Abstract
Antarctic krill (Euphausia superba) aggregate in various ways depending on a range of biological and physical factors. In some areas, typically associated with bathymetric features such as shelf edges and canyons, they may aggregate densely to form hotspots. Despite the importance of such hotspots, their development over time in demographic composition and spatial distribution is not well understood. A fishing vessel during regular operation was used for collection of krill demographic and acoustic data on the shelf northwest of South Orkney Islands. Results show a decrease in the proportion of subadult males, partly reflected in an increase in mature adult males. Concurrently, there was a change in the proportion of males in the sampled population from 0.8 to 0.3, indicating immigration or emigration of krill through the hotspot. A clear trend was observed in the diurnal vertical distribution with deeper and more vertically compact swarms during the day. However, some days displayed very small differences between the day and night distribution and considerable variability in the daytime depth distribution. It was noted that although fishing was carried out during the entire period of the study, there was no obvious trend in the acoustic backscatter, suggesting that the overall krill density was not changing during this period. Using a fishing vessel as a research platform has advantages for understanding the dynamics of the fishery and in quantifying biological and physical processes during actual exploitation of these resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Antarctic krill (Euphausia superba, hereafter krill) is stenothermic and specially adapted to cold water (Atkinson et al. 2006; Tarling et al. 2006). They have a vast circumpolar distribution with the Antarctic Convergence front, where the cold Antarctic water meets the warmer sub Antarctic waters, generally defining their northern distribution limit.
Despite a widespread distribution, it is well documented that krill aggregate in hotspots in relation to shelf breaks and other shelf associated bathymetric features (Huntley and Niiler 1995; Constable and Nicol 2002). Such areas likely provide both good food availability and shelter from offshore currents heading toward less productive areas (Hazen et al. 2013). The hotspots are likely of high importance for the entire pelagic ecosystem and are typically adjacent to important breeding areas for land-based krill predators (Constable and Nicol 2002). The distribution of fishing effort through the last 10 years shows that also the krill fishing industry is highly concentrated around certain specific shelf break locations with particularly high krill concentrations. Despite the importance of such hotspots, their dynamics over time with respect to krill spatial distribution, abundance and population characteristics is not well described or understood. Particularly interesting is the dynamics around the short summer months when their fat stores are deposited (Quetin and Ross 2001) coinciding with the energy demanding reproduction (Siegel 2012).
Krill become sexually mature by their third summer and have a lifespan of 6–7 years (Ettershank 1983; Rosenberg et al. 1986). The timing of spawning for krill is dependent on several processes including photoperiod (Brown et al. 2010), food availability and also other interacting factors such as ice extent and the presence of polynyas (Siegel 2012). Spawning may occur late in November and finish as early as January or can start as late as February and last until April (Siegel and Loeb 1995; Spiridinov 1995; Siegel 2012). Mature males produce sperm-filled spermatophores, which are transferred and attached to the females thelycum via the male petasmae, a complex organ developed especially for this purpose from the endopods of the first pair of pleopods (Makarov and Denys 1981). Krill can produce >12,000 eggs year−1 (Tarling et al. 2007) and are capable of reproducing for up to five seasons (Ettershank 1983).
Although high abundance regions are well known for krill (Constable and Nicol 2002), the spatial distribution of krill is quite variable and difficult to predict. Under given circumstances, they drift with the currents as true planktonic organisms and at other times they are able to relocate horizontally, swimming at higher speeds for extended periods of time (Marr 1962; Kanda et al. 1982), seemingly unaffected by local currents. They also have a diel vertical migration which may be strong or absent (Hamner and Hamner 2000) and may even use the bottom as a habitat (Schmidt et al. 2011). At a small scale, krill are typically distributed in aggregations or swarms believed to facilitate reproduction and feeding; this may also reduce the chance of predation (Hamner et al. 1983; Ritz 2000; Evans et al. 2007) and/or offer other locomotive energetic advantages (Ritz 2000). The features of such swarms including structure and morphology are extremely variable, and it has been shown that the spatial distribution of krill at the swarm level can be linked to demographic composition and the proportion of different maturity stages (Watkins et al. 1992). Hence, demographic composition and spawning state may be important elements for understanding distribution.
The water off the South Orkney Islands is a krill hotspot area and subject to extensive fisheries (Nicol et al. 2012). Within such a restricted geographic region, it is expected that population characteristics could be preserved over an extended period of time (Hazen et al. 2013). The purpose of the present study was to follow the krill stock here to provide detailed information on seasonal development of sexual maturation, sex ratio and other population parameters such as abundance and distribution. We also aimed to explore the potential of carrying out ecological research from a commercial krill fishing vessel within a krill hotspot. A particular advantage of such vessels may be that they provide a unique opportunity to sample regularly at quasi-stationary positions for extended periods and within a reasonable restricted region. This can allow different perspectives, data to be acquired and processes to be monitored compared to traditional scientific survey approaches, that most often consist of regularly spaced transects, forming a grid with fixed position of stations for determination of specific population parameters, distribution and biomass.
Materials and methods
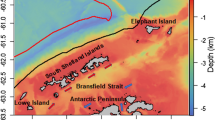
The platform used for the investigations was the FV Saga Sea (Aker Biomarine ASA), a Norwegian 92-m and 6000-hp commercial ramp trawler. Fishing occurred along the northern shelf edge off South Orkney Islands (Fig. 1). The area is characterized by a steep slope from the shelf break to abyssal depths of around 3–4000 m, parallel to the east–west axis of the Coronation Island (South Orkneys largest island). There are two north–south-oriented canyons or troughs along this axis, the Monroe and Coronation Troughs (Dickens et al. 2014). These areas are likely important for retention of krill advected along the shelf and slope region from areas further west and south-west, or via deeper currents from the Weddell Sea region flowing east and turning north in a counter clockwise fashion around the South Orkney plateau (cf. Gordon et al. 2001).
Data collection was carried out during regular fishing operations from 28 January to 2 March 2009, except between 8 and 11 February when the vessel undertook other logistical operations further north. The vessel operated two 16-mm meshed and 220-m-long trawls with continuous pumping through a 20-cm diameter and 300-m-long vacuum hose from the codends to the production deck, which enabled sampling of krill specimens at a predictable and regular basis. When there is no severe catch accumulation in the codend, it takes 10–12 min for the krill to travel from the trawl mouth to the production deck.
In total 55 samples of krill (n > 100 ind.) were acquired at regular intervals during both daytime (day: sun angle > 0°) and night time hours over the investigation period. A batch of krill specimens was collected at random directly from the end of the vacuum hose, where the catch is transferred onto a sieve to separate out most of the seawater before entering a buffer tank below deck.
Body length of krill was measured on fresh material from 7521 individuals (±1 mm) from the anterior margin of the eye to tip of telson excluding the setae, according to the “Discovery method” used in Marr (1962). From these, the sex and maturity stage were determined for a total of 4678 individuals using the classification methods outlined by Makarov and Denys (1981). Males were divided into three sub adult stages: MIIA1 (petasma vesicles are not divided, but appears with a small bump or bubble at the root), MIIA2 (petasma has developed the bubble to a split with one or two fingers) and MIIA3 (petasma root with two short fingers and an incipient formation of wings on the opposite hold) and two adult stages: MIIIA (petasma fully developed with swollen fingers and with a wing overlap, ductus ejaculatori are also visible ventrally, but these are sealed and spermatophores cannot be squeezed out) and MIIIB (petasma as for MIIIA, ductus ejaculatori has spermatophores that can be pressed out, or with the duct passage open where spermatophores are already deposited). Females were divided into one sub adult stage: FIIB (thelycum is small and colorless) and five adult stages: FIIIA (thelycum is fully developed for spawning, red pigmented and strongly chitinized), FIIIB (thelycum as FIIIA but fertilized with spermatophores), FIIIC (also with spermatophores, mature eggs or large ovaries visible under carapax, but carapax is not swollen), FIIID (with spermatophores, carapax is swollen and this swelling extends into the first abdominal segment) and FIIIE (fully spawned, the ovaries are small and the carapax is hollow). Unlike all other stages, the juveniles had no visible sexual characteristics (no visible petasma or thelycum).
In order to investigate temporal development and variability in population size frequency distribution, we have analyzed individual station-based data using mixture distribution analyses (i.e. MacDonald and Pitcher 1979; MacDonald and Green 1988) and the “mixdist” package in the R-statistical environment (www.r-project.org). The aim of this analysis was to inspect individual size frequency distributions excluding small krill that were caught more or less at random (krill with body length <33 mm). It is realized that the dominant “size group” of krill ≥33 mm most probably consists of several year classes. However, it was not intended to reveal and separate generations, but to look at the overall size distribution and estimate the overall mean body length and sigma (standard deviation) to see how these parameters and a composite distribution can aid to understand overall population variability. To visually aid the interpretation of the size distributions, we also pooled the data into 5-day slots, in total 7 periods (P1–P7). Not all periods contained data all days (see above). To optimize the goodness-of-fit (Chi-squared ANOVA), a normal distribution model and best fitting constraints for SDs were used, following the instructions of Du (2002).
For the collection of acoustic data, a Simrad ES60 echo sounder system logged data continuously at two frequencies, 38 and 120 kHz. The system was not calibrated so the logged data represent relative levels of acoustic backscatter. However, a post-calibration of the system was carried out off Brindisi, Italy, later that year using standard sphere calibration with a 38.1-mm tungsten carbide sphere (Foote et al. 1987) and showed that the transducers worked according to specifications, with no malfunctions. The echo sounder was operating with a ping interval of 1 per second. Vessel speed during towing was around two knots. Acoustic data were acquired down to 500 m depth at both frequencies.
Measurements of volume backscattering strength (Sv; m2/m3) from the ES60 were post-processed using the Large-Scale Survey System (LSSS) software (Korneliussen et al. 2006) and exported as average values of Nautical Area Scattering Coefficient (NASC; m2/nmi2) (MacLennan et al. 2002) in bins of 5 m vertical and 50 ping horizontal resolution. The acoustic targets assumed to be krill were separated from other targets following the CCAMLR protocol for target discrimination. This method takes advantage of the predictable frequency-dependent volume backscattering strength for krill within a specified range of body lengths. Given a range of body lengths, the corresponding range of ΔSv-values (Sv,120–Sv,38) can be predicted and used to discriminate krill from other targets. We used the krill length distribution found during the survey to calculate ΔSv (SC-CAMLR 2005; Reiss et al. 2008). The method was applied to each exported sample bin, and if ΔSv fell within the range estimated for krill targets, it was included as krill. The target strength (TS) predictions of krill applied to calculate values of Sv at both frequencies were derived from the simplified Stochastic Distorted Wave Born Approximation (sSWDBA) (Conti and Demer 2006), but parameterized according to Calise and Skaret (2011). The ΔSv finally applied was based on a krill length range calculated in 10-mm bins based on krill TS predictions from a 95 % PDF of krill length distribution based on the catches (SC-CAMLR 2009). The discrimination based on frequency response is strictly not valid when derived from uncalibrated systems, but later calibrations have shown that the original gain settings were not far off (<1 dB) suggesting a stable system over time. In addition, the vessel was operating in areas where krill make up a vast majority of the plankton abundance, and the other main contributors to backscatter are air breathing predators with a markedly different acoustic frequency response signature compared to krill.
A SAIV CTD (mod SD204, Environmental Sensors and Systems, Bergen, Norway) was mounted on the starboard trawl beam, in order to record data on temperature, salinity and depth. Samples were obtained at 1-min intervals. Later examination of the salinity data showed that they were corrupted due to a sensor failure and thus omitted from further analysis.
Water samples (20 ml, scintillation vials PE) for nitrate and phosphate analysis were collected from the surface (0 m) on each krill sampling location (within 5 min after sampling the krill). All water samples were fixed with chloroform (1 %) and stored in a refrigerator until analyses on shore. The samples were analyzed, following the methods of Bendschneider and Robinson (1952) and Grasshoff (1965), on a SKALAR auto-analyzer equipped with matrix detectors and manifolds developed at the Norwegian Institute of Marine Research.
Surface water samples were also obtained for chlorophyll a and phaeopigment analyses. The water (263 ml) was filtered using glass fiber filters (Munktell 0.45 µm pore size) that were stored at −20 °C until analysis on shore. The assay was performed by extraction with 90 % acetone followed by centrifugation, and the measurements were taken with a fluorometer (model 10 AU, Turner designs Inc., Sunnyvale, Ca., USA), according to Welshmeyer (1994) and Jeffrey and Humphrey (1975).
The fishing vessels along track positions were coupled to the elevation data from the new bathymetric high-resolution grid (300 m) of the continental shelf surrounding the South Orkney Islands, northeast of the Antarctic Peninsula (Dickens et al. 2014). The original data were downloaded from the Marine Geoscience Data System (MGDS) as a gridded file in GEOTIFF format. It was imported to ArcGIS, converted from raster to ascii and then imported to Fledermaus where it was converted to decimal latitude, longitude and elevation (bottom depth) using WGS84. Thereafter, a simple algorithm was developed to find the geographic position and associated bottom depth that most closely corresponded to individual vessel positions throughout the study period.
Relationships in demographic parameters and between acoustic and environmental elements were explored using analysis of variance (ANOVA, General Linear Models (GLM) and NPAR1WAY), Spearman’s rank correlation coefficient (CORR) and simple linear regressions analysis were executed using the statistical softwares R v.2.14.2 (R Development Core Team 2013, http://www.rproject.org) and SAS (Institute Inc., Box 8000 Cary, NC, USA).
Results
The body size of krill ranged from 16 to 58 mm with an average of 47.1 ± 4.5 (SD) mm. From the total catch, 2 % were juveniles, 17 % sub adults and 81 % adults. The overall sex ratio of males versus females was 1:2 and was dominated by adult females at stage FIIIA (37 %) and FIIIB (19 %) (Table 1; Fig. 2). We observed reasonable large variability in mean body lengths over the study period (Figs. 2, 3). This variability was higher during the first part of the fishing period, days 29–40, compared to the latter period, days 42–61 (Fig. 3). The mean body length was positively correlated with yearday (t = 6.3771, df = 52, p value = 4.864e−08, r corr = 0.6625) being probably related to growth as the animals significantly increased in size with time. There was no overall statistical relationship between mean body length and depth of sampling (t = 1.3035, df = 52, r corr = 0.1779, p = 0.1981). However, patterns derived visually from Fig. 3 indicate periods when sampling depth becomes shallower and is seemingly associated with a slight decrease in average body size. This was particularly evident for yeardays ~30–35, ~44–52 and ~55–58. Another interesting feature revealed through these analyses is that there was a negative correlation between mean body length and associated standard deviation (t = −7.0572, df = 53, p = 3.649e−09, r corr = −0.6960), that is, the greater the mean body length, the less the spread around the mean. Hence, the main krill distribution (cf. Figs 5, 6) becomes more narrow and homogenous when the krill becomes larger later in the fishing period.
Estimated mean length (green squares) for the dominant size distribution of Euphausia superba ≥33 mm body length and depth of sampling (blue filled circles) versus year day during the study period. Sampling depth determined from the trawl sensor, considering a 10–12 min delay for the krill to travel from the cod-end to the trawl deck
Clear changes in the maturity stage composition were observed during the study period (Fig. 4). The occurrence of all the sub adult male stages decreased, in particular the occurrence of the stages MIIA2 and MIIA3. Concurrently, occurrence of both male adult stages increased (MIIIA and MIIIB), but not at the same rate so the net sum was a proportional decrease in males relative to females. The occurrence of the FIIB or FIIIA could not be explained by a linear model. The occurrence of the female stages FIIIB and FIIIC increased, while the occurrence of FIIID-FIIIE decreased, but these two stages never made up high proportions in the samples (Table 1). It is noted that the proportion of FIIIA over the course of the study did not change gradually like most of the other stages, but peaked noticeably between yeardays 45–50. From the total catch, 2 % were juveniles and no change in occurrence was found for this stage (R 2 = 0.51; p = 0.6). Differences in samples taken during day versus night hours that could suggest diel demographic separations were not found for the overall body length (GLM, F = 1.3; p = 0.3) nor male/female ratio (NPAR1WAY, F = 0.0; p = 1.0).
Proportions of krill maturity stages as a function of yearday for krill sampled off the South Orkney Islands, during January–March 2009. The lines are fitted using spline smoothing, and coefficients are output from Pearson’s product-moment correlation tests investigating the relationship between the given maturity stage and day of the year
There was a marked diel variation in the depth distribution of the krill during the investigation period with krill distributed closer to the surface during nighttime than daytime (Wilcoxon’s ranked sum test on averaged daily values, p < 0.0001; Fig. 5). During nighttime, the majority of the krill was distributed in the upper 50 m. The range in depth distribution was also higher during daytime than nighttime (Wilcoxon’s ranked sum test on daily range of occurrence, p < 0.05). During daytime, krill were recorded from the surface down to 250 m depth. The results show that krill were found in more narrow vertical swarms during daytime than nighttime, but overall spread more over the water column during daytime (p < 0.0001, Fig. 5). Furthermore, the number of vertically integrated single samples (50 pings average) with krill recordings, but with empty neighbor samples, was significantly higher during daytime (3419 of 5899) than nighttime (2258 of 6640) (Chi-squared test, χ 2 ~ 722, p < 0.0001). This strongly indicates a more patchy distribution of krill during daytime.
Depth distribution of krill recordings throughout the entire study period illustrating the diel cycle based on acoustic recordings. The diel cycle is categorized into 1-h periods, and each horizontal bar reflects the weighted vertical distribution center of acoustic recordings along a 50 ping sample period. The length of the bar is proportional to the value of the NASC (m2/nmi2). The red dots denote the mean depth distribution weighted according to the value of the NASC. The blue dots denote mean fishing depth, estimated from a CTD sensor fixed to the trawl opening. The number on the top of the panel denotes average NASC for the given 1-h period
There were no marked trends in the average daily bottom depth where the fishery was conducted (Fig. 6). However, the original bottom depth values linked to the high-resolution acoustic data shows that the highest krill abundances, NASC (m2/nmi2) where observed associated with the shelf and slope region from approximately 250–1000 m, dropping off slowly toward deeper waters and were nearly absent around 3000 m and beyond.
Depth distribution of krill recordings over the investigation period during daytime (upper panel) and nighttime (middle panel) and average daily bottom depth with standard deviation (lower panel). The investigation period is categorized into 1-day periods, and for the krill distribution plots, each horizontal bar represents the center of the vertical distribution for a 50 ping sample period, and the length of the bar is weighted according to the value of the NASC. The red dots denote the mean depth distribution, also weighted according to the value of the NASC. The blue dots denote mean fishing depth, estimated from a CTD sensor fixed to the trawl opening. Note that the data collection from the CTD started on yearday 32 and that data lack for some periods without fishing. The number on the top of the panel denotes average NASC for a given yearday. Note that the x-axis is not linear since there were days of no sampling
It is interesting to note the day-to-day variation in vertical distribution, in particular during daytime. There seemed to be no apparent trend in the depth distribution of krill over the investigation period, but there were indications of a shallower krill distribution after yearday 40 with few recordings below 150 m (Fig. 6). During the investigation period, the depth distribution was centered as high as 25 m (yearday 61) and as low as 170 m (yearday 38), and the depth range went from a unimodal distribution centered around 50 m (yearday 58) to a bimodal or almost uniform distribution spanning up to 200 m (yearday 51). During some days, the depth distribution was very similar between night and day (yearday 42–47). During nighttime, there was less variability with most recordings in the upper 75 m, with the exception of a few days with parts of the distribution also deeper down. Also the total backscatter showed a marked day-to-day variability, but no apparent trend over time (Fig. 6).
An interesting observation is the significant correlation between night abundance and subsequent day abundance of krill underneath the vessel (t = 3.1226, df = 29, r corr = 0.5016, p value = 0.00404), while no correlation was found between day and subsequent day abundance.
The nutrient analysis showed an average nitrate concentration of 24.3 ± 1.1 (SD) µmol/l, and the phosphate concentration was 1.9 ± 0.1 (SD) µmol/l. Change over time was found (p > 0.2) in neither nitrate nor phosphate concentrations. The chlorophyll a concentration at the surface waters ranged from 0.09 to 1.31 mg/m3 and decreased over time during the study period (Fig. 7). Also the phaeopigment concentration, which ranged from 0.09 to 0.41 mg/m3, decreased during the study period. The wind mixing layer was limited to approximately the upper 50 m of the water column. Temperature was reasonably stable during the period both in surface waters (10–20 m; 0.8 ± 0.2(SD) °C) and below the thermocline (90–100 m; 0.1 ± 0.3(SD) °C) (Fig. 8).
Boxplots showing the concentrations of chlorophyll a and phaeopigments at the surface (0 m) during the 7 periods of the study from January–March (P1–P7) 2009 in the waters off South Orkney Islands. The boxes indicate median values and the first and third quartiles. Whiskers extend to the most extreme data point which extends <1.5 times the length of the box. Width of the boxes is proportional to the square root of N
Discussion
The study is based on data collected from a krill fishing vessel during normal operation and demonstrates the possibilities of using such platforms for scientific purposes. It is important to keep in mind, however, that the area sampled in our study reflects the preferred fishing locations of the vessel. The area of effort was limited to a ca. 75 km strip of the shelf break on the north-west side of the South Orkney Islands (Fig. 1). The fishing vessels usually aim to maximize their profit, and even though there are several elements which potentially influence their revenue, the most obvious is predictable high concentrations of krill. High concentrations of krill in association with shelf break areas have been documented in several studies (e.g., Constable and Nicol 2002; Santora et al. 2011), and the krill fishing pattern during the last decade shows that a few areas in relation to shelf breaks are subject to a particularly high fishing effort (Nicol et al. 2012). Our results show that the depth with highest acoustic densities corresponds with the actual fishing depth, indicating that the “Saga Sea” deliberately targets the highest krill concentrations also vertically. If the intent is to estimate krill abundance over a larger region the data from the vessel are obviously biased, given that the vessel to a large extent is able to localize and follow the highest krill concentrations, limiting its immediate use for abundance estimation over the hotspot region.
The so-called “ad hoc” sampling approach from the krill fishing vessels using the continuous pumping technology has drawbacks. One of these is the limited possibility of sampling the whole vertical range of krill within the water column, since trawling basically is performed for prolonged periods in the same depth, targeting acoustic registrations of krill that are often confined to specific depth layers. However, it is demonstrated in Figs. 5 and 6 that krill could be dispersed over a 200 m depth range during certain times of day, meaning that it is still likely that a representative sample of the population could be obtained using this sampling approach at a given geographic locality. However, the highly dynamic environmental conditions, in part governed by complex bottom topography in the krill hotspot area, do suggest that frequent changes in krill abundance and vertical distribution could be a rule rather than the exception, thus favoring particular attention with respect to the interpretation of results based on such a sampling strategy.
The most pronounced signal in distribution pattern found in the present study was the diel difference, incorporating both depth distribution and vertical extension of the krill swarms. Krill diel vertical migration is variable, most certainly depending on a range of factors including latitudinal light conditions and shelf areas of the Southern Atlantic typically have marked day night differences, normally linked to anti-predatory behavior (Hamner and Hamner 2000; Klevjer et al. 2010). Deep waters provide shelter from visual predators diving from the surface, hence could explain deep daytime distributions. At nighttime, with assumingly lower pressure from visual predators, the distribution was shallower and the krill were also less compact in the vertical extension. Seals and penguins attacking from the surface have been estimated to represent around 80 % of the krill consumption in the Scotia Sea (Murphy et al. 2007). The predation pressure was not quantified in our study, but at the time of our investigations in late summer, the South Orkneys is known for a high abundance of land dependant predators, in particular penguins and Antarctic fur seals (Arctocephalus gazella) (Poncet and Poncet 1985, Lewis Smith 1988).
Despite the generally clear signal of diel variation in vertical distribution, there were some interesting exceptions where only marginal differences in depth distribution between night and day occurred. The daytime vertical distribution observed was also very variable despite the relatively small area studied and short investigation period. These patterns could be related to variable local environmental conditions such as bottom topography, currents and predator abundance within the region the vessel operated (Ritz 2000; Klevjer et al. 2010). The effect of the two latter factors could unfortunately not be assessed and averaged bottom topography showed no apparent trend or relationship to krill vertical distribution pattern. Despite this, the original high-resolution bottom topography data underline the importance of the shallow shelf and slope region, since the highest values of total integrated sA (NASC) were observed within the bottom depth range 250–1000 m thereafter being slowly reduced.
The krill abundance measured as total integrated sA (NASC) over the water column, also showed great variation over the study period, but varied in a more systematic manner than the daytime vertical distribution, with a couple of extreme values during the course of the investigations. The daytime and nighttime abundance also co-varied, indicating that krill availability to acoustic detection was similar during day and night, but there were no such relationship between consecutive days. This simple pattern could be understood from the fact that an actively fishing vessel will not move much from day to night, but the probability of being further away the consecutive day, hence experiencing a considerably change in krill distribution, abundance and physical conditions, is higher. It could also be other strategic fishing master decisions that might aid to explain this pattern, although it is beyond the scope of this investigation to open this debate. Another relevant issue is the potential for the fishing vessel to regularly be exposed to various types of currents since it most of the time operates very close to the shelf break that are most certainly a site of strong currents and changing topography that easily could affect the abundance of krill. Therefore, the level of biomass on one day is not a significant predictor of that found the next day.
During the entire investigation period, we sampled adult krill in maturity stages which indicated active mating. However, the proportion of the different stages and the time of the year indicate that the monitored krill population was past peak spawning (Fig. 4), which is also supported by findings presented in Sologub and Remeslo (2011) from a similar area and with similar timing.
Krill are expected to molt once in approximately 20–30 days at a temperature of −1 to +1 °C depending probably on food supply, season, physiological condition including degree of maturity and overall size of an animal (Buchholz 1991; Nicol et al. 1992; Brown et al. 2010). Keeping in mind that regression to previous maturity stages may occur (Thomas and Ikeda 1987), this suggests that during the approximate 1-month study we conducted in the South Orkney area in 2009, some changes in the size of the animals (cf. information on growth given by Nicol et al. 1992) and the “population” maturity stage composition are expected if it is the same population that is sampled over the course of the study period. We found trends mainly corresponding to gradual changes in maturity stage composition of the krill over the sampling period. Even though there was some station-to-station variability, the steady change observed suggests that it was the same population that was sampled over the course of the investigation period and that it went through a gradual change in demographic composition. Previous studies on demographic composition of krill have typically found large variability even if collected within the same seasons and/or area (Marr 1962; Witek et al. 1981; Fevolden and George 1984; Siegel 1986; Watkins et al. 1992; Krafft et al. 2010, 2012). The clearest change we observed was the decrease in subadult males and the decrease in overall male to female ratio over the investigation period. These results are consistent with what was presented in Sologub and Remeslo (2011) from a similar period and area. There could be several explanations for the changes that are not mutually exclusive: The male subadult component of the population may gradually disappear out of the sampled waters and/or an influx of krill to the sampled area may result in increases in particularly the female component of the sampled krill.
It has been discussed that krill segregate seasonally according to size (Nicol 2006), and the results in Watkins et al. (1992) indicated that such segregation according to demography may even occur at the swarm scale. Given that krill may have a high degree of self-locomotion, with adults maintaining swimming speeds between 10 and 15 cm/s over time scales of hours to days (Marr 1962; Kanda et al. 1982), active migration may play an important role in establishing spatial segregation in krill size classes (Siegel 1988). It is not clear whether the demographic change observed in our study was the result of segregation, but there are environmental circumstances supporting such segregation. Firstly, subadult males are not sexually active and therefore not dependent on swarming pelagically for reproductive purposes (Hamner et al. 1983), secondly, the decrease in subadult males also coincided with the decrease in chlorophyll a and phaeopigment concentrations, indicating that food availability in the upper 50 m of the surface decreased and thirdly, the predation pressure from air breathing predators like Antarctic fur seals and penguins, which are abundant in the area, is highest in the upper 200 m. An interesting possibility is that part of the krill population segregate and go down to the bottom (Schmidt et al. 2011), with the possibility of feeding on accumulated phytoplankton (see, e.g., Pape et al. 2013) and largely being sheltered from air breathing predators. However, the acoustic recordings did not indicate regular occurrences of krill in particular depth layers which could suggest a systematic segregation of one krill population component relative to others. On the other hand, these groups (subadult males) would not necessarily be detected by the echosounders, especially if they were dispersed.
An additional factor that could play a role with respect to the reduction in the proportion of subadult males is that they could have been subject to a higher mortality rate than other parts of the population. Since the krill population apparently was past peak spawning and food availability decreased over the period, a particular part of the population that could be vulnerable is the males, and especially subadults that probably have been in rapid growth have built few fat reserves and which at this stage in the spawning cycle will struggle to attain maturity. The ideas and findings by Kawaguchi et al. (2007) that “male krill grow fast and die young” might suggest that these males during this part of the season could be particularly vulnerable and exposed to increased mortality.
Although there seems to be no apparent reason for considering the observed krill stock in the region as consisting of subpopulations, it is realized that oceanographic processes as well as small-scale population characteristics can contribute to the variability observed during this study. An interesting observation is the consistent shallow sampling and shallow distribution of krill both day and night during yeardays 45–50 (Fig. 3), coinciding with a peak in the occurrence of FIIIA (adult females with no spermatophores) (Fig. 4), followed by an increase in FIIIB (adult females with spermatophores) from day 50 onwards. It is also realized that the mean length of the main size distribution becomes somewhat reduced during the same period, and a similar feature is also observed during yeardays 30–35 and 55–60, possibly associated with shallower fishing.
It is speculated that what we observed during yeardays 45–50 could be temporarily an isolated part of the population slightly unsynchronized with the broader pattern of population or reproductive development, which suddenly became accessible to sampling during this period. An interesting observation is the extremely high abundance values found on two separate occasions (yearday 37 and 47). Given that the changes in density were so sudden, they are likely not caused by flux events, but probably the result of natural variability in swarming behavior. The high variability observed both with regard to krill abundance, vertical structure and partly biological parameters, underlines the complexity of the study region.
The explanation that the change in demographic composition is due to influx, could not be investigated and verified with our data. The initial temperature data being recorded in the easternmost study region suggests that the water column here to approximately 100 m depth was the coldest, being slightly warmer as the vessel moved westwards. This could be a seasonal affect or due to change in water mass characteristics. Colder deep water could be introduced from the east along the northern slope of South Orkney by a branch of the Weddel Front originating south of the island (Heywood et al. 2004; Thompson et al. 2009). The general flow pattern in the South Orkney area is strongly influenced by the southern Antarctic Circumpolar Current (ACC) front and the southern boundary of the ACC that flows eastward very close to the edge of the continental shelf west of the Antarctic Peninsula (Orsi et al. 1995). These currents could transport krill from the Antarctic Peninsula area to the South Orkneys (Hofmann et al. 1998), while other currents could transport krill from the Weddell Sea as Siegel et al. (1990) and Sologub and Remeslo (2011) suggest as an additional advective pathway for the krill population in the South Orkney region. The South Orkney Islands are located in the Subpolar-zone north of the Weddell Gyre (Crelier et al. 2010), an area significantly influenced by the Weddell-Scotia Confluence (WSC), often located to the north of South Orkney (Patterson and Sievers 1980; Deacon 1982), and being a zone where waters from the Scotia Sea and Weddell Sea meet and interact. However, the extent of krill transport and possibly origin of the South Orkney krill population during our study period are not known. For future management purposes, these issues along with the potential for retention of the krill stock in this region remain important and should be key elements in future studies of the South Orkney krill population dynamics.
Concluding remarks
This study shows the development in krill demography and distribution in a krill hotspot area of likely high importance for the pelagic ecosystem, land-based predators, fisheries and fisheries management. A high day-to-day variability in krill distribution and density even within the small area sampled were recorded. There was also a change in krill population demography, but more gradual, in particular a decrease in subadult males and a decrease in the male to female ratio suggesting segregation, influx or selective mortality of males. This study demonstrates for the first time that fishing vessels can be employed as research platforms to produce scientifically valuable information from a krill hotspot on the variability in krill abundance, vertical structure, population-specific characteristics and some simple environmental variables. In order to further understand the dynamics in krill hotspots, it will be of high importance in future studies to investigate the mechanisms behind the observations presented here. That is how current patterns, retention mechanisms and fisheries activities affect; (1) absolute densities of krill, (2) intra-specific relationships, (3) aggregation and dispersion and (4) demography and other population characteristics. From a management perspective, it will be critical to apply such knowledge and assimilate data on population variability of such small-scale hotspots to assess consequences for the predators potentially competing with the fishery for krill and optimize survey design and sampling approaches to improve abundance estimation and quantitative information on krill flux.
References
Atkinson A, Shreeve RS, Hirst AG, Rothery P, Tarling GA, Pond DW, Korb RE, Murphy EJ, Watkins JL (2006) Natural growth rates in Antarctic krill (Euphausia superba): II. Predictive models based on food, temperature, body length, sex, and maturity stage. Limnol Ocean 51:973–987
Bendschneider K, Robinson RI (1952) A new spectrophotometric method for the determination of nitrite in seawater. J Mar Res 2:87–96
Brown M, Kawaguchi S, Candy S, Virtue P (2010) Temperature effects on the growth and maturation of Antarctic krill (Euphausia superba). Deep Sea Res II 57:672–682
Buchholz F (1991) Moult cycle and growth of Antarctic krill Euphausia superba in the laboratory. Mar Ecol Prog Ser 69:217–229
Calise L, Skaret G (2011) Sensitivity investigation of the SDWBA Antarctic krill target strength model to fatness, material contrasts and orientation. CCAMLR Sci 18:97–122
Constable AJ, Nicol S (2002) Defining smaller-scale management units to further develop the ecosystem approach in managing large-scale pelagic krill fisheries in Antarctica. CCAMLR Sci 9:117–131
Conti SG, Demer DA (2006) Improved parameterization of the SDWBA for estimating krill target strength. ICES J Mar Sci 63:928–935
Crelier AM, Dadon JR, Isbert-Perlender HG, Nahabedian DE, Daponte MC (2010) Distribution patterns of chaetognata, polychaeta, pteropoda and salpidae off South Georgia and South Orkney Islands. Braz J Ocean 58:287–298
Deacon GER (1982) Physical and biological zonation of the Southern Ocean. Deep Sea Res 29:1–15
Dickens WA, Graham AGC, Smith JA, Dowdeswell JA, Larter RD, Hillenbrand C-D, Trathan PN, Arndt JE, Kuhn G (2014) A new bathymetric compilation for the South Orkney Islands region, Antarctic Peninsula (49°–39°W to 64°–59°S): Insights into the glacial development of the continental shelf. Geochem Geophys Geosyst 15(6):2494–2514. doi:10.1002/2014GC005323
Du J (2002) Combined algorithms for fitting finite mixture distributions. MS thesis, McMaster University, Hamilton
Ettershank G (1983) Age structure and cyclical annual size changes in Antarctic krill (Euphasia superba). Polar Biol 2:189–193
Evans SR, Finnie M, Manica A (2007) Shoaling preferences in decapods crustacean. Anim Behav 74:1691–1696
Fevolden SE, George RY (1984) Size frequency pattern of Euphausia superba in the Antarctic Peninsula waters in the austral summer of 1983. J Crustac Biol 4:107–122
Foote KG, Knudsen HP, Vestnes G, MacLennan DN, Simmonds EJ (1987) Calibration of acoustic instruments for fish density estimation: a practical guide. ICES Coop Res Rep 144, ICES, Copenhagen
Gordon AL, Visbeck M, Huber B (2001) Export of Weddell Sea deep and bottom water. J Geophys Res 106(5):9005–9017
Grasshoff K (1965) On the automatic determination of phosphate, silicate and fluoride in sea water. Coun Met Int Coun Explor Sea 129:1–3
Hamner WH, Hamner PP (2000) Behaviour of Antarctic krill (Euphausia superba): schooling, foraging, and antipredatory behavior. Can J Fish Aquat Sci 57:192–202
Hamner WM, Hamner PR, Strandd SW, Gilmer RW (1983) Behaviour of Antarctic krill, Euphausia superba: Chemoreception, feeding, schooling, and molting. Science 220:433–435
Hazen EL, Suryan RM, Santora JA, Bograd SJ, Watanuki Y, Wilson RP (2013) Scales and mechanisms of marine hotspot formation. Mar Ecol Prog Ser 487:177–183
Heywood KJ, Garabato ACN, Stevens DP, Muench RD (2004) On the fate of the Antarctic Slope Front and the origin of the Weddell Front. J Geophys Res 109:1–13. doi:10.1029/2003JC002053
Hofmann EE, Klinck JM, Locarnini RA, Fach B, Murphy E (1998) Krill transport in the Scotia Sea and environs. Antarct Sci 10:406–415
Huntley ME, Niiler PP (1995) Physical control of population dynamics in the Southern Ocean. ICES J Mar Sci 52:457–468
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, cl and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Plantzen 167:191–194
Kanda K, Takagi K, Seki Y (1982) Movement of larger swarms of Antarctic krill Euphausia superba off Enderby Land during 1976–1977 season. J Tokyo Univ Fish 68:24–42
Kawaguchi S, Finley LA, Jarman S, Candy SG, Ross MS, Quetin LB, Siegel V, Trievelpiece W, Naganobu M, Nicol S (2007) Male krill grow fast and die young. Mar Ecol Prog Ser 345:199–210
Klevjer TA, Tarling GA, Fielding S (2010) Swarm characteristics of Antarctic krill Euphausia superba relative to the proximity of land during summer in the Scotia Sea. Mar Ecol Prog Ser 409:157–170
Korneliussen RJ, Ona E, Eliassen I, Heggelund Y, Patel R, Godø OR, Giertsen C, Patel D, Nornes E, Bekkvik T, Knudsen HP, Lien G (2006) The large scale survey system—LSSS. In: Proceedings of 29th Scandinavian symposium physical acoustics, 29 Jan–01 Feb 2006, Ustaoset, Norway
Krafft BA, Melle W, Knutsen T, Bagøien E, Broms C, Ellertsen B, Siegel V (2010) Distribution and demography of Antarctic krill in the Southeast Atlantic sector of the Southern Ocean during the austral summer 2008. Polar Biol 33:957–968
Krafft BA, Skaret G, Knutsen T, Melle W, Klevjer T, Søiland H (2012) Antarctic krill swarm characteristics in the Southeast Atlantic sector of the Southern Ocean. Mar Ecol Prog Ser 465:69–83
Lewis Smith RI (1988) Destruction of Antarctic terrestrial ecosystems by a rapidly increasing fur seal population. Biol Conserv 45:55–72
MacDonald P, Green P (1988) Users guide to program MIX: an interactive program for fitting mixtures of distribution. Ichthus Data Systems, Hamilton
MacDonald PDM, Pitcher TJ (1979) Age-groups from size-frequency data: a versatile and efficient method of analyzing distribution mixtures. J Fish Res Board Can 36:987–1001
MacLennan DN, Fernandes PG, Dalen J (2002) A consistent approach to definitions and symbols in fisheries acoustics. ICES J Mar Sci 59:365–369
Makarov RR, Denys CJ (1981) Stages of sexual maturity of Euphausia superba. BIOMASS 11:1–13
Marr J (1962) The natural history and geography of the Antarctic krill Euphausia superba. Discov Rep 32:33–464
Murphy EJ, Watkins JL, Trathan PN, Reid K, Meredith MP, Thorpe SE, Johnston NM, Clarke A, Tarling GA, Collins MA, Forcada J, Shreeve RS, Atkinson A, Korb R, Whitehouse MJ, Ward P, Rodhouse PG, Enderlein P, Hirst AG, Martin AR, Hill SL, Staniland IJ, Pond DW, Briggs DR, Cunningham NJ, Fleming AH (2007) Spatial and temporal operation of the Scotia Sea ecosystem: a review of large-scale links in a krill centred food web. Philos Trans R Soc Lond B Biol Sci 362:113–148
Nicol S (2006) Krill, currents, and sea ice: Euphausia superba and its changing environment. Bioscience 56:111–120
Nicol S, Stolp M, Cochran T, Geijsel P, Marshal J (1992) Growth and shrinkage of Antarctic krill Euphausia superba from the Indian Ocean sector of the Southern Ocean during summer. Mar Ecol Prog Ser 89:175–181
Nicol S, Foster J, Kawaguchi S (2012) The fishery for Antarctic krill-recent developments. Fish Fish 13:30–40
Orsi AH, Whitworth T III, Nowlin WD (1995) On the meridional extent and fronts of the Antarctic circumpolar current. Deep Sea Res I 42:641–673
Pape E, Jones DOB, Manini E, Bezerra TN, Vanreusel A (2013) c. PLoS ONE 8(4):e59954. doi:10.1371/journal.pone.0059954
Patterson SL, Sievers HA (1980) The Weddell-Scotia confluence. J Phys Oceanogr 10:1584–1610
Poncet S, Poncet J (1985) A survey of penguin breeding populations at the South Orkney Islands. Br Antarct Surv Bull 68:71–81
Quetin LB, Ross RM (2001) Environmental variability and its impact on the reproductive cycle of Antarctic krill. Am Zoo 41:74–89
R-Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL:http://www.R-project.org/
Reiss CS, Cossio AM, Loeb V, Demer DA (2008) Variations in the biomass of Antarctic krill (Euphausia superba) around the South Shetland Islands, 1996–2006. ICES J Mar Sci 65:497–508
Ritz DA (2000) Is social aggregation in aquatic crustaceans a strategy to conserve energy? Can J Fish Aquat Sci 57:59–67
Rosenberg AA, Beddington J, Basson M (1986) Growth and longevity of krill during the first decade of pelagic whaling. Nature 324:152–154
Santora JA, Sydeman WJ, Schroeder ID, Wells BK, Field JC (2011) Mesoscale structure and oceanographic determinants of krill hotspots in the California current: implications for trophic transfer and conservation. Prog Ocean 91:397–409
SC-CAMLR (2005) Report of the twenty-fourth meeting of the scientific committee (SC-CAMLR-XXIV). CCAMLR, Hobart, Australia, 647
SC-CAMLR (2009) Report of the fourth meeting of the subgroup on acoustic survey and analysis method (SG-ASAM). In: Report of the twenty-eight meeting of the scientific committee (SC_CAMLR-XXVIII/8). CCAMLR, Hobart Australia, 33
Schmidt K, Atkinson A, Steigenberger S, Fielding S, Lindsay MCM, Pond DW, Tarling GA, Klevjer TA, Allen CS, Nicol S, Achterberg EP (2011) Seabed foraging by Antarctic krill: implications for stock assessment, bentho-pelagic coupling, and the vertical transfer of iron. Limnol Oceanogr 56:1411–1428
Siegel V (1986) Structure and composition of the Antarctic krill stock in the Bransfield Strait (Antarctic Peninsula) during the Second International BIOMASS Experiment (SIBEX). Arch FishWiss 37:51–72
Siegel V (1988) A concept of seasonal variation of krill (Euphausia superba) distribution and abundance west of the Antarctic Peninsula. In: Sahrhage D (ed) Antarctic Ocean and resources variability. Springer, Berlin, pp 219–230
Siegel V (2012) Krill stocks in high latitudes of the Antarctic Lazarev Sea: seasonal and interannual variation in distribution, abundance and demography. Polar Biol 35:1151–1177
Siegel V, Loeb V (1995) Recruitment of Antarctic krill (Euphausia superba) and possible causes for its variability. Mar Ecol Prog Ser 123:45–56
Siegel V, Bergstrøm B, Strømberg JO, Schalk PH (1990) Distribution, size frequencies and maturity stages of krill, Euphausia superba, in relation to sea-ice in the northern Weddell Sea. Polar Biol 10:549–557
Sologub DO, Remeslo AV (2011) Distribution and size-age composition of Antarctic krill (Euphausia superba) in the South Orkney Island region (CCAMLR subarea 48.2). CCAMLR Sci 18:123–134
Spiridinov VA (1995) Spatial and temporal variability in reproductive timing of Antarctic krill (Euphausia superba Dana). Polar Biol 15:161–174
Tarling GA, Shreeve RS, Hirst AG, Atkinson A, Pond DW, Murphy EJ, Watkins JL (2006) Natural growth rates in Antarctic krill (Euphausia superba). I. Improving methodology and predicting intermolt period. Limnol Oceanogr 51:959–972
Tarling GA, Cuzin-Roudy J, Thorpe SE, Shreeve RS, Ward P, Murphy EJ (2007) Recruitment of Antarctic krill Euphausia superba in the South Georgia region: adult fecundity and the fate of larvae. Mar Ecol Prog Ser 331:161–179
Thomas PG, Ikeda T (1987) Sexual regression, shrinkage, re-maturation and growth of spent female Euphausia superba in the laboratory. Mar Ecol 95:357–363
Thompson AF, Heywood KJ, Thorpe SE, Renner AHH, Trasvina A (2009) Surface circulation at the tip of the Antarctic Peninsula from drifters. J Phys Ocean 39:3–26. doi:10.1175/2008JPO3995.1
Watkins JL, Buchholz F, Priddle J, Morris DJ, Ricketts C (1992) Variation in reproductive status of Antarctic krill swarms; evidence for a size-related sorting mechanism? Mar Ecol Prog Ser 82:163–174
Welshmeyer NA (1994) Fluorometric analysis of chlorophyll-α in the presence of chlorophyll b and phaeopigments. Limnol Oceanogr 39:1985–1992
Witek Z, Kalinowski J, Grelowski A, Wolnomiejski N (1981) Studies of aggregations of krill (Euphausia superba). Meeresforsch 28:228–243
Acknowledgments
This study is part of AKES (Antarctic Krill and Ecosystem Studies), which is supported by the Royal Norwegian Ministry of Fisheries and Coastal affairs, the Institute of Marine Research, the University of Bergen, the Norwegian Antarctic Research Expeditions (NARE), the Norwegian Research Council, StatoilHydro and the Norwegian Petroleum Directorate. We would also like to extend our gratitude to Aker BioMarine ASA and the crew of the “Saga Sea.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krafft, B.A., Skaret, G. & Knutsen, T. An Antarctic krill (Euphausia superba) hotspot: population characteristics, abundance and vertical structure explored from a krill fishing vessel. Polar Biol 38, 1687–1700 (2015). https://doi.org/10.1007/s00300-015-1735-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1735-7