, Peters KJ, Baker BJ (2006) Feeding rates of common Antarctic gammarid amphipods on ecologically important sympatric macroalgae. J Exp Mar Biol Ecol 329:55–65. doi:10.1016/j.jembe.2005.08.013
Huang YM, Amsler MO, McClintock JB, Amsler CD, Baker BJ (2007) Patterns of gammaridean amphipod abundance and species composition associated with dominant subtidal macroalgae from the western Antarctric Peninsula. Polar Biol 30:1417–1430. doi:10.1007/s00300-007-0303-1
Jażdżewska A (2011) Soft bottom sublittoral amphipod fauna of Admiralty Bay, King George Island, Antarctic. Oceanol Hydrobiol St 40:1–10. doi:10.2478/s13545-011-0001-z
Jażdżewska A, Krapp-Schickel T (2011) New data on the distribution of stenothoid amphipods (Crustacea) from Scotia Arc, West Antarctic. Pol Polar Res 32:293–320. doi:10.2478/v10183-011-0025-5
Jazdzewski K, De Broyer C, Pudlarz M, Zielinski D (2001) Seasonal fluctuations of vagile benthos in the uppermost sublittoral of a maritime Antarctic fjord. Polar Biol 24:910–917. doi:10.1007/s003000100299
Jażdżewski K, Jurasz W, Kittel W, Preser E, Preser P, Siciński J (1986) Abundance and biomass estimates of the benthic fauna in Admiralty Bay, King George Island, South Shetland islands. Polar Biol 5:5–16
Jażdżewski K, De Broyer C, Teodorczyk W, Konopacka A (1991a) Survey and distributional patterns of the amphipod fauna of Admiralty Bay, King George Island, South Shetland Islands. Pol Polar Res 12:461–472
Jażdżewski K, Teodorczyk W, Siciński J, Kontek B (1991b) Amphipod crustaceans as an important component of zoobenthos of the shallow Antarctic sublittoral. Hydrobiologia 223:105–117
Jonasz M (1983) Particulate matter in Ezcurra Inlet: concentration and size distributions. Oceanologia 15:65–74
Kruszewski G (2002) Zlodzenie Zatoki Admiralicji—przebieg i uwarunkowania. Wydawnictwo Uczelniane Akademii Morskiej w Gdyni, Gdynia, 123 pp
Laudien J, Herrmann M, Arntz WE (2007) Soft bottom species richness and diversity as a function of depth and iceberg scour in Arctic glacial Kongsfjorden (Svalbard). Polar Biol 30:1035–1046. doi:10.1007/s00300-007-0263-5
Lowry JK (1975) Soft bottom macrobenthic community of Arthur Harbor, Antarctica. In: Pawson DL (ed) Biology of the Antarctic Seas V, Antarctic research series, vol 23. American Geophysical Union, Washington, pp 1–19
Magurran AE (2004) Measuring biological diversity. Blackwell, Oxford, p 256
Marsz A (1983) From surveys of the geomorphology of the shores and bottom of the Ezcurra Inlet. Oceanologia 15:209–220
Martín A, Miloslavich P, Díaz Y, Ortega I, Klein E, Troncoso J, Aldea C, Carbonini AK (2016) Intertidal benthic communities associated with the macroalgae Iridaea cordata and Adenocystis utricularis in King George Island, Antarctica. Polar Biol 37:207–220. doi:10.1007/s00300-015-1773-1
Mellin C, Delean S, Caley J, Edgar G, Meekan M, Pitcher R, Przeslawski R, Williams A, Bradshaw C (2011) Effectiveness of biological surrogates for predicting patterns of marine biodiversity: a global meta-analysis. PLoS One 6:e20141
Mills EL (1967) The biology of an ampeliscid amphipod crustacean sibling species pair. J Fish Res Board Can 24:305–355
Moore PG (1977) Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanogr Mar Biol 15:225–363
Munn R, De Broyer C, Siciński J, Dauby P (1999) Seasonal variation of distribution and new records of benthic amphipods (Crustacea) from Admiralty Bay, King George Island, West Antarctic. In: Repelewska-Pękalowa J (ed) Polish polar studies. Department of Geomorphology of the Maria Curie-Skłodowska University, Lublin, pp 371–378
Myers AA, Lowry JK (2003) A phylogeny and a new classification of the Corophiidea Leach, 1814 (Amphipoda). J Crustacean Biol 23:443–485
Nyssen F, Brey T, Dauby P, Graeve M (2005) Trophic position of Antarctic amphipods: enhanced analysis by a 2-dimensional biomarker assay. Mar Ecol-Prog Ser 300:135–145. doi:10.3354/meps300135
Oliver JS, Slattery PN (1985) Effects of crustacean predators on species composition and population structure of soft-bodied infauna from McMurdo Sound, Antarctica. Ophelia 24:155–175
Oliver JS, Oakden JM, Slattery PN (1982) Phoxocephalid amphipod crustaceans as predators on larvae and juveniles in marine soft-bottom communities. Mar Ecol-Prog Ser 7:179–184
Pabis K, Błażewicz-Paszkowycz M (2011) Distribution and diversity of cumacean assemblages in Admiralty Bay, King George Island. Pol Polar Res 32:341–354. doi:10.2478/v10183?011?0024?6
Pabis K, Siciński J (2010a) Distribution and diversity of polychaetes collected by trawling in Admiralty Bay—an Antarctic glacial fiord. Polar Biol 33:141–151. doi:10.1007/s00300-009-0692-4
Pabis K, Siciński J (2010b) Polychaete fauna associated with holdfasts of the large brown alga Himantothallus grandifolius in Admiralty Bay, King George Island, Antarctic. Polar Biol 33:1277–1288. doi:10.1007/s00300-010-0816-x
Pabis K, Siciński J (2012) Is polychaete diversity in the deep sublittoral of an Antarctic fiord related to habitat complexity? Pol Polar Res 33:181–197. doi:10.2478/v10183-012-0009-0
Pabis K, Sicinski J, Krymarys M (2011) Distribution patterns in the biomass of macrozoobenthic communities in Admiralty Bay (King George Island, South Shetlands, Antarctic). Polar Biol 34:489–500. doi:10.1007/s00300-010-0903-z
Pabis K, Hara U, Presler P, Siciński J (2014) Structure of bryozoan communities in an Antarctic glacial fjord (Admiralty Bay, South Shetlands). Polar Biol 37:737–751. doi:10.1007/s00300-014-1474-1
Pęcherzewski K (1980) Distribution and quantity of suspended matter in Admiralty Bay, King George Island, South Shetland Islands. Pol. Polar Res 1:75–82
Presler P (1986) Necrophagous invertebrates of the Admiralty Bay of King George Island (South Shetland Islands, Antarctica). Pol. Polar Res 7:25–61
Rauschert M (1989) Atylopsis fragilis n. sp. (Crustacea, Amphipoda, Gammaridea, Eusiridae) aus dem Sublitoral von King George (Süd-Shetland-Inseln). Mitteilungen aus dem Zoologischen Museum in Berlin 65:127–138
Rauschert M (1991) Ergebnisse der faunistischen Arbeiten im Benthal von King George Island (Südshetlandinseln, Antarktis). Berichte zur Polarforschung, p 75
Rehm P, Thatje S, Arntz WE, Brandt A, Heilmayer O (2006) Distibution and composition of macrozoobenthic communities along a Victoria-Land Transect (Ross Sea, Antarctica). Polar Biol 29:782–790. doi:10.1007/s00300-006-0115-8
Rehm P, Hooke RA, Thatje S (2011) Macrofaunal communities on the continental shelf off Victoria Land, Ross Sea, Antarctica. Antarct Sci 23:449–455. doi:10.1017/S0954102011000290
Ren X, Huang L (1991) Studies on Gammaridea and Caprellidea (Crustacea:Amphipoda) from the northwest waters off the Antarctic Peninsula. Studia Marina Sinica 32:187–323
Richardson MD, Hedgpeth JW (1977) Antarctic soft-bottom, macrobenthic community. Adaptations to a cold, stable, highly productive, glacially affected environment. In: Llano GA (ed) Adaptations within Antarctic ecosystems, proceedings of the third SCAR symposium on Antarctic Biology. Smithsonian Institution, Washington, pp 181–196
Rudowski S, Marsz A (1996) Cechy rzeźby dna i pokrywy osadowe we współcześnie kształtujących się fiordach na przykładzie Hornsundu (Spitsbergen) i Zatoki Admiralicji (Antarktyka Zachodnia). Prace Wydziału Nawigacyjnego Wyższej Szkoły Morskiej w Gdyni 3:39–81
Saiz JI, García FJ, Manjón-Cabeza ME, Parapar J, Peña-Cantero A, Saucède T, Troncoso JS, Ramos A (2008) Community structure and spatial distribution of benthic fauna in the Bellingshausen Sea (West Antarctica). Polar Biol 31:735–743. doi:10.1007/s00300-008-0414-3
Sáiz-Salinas JI, Ramos A, García FJ, Troncoso JS, San Martin G, Sanz C, Palacin C (1997) Quantitative analysis of macrobethic soft-bottom assemblages in South Shetland waters (Antarctica). Polar Biol 17:393–400
Salzwedel H, Rachor E, Gerdes D (1985) Benthic macrofauna communities in the German Bight. Veröff Inst Meeresforsch Bremerh 20:199–267
Schellenberg A (1926) Die Gammaridean der Deutschen Sudpolar-expedition 1901–1903, pp 235–414
Schellenberg A (1931) Gammariden und Caprelliden des Magellangebietes, Sudgeorgiens und der Westantarktis. Further Zoological Results of the Swedish Antarctic Expedition 1901–1903 under the direction of dr. Otto Nordenskjold 2:1–290
Siciński J (2004) Polychaetes of Antarctic sublittoral in the proglacial zone (King George Island, South Shetland Islands). Pol Polar Res 25:67–96
Siciński J, Jażdżewski K, De Broyer C, Presler P, Ligowski R, Nonato EF, Corbisier TN, Petti MAV, Brito TAS, Lavrado HP, Błażewicz-Paszkowycz M, Pabis K, Jażdżewska A, Campos LS (2011) Admiralty Bay Benthos Diversity—a census of a complex polar ecosystem. Deep-Sea Res Pt II 58:30–48. doi:10.1016/j.dsr2.2010.09.005
Siciński J, Pabis K, Jażdżewski K, Konopacka A, Błażewicz-Paszkowycz M (2012) Macrozoobenthos of two Antarctic glacial coves: a comparison with non-disturbed bottom areas. Polar Biol 35:355–367. doi:10.1007/s00300-011-1081-3
Stebbing TRR (1888) Amphipoda Gammarina. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873–76, p 1737
Szafrański Z, Lipski M (1982) Characteristics of water temperature and salinity at Admiralty Bay (King George Island, South Shetland Islands, Antarctic) during the austral summer 1978/1979. Pol Polar Res 3:7–24
Takeuchi I, Watanabe K (2002) Mobile epiphytic invertebrates inhabiting the brown macroalga, Desmarestia chordalis, under the coastal fast ice of Lützow-Holm Bay, East Antarctica. Polar Biol 25:624–628. doi:10.1007/s00300-002-0373-z
Tataranni M, Maltagliati F, Floris A, Castelli A, Lardicci C (2009) Variance estimate and taxonomic resolution: an analysis of macrobenthic spatial patterns at different scales in a Western Mediterranean coastal lagoon. Mar Environ Res 67:219–229. doi:10.1016/j.marenvres.2009.02.003
Terlizzi A, Anderson MJ, Bevilacqua S, Fraschetti S, Włodarska-Kowalczuk M, Ellingsen KE (2009) Beta diversity and taxonomic sufficiency: do higher-level taxa reflect heterogeneity in species composition? Divers Distrib 15:450–458. doi:10.1111/j.1472-4642.2008.00551.x
Thiel M, Guerra-García JM, Lancellotti DA, Vásquez N (2003) The distribution of littoral caprellids (Crustacea: Amphipoda: Caprellidea) along the Pacific coast of continental Chile. Rev Chil Hist Nat 76:297–312
Thurston MH (1974) The Crustacea Amphipoda of Signy Island, South Orkney Islands. Br Antart Surv Sci Rep 71:1–127
Włodarska M, Węsławski JM, Gromisz S (1996) A comparison of the macrofaunal community structure and diversity in two arctic glacial bays—a “cold” one off Franz Josef Land and a “warm” one off Spitsbergen. Oceanologia 38:251–283
Wlodarska-Kowalczuk M, Pearson TH (2004) Soft-bottom macrobenthic faunal associations and factors affecting species distribution in an Arctic glacial fjord (Kongsfjord, Spitsbergen). Polar Biol 27:155–167. doi:10.1007/s00300-003-0568-y
Wlodarska-Kowalczuk M, Pearson TH, Kendall MA (2005) Benthic response to chronic natura physical disturbance by glacial sedimentation in an Arctic fjord. Mar Ecol-Prog Ser 303:31–41. doi:10.3354/meps303031
Włodarska-Kowalczuk M, Kędra M (2007) Surrogacy In natural patterns of benthic distribution and diversity:selected taxa versus lower taxonomic resolution. Mar Ecol-Prog Ser 351:53–63. doi:10.3354/meps07127
Zemko K, Pabis K, Siciński J, Błażewicz M (in press) Low abundance and high species richness: the structure of the soft-bottom isopod fauna of a West Antarctic glacial fjord. Polar Biol
Zieliński K (1990) Bottom macroalgae of the Admiralty Bay (King George Island, South Shetlands, Antarctica). Pol Polar Res 11:95–131
Acknowledgements
The authors thank the participants of the IX Polish Antarctic Expedition for assistance during the sampling and the staff of the Department of Invertebrate Zoology and Hydrobiology, University of Lodz for help in sample sorting. This study was supported by the Polish Ministry of Science and Higher Education (51/N-IPY/2007/0) and partially from the internal funds of the University of Lodz. Part of the identification was done during two visits of the senior author in Institut royal des Sciences naturelles de Belgique, Brussels which were granted by SYNTHESYS programme (The European Union-funded Integrated Infrastructure Initiative grant). We thank also Krzysztof Pabis for fruitful discussions during preparation of the manuscript, the reviewers for constructive critiques and Joanna Leszczyńska who has done the language correction of the text.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jażdżewska, A.M., Siciński, J. Assemblages and habitat preferences of soft bottom Antarctic Amphipoda: Admiralty Bay case study. Polar Biol 40, 1845–1869 (2017). https://doi.org/10.1007/s00300-017-2107-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2107-2