Abstract
Microbial communities living in mosses are known to constitute early indicators of ecosystem disturbances, but little is known about their response to environmental factors in the Antarctic area. This paper presents the first major study on testate amoebae from different species of mosses occurring on King George Island (Antarctica). The objective of the study was to investigate the structure and spatial distribution of moss-dwelling testate amoebae communities and to identify the environmental factors determining the structure of their assemblages. Additionally, we compared moss-dwelling testate amoebae communities on King George Island with other isolated islands of the Antarctic. Samples were collected from different species of mosses (Polytrichastrum alpinum, Sanionia georgico-uncinata, Sanionia uncinata, and Brachythecium austrosalebrosum). Sampling was carried out three times from 17 January to 24 February 2012. The species richness and abundance of protozoa differed significantly between the stations studied with the lowest count found in Polytrichastrum and the highest in Sanionia. The total population of testate amoebae was dominated by small taxa recognised as cosmopolitan, also recorded on other islands of the maritime Antarctic. The RDA analysis showed that all variables together accounted for 84.5 % of the total variance. However, variables that significantly explained the variance in testate amoebae communities were moisture, temperature, pH, and dissolved oxygen. Further research is required to explain the impact of biotic factors influencing the presence of testate amoebae, including the abundance of bacteria, microalgae, and small metazoa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of species/environment relationships in classical ecological research has, over the past century, led to important concepts such as ecological niche and gradient. However, most studies have focused on vascular plants and animals, while microorganisms have received less attention. To understand the effect of global change on Antarctic ecosystems, knowledge of the current biogeography and ecological preferences of the composing organisms is required (Meisterfeld 1977; Vincke et al. 2004a, b; Mattheeussen et al. 2005; Anesio et al. 2009; Convey 2011; Mieczan et al. 2013).
Testate amoebae form a very sensitive group of organisms (Nguyen-Viet et al. 2007). Their short generation times make them useful indicators of environmental changes (Vincke et al. 2004a, b; Mattheeussen et al. 2005). Testate amoebae, being inherently aquatic, restructure their communities in response to environmental changes in, for example, ground water table, soil moisture, pH, concentrations of nutrients and organic matter (Mitchell et al. 2000; Bobrov et al. 2013; Mieczan et al. 2013). These microorganisms are abundant in soils, lakes, and peatlands (Tolonen et al. 1992; Mitchell et al. 2000). They produce a decay-resistant test, or shell, that protects the cell from desiccation. The shell may be proteinaceous, siliceous, or calcareous and may incorporate extraneous materials such as mineral grains, fungal hyphae, and diatoms (Odgen and Hedley 1980). Their well-defined ecological preferences in relation to important ecological variables in different type of ecosystems have made them useful in biomonitoring (Charman 1997; Mitchell et al. 2000). They are commonly used as model organisms in population ecology, ecotoxicology, and palaeoecology owing to their cosmopolitan dispersion and species-specific ecological preferences and capability to adapt to environmental changes. Testate amoebae are excellent indicators of calcite precipitation (Casper and Schönborn 1985) and heavy metal pollutants (Nguyen-Viet et al. 2007). Previous studies have shown that the abundance of each taxon, and hence the structure of communities, is controlled by a set of environmental variables. Moisture conditions have often been identified as the most important factor controlling testate amoebae community composition (Mitchell et al. 2000; Booth 2002; Kishaba and Mitchell 2005; Opravilová and Hájek 2006; Bobrov et al. 2013). However, the responses of testate amoebae species to other environmental factors such as pH and nutrients are less well known. According to Bobrov et al. (1999), Mitchell et al. (2000), Booth (2002) and Tsyganov et al. (2012), the composition of testate amoebae communities is primarily controlled by the moisture regime and to a lesser extent by pH. Mieczan (2007a, b) found that moisture conditions, pH, and the content of total organic carbon in water correlated positively with the total numbers and biomass of testate amoebae in microsites dominated by Sphagnum. In addition, Tolonen et al. (1992) found that testate amoebae were correlated with the concentration of total nitrogen. Even soil and water temperature can codetermine the distribution pattern of the testacean fauna. Testate amoebae require a minimum temperature at a specific time of the year to reproduce successfully. In addition, factors such as light, oxygen, and food availability may also affect testate amoebae communities (Charman et al. 2000). The ecology of most species is still rather poorly known although there have been a number of recent studies in several regions of the world, including Finland (Tolonen et al. 1992), Canada (Charman and Warner 1997), Western Russia (Bobrov et al. 1999), and Poland (Lamentowicz and Mitchell 2005; Mieczan et al. 2007a, b, 2012). Most ecological studies on testate amoebae were done in the Arctic habitats (Beyens et al. 1986, 1990, 1992; Tsyganov et al. 2011, 2012). By contrast, little or no attention has been given to the abundance and structure of testate amoebae in the South Shetland Islands (Antarctic Peninsula). Not investigated, however, was the distribution of testate amoebae with respect to individual moss species. In Antarctic ecosystems, studies on testate amoebae have been particularly conducted in soil environments, among others on the Bailey Peninsula (Petz 1997) and in Dronning Maud Land (Smith 1992), or in lake ecosystems and small ponds (Beyens et al. 1995). As shown by the study by Davis (1981), in polar environments, factors determining the occurrence of testate amoebae particularly include habitat humidity and temperature. It also seems that bio-geographical factors can have a substantial effect on the occurrence of testate amoebae.
Recently, a lot of attention has been given to the factors influencing geographical distribution of these protists. Most studies show that microorganisms (e.g. testate amoebae) are disseminated worldwide by wind and on the legs of birds (Smith and Wilkinson 1987). An alternative hypothesis suggests the existence of geographical barriers for larger and heavier species. In addition, hardly any data are available regarding the occurrence of the protozoans among particular species of mosses. Moss-dwelling testate amoebae have been so far studied in the region of Ile de la Possession (Crozet Archipelago, sub-Antarctica), where 83 taxa of protozoans were recorded (Vincke et al. 2004a), and in South Georgia, where the occurrence of 87 taxa of testate amoebae was observed (Vincke et al. 2006). Because numerous moss species show a considerable gradient determined by changing hydrological and trophic conditions, it seems that similar diversity should be expected in the case of testate amoebae inhabiting particular species of the plants.
The main objectives of the study were to determine: (1) whether testate amoebae assemblages showed clear preferences towards particular species of mosses, (2) which physical and chemical parameters of the habitat determined the species diversity and abundance of the microorganisms to the highest degree, (3) whether considerable differences occurred between the taxonomic composition of testate amoebae inhabiting King George Island and those inhabiting other isolated islands of the maritime Antarctic.
Materials and methods
Study site
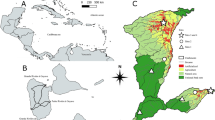
Samples were collected from different species of mosses on King George Island (South Shetland Islands, Antarctic Peninsula, 62°10′S, 58°28′W) (Fig. 1). Approximately 94 % of the island is covered with ice, and the peak of the ice cap extends to about 650 m above sea level. The island’s climate is characterised by a rapid succession of eastward moving low-pressure systems that transport relatively warm, humid air towards the coast of Antarctica (Bintanja 1995). These systems explain the relatively high annual mean temperature (2.0 °C) and humidity level (82 %) at Arctowski Station, situated on the south-eastern side of the island (Martinov and Rakusa-Suszczewski 1989). During summer, the mean temperature is well above zero, and precipitation varies from 500 mm year−1 at sea level to approximately 2,000 mm year−1 at the summit of the island (Martinov and Rakusa-Suszczewski 1989). Our study covered testate amoebae associated with moss species dominant in the vicinity of the Arctowski Polish Antarctic station. These included: the moss hummock subformation composed by Brachythecium austrosalebrosum (Müll. Hal.), occurring along rapid streams; the tall moss turf subformation with predominant Polytrichastrum alpinum (Hedw.); and the moss carpet subformation with predominant Sanionia georgico-uncinata (Müll. Hal.) and S. uncinata (Hedw.). The selected sites formed a clear gradient in terms of humidity conditions. Sanionia uncinata and S. georgico-uncinata occupied the most humid habitats. Brachythecium austrosalebrosum was in contact with running water throughout the growing seasons, while Polytrichastrum alpinum occupied periodically flooded sites with numerous drainage lines (Table 2).
Field sampling and testate amoebae analyses
Samples for testate amoebae analysis were collected from different species of mosses (P. alpinum, S. georgico-uncinata, S. uncinata, and B. austrosalebrosum). Sampling was carried out three times from 17 January to 24 February 2012. The vegetation was sampled in 0.5 × 0.5 m plots. A long knife was used to cut out plants from the vegetation. Each sample was packed into a cylindrical plastic container (10 cm in diameter), which was driven into the moss carpet and cut with a knife. During a sampling session, three samples were collected from each moss. All samples were stored in a cooler and transported within 1 day to the laboratory. Microorganisms were identified in four subsamples. Testate amoebae were isolated from moss samples using standard methods (Hendon and Charman 1997): by boiling and sieving the samples through nested sieves of 250 and 10 µm in size, mounted in glycerol, and testate amoebae were identified and counted at ×500 magnification (Zeiss Axioskop). The relative abundance of each taxon was calculated as a percentage of the total count. The abundance of microorganisms was calculated based on 1 g wet mass of plant material. Biovolumes of testate amoebae community were estimated by assuming geometric shapes and converting to carbon using the following conversion factor: 1 µm3 = 0.11 × 10−6 µg C (Gilbert et al. 1998). Quantitative sampling and counting were performed with classical limnological methods using the Utermölh (1958) technique. Morphological identifications of testate amoebae are mainly based on the works by Odgen and Hedley (1980), Charman et al. (2000), and Clarke (2003).
The moisture content of the sampled mosses was determined with reference to the F-classification of Jung (1936): FI—submerged mosses; FII—free-floating mosses, partly submerged, partly floating; FIII—very wet: water drips from sample without pressure; FIV—wet water drips after slight pressure; FV—semi-wet: water drips after moderate pressure; FVI—moist: little water produced after high pressure; FVII—semi-dry: only a few drops of water can be squeezed out; FVIII—dry: no water (Meisterfeld 1977).
Physical and chemical variables
Water samples for chemical analysis were taken simultaneously with microbial samples. Temperature, oxygen, pH, and electrical conductivity (EC) were determined in situ using a multifunction device equipped with an integrated head (CX-461, Elmetron, Poland). Total organic carbon (TOC) was determined using the multiparametric UV analyser (Secomam, France). The remaining factors (total nitrogen Ntot, total phosphorus Ptot, ammonium nitrogen N-NH4, and dissolved orthophosphates P-PO4) were analysed in the laboratory using a spectrophotometer VEGA 400 equipped with a thermoreactor (Spectroquant TR320, Merck, Germany) and test kits for rapid chemical analysis (LCK 304 ammonium, LCK 349 phosphate). Concentrations of total phosphorous and dissolved orthophosphates were determined using a spectrophotometric method (with molybdate method after mineralisation with a mixture of HNO3 and H2SO4), and concentrations of ammonium nitrogen were analysed using Niessler’s method and those of total nitrogen using Kjeldahl’s method (Golterman 1969).
Statistical analyses
Diversity analysis (Shannon–Wiener diversity index, log10-based) was performed using the Multivariate Statistical Package MVSP (2002, Kovach Computing Services). Full-factorial ANOVA was used to test for significant effects of the species of mosses and the time on testate amoebae species richness and abundance. Ordination techniques were used to describe relationships between the abundance of testate amoebae in different mosses and environmental variables (Ter Braak 1988, 1994; Lepš and Šmilauer 2003). The length of the gradient indicated by a de-trended correspondence analysis of amoebae abundance was 2.62 SD, which suggests that principal component analysis (PCA) and redundancy analysis (RDA) are appropriate methods (Ter Braak 1994). PCA was performed in order to specify separation of species among moss habitats. RDA was used to recognise the most important environmental variables, which determine abundances of testate amoebae in distinct habitats. During the analysis, factors such as N-NH4, P-PO4, and Ptot showed negligible variations, so they were omitted in the further procedure. The ordination analyses were performed using CANOCO 4.5 for Windows.
The Monte Carlo permutation test was used to clarify significant variables (Lepš and Šmilauer 2003). The variables whose level of significance exceeded P = 0.05 were presented on the plots. All testate amoebae data were log + 1 transformed to normal distribution. The analysis was performed using CANOCO 4.5 for Windows.
Results
Testate amoebae species richness and abundance
A total of 15 testate amoebae species were identified in the sample (Table 1). The highest species richness (15 taxa) occurred in microsites dominated by S. uncinata. In habitats dominated by B. austrosalebrosum and S. georgico-uncinata from 11 to 13, testate amoebae taxa were noted. Decidedly lower numbers of taxa (8) were observed in P. alpinum. From 6 to 12, species were counted per sample (Table 1). The most frequently occurring species were Trinema lineare, Corythion dubium, and Centropyxis aerophila. The species distribution pattern also showed a higher variation in acid habitats, from wet assemblages with Trinema lineare to Corythion dubium assemblages in drier microhabitats. The species richness alone differed significantly (Mann–Whitney test with Bonferroni correction for multiple comparisons; P < 0.05) between mosses. The diversity analysis revealed a mean Shannon–Wiener diversity index (H) of 2.45. The highest diversity was measured in S. uncinata (H = 2.8), and the lowest was observed in P. alpinum (H = 0.65). Testate amoebae abundances were also significantly related to the moss species, with the lowest numbers found in P. alpinum (9 ± 2 ind g−1) and the highest in S. uncinata (98 ± 6 ind g−1) (ANOVA, F = 18.5, P = 0.001). Testate amoebae biomass, corresponding with abundances, was significantly higher in microsites dominated by S. uncinata (473 ± 11 µg C g−1) than in microsites dominated by Polytrichastrum (134 ± 7 µg C g−1) (ANOVA, F = 21.2, P = 0.001). The community composition of testate amoebae varied greatly between microhabitats. T. lineare, Centropyxis aerophila and Nebela lageniformis dominated in B. austrosalebrosum, S. georgico-uncinata, and S. uncinata. In Polytrichastrum, the community was predominantly composed of C. dubium.
Environmental variables
Water temperature varied among sites and samples, ranged from −0.05 to 2.5 °C (ANOVA, F = 16.5, P = 0.001). Statistically significant differences among the studied habitats were found for electrical conductivity, Ptot, Ntot, N-NH4, and P-PO4 (ANOVA, F = 14.21–16.22, P = 0.001). In the samples, the pH gradient ranged from 3.4 to 5.2 with a electrical conductivity ranged of 6.5–9.8 μS cm−1 and TOC ranged of 5.2–7.5 mg C l−1. Concentrations of Ptot and P-PO4 were highest in the microhabitats dominated by Sanionia and B. austrosalebrosum; however, the remaining parameters (conductivity, Ntot, and N-NH4) showed the highest values in the sites dominated by P. alpinum. Descriptions of the sampling sites with coordinates, parameters measured, and identified moss species are given in Table 2.
Species–environment correlations
In the PCA diagram, the first two axes separated testate amoebae among moss habitats. Axis 1 (λ = 0.63) and Axis 2 (λ = 0.088) accounted for 71.9 % of the total variance in the amoebae-related data. Their abundances were most strongly correlated with the main direction of variation (Axis 1), with samples collected from P. alpinum. That habitat was clearly separated in the ordination diagram, and the amoebae typical of the habitat were the genera of Euglypha, Amphitrema sp., Assulina muscorum, and Difflugia bryophila (Fig. 2a, b).
Principal components analysis (PCA) for axes 1 and 2 showing: a species communities of testate amoebae and b habitats of mosses. Samples collected are marked with symbols: circle Sanionia uncinata, Triangle Sanionia georgico-uncinata, square Brachythecium austrosalebrosum, diamond Polytrichastrum alpinum. Species codes: Amp.sp—Amphitrema sp., Ass.mus—Assulina muscorum, Arc.are—Arcella arenaria, Cen.aer—Centropyxis aerophila, Cor.dub—Corythion dubium, Dif.bry—Difflugia bryophila, Dif.pul—Difflugia pulex, Eug.com—Euglypha compressa, Eug.lae—Euglypha laevis, Eug. rot—Euglypha rotunda, Eug.str—Euglypha strigosa, Eug.tub—Euglypha tuberculata, Neb.col—Nebela collaris, Neb.lon—Nebela lageniformis, Tri.lin—Trinema lineare
The direct relationships between abundance of testate amoebae and environmental variables were specified using redundancy analysis (RDA). Axis 1 (λ = 0.567) accounted for 84.5 % of total variance in community structure and showed high correlation with F (moisture) and pH. Axis 2 (λ = 0.006) only accounted for 8.8 % of total variance in amoebae species structure. The Monte Carlo permutation test showed the significance of three variables: moisture (λ = 0.51, F = 35.64, P = 0.002), O2 (λ = 0.05, F = 3.95, P = 0.004), and pH (λ = 0.03, F = 2.7, P = 0.01). On the ordination plot, the direct effect of the variables on the abundance of the studied communities can be seen; as a result, the habitats studied are distributed separately on the ordination diagram, with samples collected from P. alpinum being located in the right part of the ordination space along with increasing values of F and pH, and B. austrosalebrosum placed in the top left part along with decreasing values of temperature and O2. All the species typical of the habitat of P. alpinum corresponded with high gradient of moisture (F = VI–VII) and pH, while Arcella arenaria and T. lineare species showed a positive relation with the rising gradient of O2 (Fig. 3a, b).
Redundancy analysis (RDA) biplots for spatial distribution of testate amoebae showing a studied communities and environmental variables, b samples collected in distinct habitats and environmental variables. Arrows marked as bolded indicate significant parameters in Monte Carlo permutation test at P < 0.05. Sample and species codes—see Fig. 2
Discussion
Testate amoebae communities on mosses
In the study testate amoebae species, richness was significantly related to the type of microenvironment. The highest richness occurred in habitats dominated by B. austrosalebrosum, S. uncinata, and S. georgico-uncinata. Decidedly lower numbers of taxa were observed in P. alpinum. The especially low variety of testate amoebae among Polytrichastrum is probably a consequence of the low level of moisture in this microenvironment. In the present study, the occurrence of from 3 to 14 species of testate amoebae was ascertained in a single species of moss. Vincke et al. (2004a) ascertained the occurrence of 83 species of testate amoebae among Sanionia and Racomitrium moss species in 109 samples. By comparison, in a study on moss-dwelling testate amoebae, Vincke et al. (2006) recorded a total of 71 species. However, this represented 24 different moss species growing on a range of substrates. Matsuda (1968) studied testate amoebae communities colonising the Bryum moss and recorded 12 species in Syowa Station, Antarctica. Such a low diversity may be attributed to the extremely dry conditions of the sampled habitat. The number of species of testate amoebae increased along with increasing moisture conditions. This compares well to other studies. Vincke et al. (2004a) and Mieczan et al. (2012) observed a clear growth in the variety of species testate amoebae in mosses located in the wettest habitats. Warner (1987) found that an increase in species diversity was connected to an increase in the moisture content of the habitat. Leaving the aquatic moss samples (FII) aside, Werner's observation was confirmed by our study. Species richness and abundance of testate amoebae were highest in moss samples with FIII moisture values and decreased towards FVII mosses.
Environmental controls on testate amoebae communities
According to the study results, testate amoebae respond to major environmental gradients on King George Island the same as in other parts of the world (Beyens et al. 1986). The strongest correlation was found between testate amoebae assemblages and both humidity and pH. In Sphagnum peatlands, testate amoebae assemblages were related to Ptot and Ntot (Mitchell et al. 2000; Jauhiainen 2002), and to a combination of physical variables, e.g. moisture and chemical variables such as pH, N, and DOC (Tolonen et al. 1994; Opravilová and Hájek 2006). Nutrients seem to indirectly determine the prevalence of testate amoebae through the control of food abundance (mainly bacteria, fungi, or protists). The RDA analysis showed that humidity and pH were the dominant factors controlling distribution patterns in testate amoebae assemblages and that the total variance by each RDA was similar. This is reflected in an increase in the contribution of species typical of relatively dry environments. The present study shows a significant correlation between testate amoebae and the type of microenvironment. In microhabitats dominated by S. uncinata and S. georgico-uncinata, amoebae species, such as T. lineare, A. arenaria, N. collaris, and D. bryophila, were recorded in high numbers. Habitats dominated by B. austrosalebrosum and P. alpinum were mostly colonised by testate amoebae located at the dry end of the water table gradient, such as C. dubium and E. tuberculata. Some researches refer to T. lineare and A. arenaria as xerophilous species (Bobrov et al. 2002; Payne and Mitchell 2007). This might indicate that these species have wide ecological preferences and can be found in both dry and moist biotopes. According to research by Vincke et al. (2004a), T. lineare is a cosmopolitan species occurring both in surface waters and soil. Genus Difflugia has also been relatively frequently recorded in various types of aquatic ecosystems. Nebela is particularly related to humid microhabitats dominated by mosses (Vincke et al. 2006). C. dubium is usually found in dry microhabitats and is frequently recognised as an acidophilic species (Bonnet 1981). Euglypha is described as a eurytopic species, relatively frequently occurring in the soil (Vincke et al. 2004a). Moreover, in the scope of the present study, irrespective of the microhabitat, a slight contribution of testate amoebae taxa containing zoochlorellae was observed (e.g. Amphitrema). This may be related not only to the microhabitat conditions but also to the high UV radiation observed in the area. This factor can substantially affect the abundance of testate amoebae. Taxa containing zoochlorellae are particularly sensitive (Searles et al. 2001). Similar patterns were also observed in other climatic zones, e.g. in the Rocky Mountains in North America (Booth and Zygmunt 2005). Temperature is another factor likely to substantially influence testate amoebae succession. According to Charman et al. (2000), the growth and reproduction of freshwater testate amoebae were strongly correlated with temperature. On King George Island, temperature had a significant influence on the number of testate amoebae. Many testate amoebae occur in waters with a broad temperature range or are eurythermic, and higher temperature usually causes an abundant growth of protists.
Moss-dwelling testate amoebae on islands of the Antarctic
The species diversity of testate amoebae (15 taxa) was comparable with the number of taxa recorded in the areas of the continental Antarctic (Smith 1992), although it was substantially lower than that recorded on other islands of the maritime Antarctic (Vincke et al. 2006) (Table 3). Research conducted by Vincke et al. (2006) showed the presence of 71 taxa of the protozoans in the area of South Georgia. Research conducted in sub-Antarctic Ile de la Possession (Crozet Archipelago) shows considerably higher species diversity of testate amoebae (88 taxa + 34 non-identified taxa) (Vincke et al. 2004a). Todorov and Golemansky (1996) recorded the occurrence of 24 taxa of testate amoebae on Livingston Island, South Shetland Islands. Such considerable differences in taxonomic richness between King George Island and South Georgia can among other things result from the fact that the testate amoebae were analysed in a higher number of moss species. On the other hand, some of the study sites on King George Island were located in the zone of periodical contact with marine waters, flowing into a part of the moss carpet in strong onshore winds. Such extreme conditions could also result in a decrease in the species diversity of the microorganisms. The dominance structure of amoebae is very similar between, among other islands, King George Island, South Georgia, and Ile de la Possession. The dominant species are T. linare and C. aerophila, constituting from 4 to 45 % of the total numbers. Trinema linare reached 17 % on South Georgia and 32 % on Ile de la Possession (Vincke et al. 2004a). On South Georgia, the contribution of Centropyxis is increased. This variety may result from the habitat preferences of the species. Trinema occurs frequently in dry and wet habitats, and Centropyxis is recognised as a hydrophilic species (de Graaf 1956). A high contribution was also reached by C. dubium. This species (r-strategist) was relatively frequently observed in microhabitats dominated by mosses in the area of the maritime Antarctic (Convey 1996). It was recorded, among other sites, in microhabitats dominated by Cephaloziella varians on the Bailey Peninsula (Wilkes Land) or Adelaide Island (Newsham 2010), and on Livingston Island (South Shetland Islands) (Todorov and Golemansky 1996). Therefore, irrespective of the geographical distance between sub-Antarctic islands, the species composition of testate amoebae is relatively similar. Differences in the taxonomic richness itself may result from local microhabitat conditions (such as physical and chemical parameters of a habitat, or moss species). Moreover, the study area is dominated by small-sized taxa (frequently below 100 µm), often recognised as cosmopolitan. According to the theory by Wilkinson (2001), small taxa migrate considerably more easily and colonise a much higher number of habitats.
Conclusions
In view of the above, it seems that in polar environments, the relationship between testate amoebae and species of mosses does not necessarily imply a direct ecological link between the two types of organisms, but is explained by the fact that the humidity conditions of the environment primarily define the niches of the moss species. Irrespective of the distribution of testate amoebae, their abundance was largely limited by humidity, temperature, and pH, as well as by dissolved oxygen to a somewhat lesser degree. The total population of testate amoebae was dominated by small taxa recognised as cosmopolitan, also recorded on other islands of the maritime Antarctic. Such data are used to determine the habitat preferences of testate amoebae and the biogeography of the organisms. The full analysis of the effect of environmental factors on the distribution of testate amoebae indicates a need for future research, also involving the identification of trophic factors, which may determine the occurrence of the organisms, among other things, the abundance of algae, bacteria, flagellates, or ciliates.
References
Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B (2009) High microbial activity on glaciers: importance to the global carbon cycle. Glob Chang Biol 15:955–960. doi:10.1111/j1365-2486.2008.01758
Beyens L, Chardez D, Delandtsheer R, Debock P, Jacques E (1986) Testate amoebae populations from moss and lichen habitats in the Arctic. Polar Biol 5:165–173. doi:10.1007/BF00441696
Beyens L, Chardez D, de Baere D, Jacques E (1990) Ecology of terrestrial testate amoebae assemblages from coastal lowlands on Devon Island (NWT, Canadian Arctic). Polar Biol 10:431–440. doi:10.1007/BF00233691
Beyens L, Chardez D, Baere D, de Bock P (1992) The testate amoebae from the Søndre Strømfjord region (West-Greenland): their biogeographic implications. Arch Protistenkd 142:5–13. doi:10.1016/S0003-9365(11)80092-8
Beyens L, Chardez D, de Baere D, Verbruggen C (1995) The aquatic testate amoebae fauna of the Stomness Bay area, South Georgia. Antarct Sci 1:3–8. doi:10.1017/S0954102095000022
Bintanja R (1995) The local surface energy balance of the Ecology Glacier, King George Island, Antarctica: measurements and modelling. Antarct Sci 3:315–325. doi:10.1017/S0954102095000435
Bobrov AA, Charman DJ, Warner BG (1999) Ecology of testate amoebae (Protozoa: Rhizopoda) on peatlands in western Russia with special attention to niche separation in closely related taxa. Protistol 150:125–136. doi:10.1016/S1434-4610(99)70016-7
Bobrov AA, Charman DJ, Warner BG (2002) Ecology of testate amoebae from oligotrophic peatlands: specific features of polytypic and polymorphic species. Biol Bull 29:605–617
Bobrov AA, Wetterich S, Beermann F, Schneider A, Kokhanova L, Schirrmeister L, Pestryakova LA, Herzschuh U (2013) Testate amoebae and environmental features of polygon tundra in the Indigirka lowland (East Siberia). Polar Biol 36:857–870. doi:10.1007/s00300-013-1311-y
Bonnet L (1981) Thecamoebiens (Rhizopoda, Testacea). C.F.F.R.A. Biol Sols 48:23–32
Booth RK (2002) Testate amoebae as paleoindicators of surface-moisture changes on Michigan peatlands: modern ecology and hydrological calibration. J Paleolimnol 28:329–348. doi:10.1023/A:1021675225099
Booth RK, Zygmunt JR (2005) Biogeography and comparative ecology of testate amoebae inhabiting Sphagnum-dominated peatlands in the Great Lakes and Rocky Mountain regions of North America. Divers Distrib 11:577–590. doi:10.1111/j.1366-9516.2005.00154.x
Casper SJ, Schönborn W (1985) Difflugia limnetica (Levander) Penard (Protozoa: Testacea) as indicator organism of calcite precipitation in Lake Stechlin, GDR. Arch Protistenkd 130:305–311. doi:10.1016/S0003-9365(85)80041-5
Charman DJ (1997) Modelling hydrological relationships of testate amoebae (Protozoa: Rhizopoda) on New Zealand peatlands. J R Soc N Z 27:465–483. doi:10.1080/03014223.1997/9517549
Charman DJ, Warner BG (1997) The ecology of testate amoebae (Protozoa: Rhizopoda) in oceanic peatlands in Newfoundland, Canada: modeling hydrological relationships for paleoenvironmental reconstruction. Ecoscience 4:555–562
Charman DJ, Hendon D, Woodland W (2000) The identification of testate amoebae (Protozoa: Rhizopoda) in peats. Quatenary Research. Technical Guide
Clarke KJ (2003) Guide to the identification of soil protozoa: testate Amoebae. Freshwater Biological Association, UK
Convey P (2011) Antarctic terrestrial biodiversity in a changing world. Polar Biol 34:1629–1641. doi:10.1007/s00300-011-1068-0
Davis RC (1981) Structure and function of two Antarctic terrestrial moss communities. Ecol Monogr 51:125–143. doi:10.2307/2937260
De Graaf F (1956) Studies on Rotatoria and Rhizopoda from the Netherlands. Biol J Dodone 23:145–217
Gilbert D, Amblard C, Bourdier G, Francez AJ (1998) The microbial loop at the surface of a peatland: structure, functioning and impact of nutrients inputs. Microb Ecol 35:89–93
Golterman HL (1969) Methods for chemical analysis of freshwaters. Blackwell Scientific Publications, Oxford
Hendon D, Charman DJ (1997) The preparation of testate amoebae (Protozoa: Rhizopoda): samples from peat. Holocene 7:199–205. doi:10.1177/09596836970070020
Jauhiainen S (2002) Testacean amoebae in different types of mire following drainage and subsequent restoration. Eur J Protistol 38:59–72. doi:10.1078/0932-4739-00748
Jung W (1936) Thekamőben ursprünglicher lebender deutscher Hochmoore. Abh Landesmus Provinz Mus Naturkunde 7:1–87
Kishaba K, Mitchell EAD (2005) Changes in testate amoebae (Protists) communities in a small raised bog: a 40-year study. Acta Protozool 44:1–12
Lamentowicz M, Mitchell EAD (2005) The ecology of testate amoebae (Protists) in Sphagnum in north-western Poland in relation to peatland ecology. Microb Ecol 50:48–63. doi:10.1007/s00248-004-0105-8d
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. University Press, Cambridge
Martinov V, Rakusa-Suszczewski S (1989) Ten years of climate observations at the Arctowski and Bellingshausen Station (King George Islands, South Shetlands, Antarctis. In: Breymeyer A (ed) Global change regional research centres: scientific problems and concept developments, PAS Warsaw, pp 80–90
Matsuda T (1968) Ecological study of the moss community and microorganisms in the vicinity of Syowa Station, Antarctica. JARE Sci Rep 1:29–58
Mattheeussen R, Ledeganck P, Vincke S, van de Vijver B, Nijs I, Beyens L (2005) Habitat selection of aquatic testate amoebae communities on Qeqertarsuq (Disko Island), west Greenland. Acta Protozool 44:253–263
Meisterfeld R (1977) Die horizontale und vertikale Verteilung der Testacean (Rhizopoda: Testacea) in Sphagnum. Arch Hydrobiol 79:319–356
Mieczan T (2007a) Epiphytic protozoa (Testate amoebae, Ciliates) associated with Sphagnum in peatbogs: relationship to chemical parameters. Pol J Ecol 55:79–90
Mieczan T (2007b) Seasonal patterns of testate amoebae and ciliates in three peatbogs: relationship to bacteria and flagellates (Poleski National Park, Eastern Poland). Ecohydrol Hydrobiol 1:295–305. doi:10.1016/S1642-3593(07)70191-X
Mieczan T, Tarkowska-Kukuryk M, Bielańska-Grajner I (2012) Hydrochemical and microbiological distinction and function of ombrotrophic peatland lagg as ecotone between Sphagnum peatland and forest catchment (Poleski National Park, eastern Poland). Ann Limnol Int J Limnol 48:323–336. doi:10.1051/limn/2012022
Mieczan T, Górniak D, Świątecki A, Zdanowski M, Tarkowska-Kukuryk M (2013) The distribution of ciliates on Ecology Glacier (King George Island, Antarctica): relationships between species assemblages and environmental parameters. Polar Biol 36:249–258. doi:10.1007/s00300-012-1256-6
Mitchell EAD, Buttler A, Grosvernier Ph, Hydin H, Albinsson C, Greenup AL, Heijmans MMPD, Hoosbeek MR, Saarinen T (2000) Relationships among testate amoebae (Protozoa), vegetation and water chemistry in five Sphagnum-dominated peatlands in Europe. Res New Phytol 145:95–106. doi:10.1046/j.1469-8137.2000.00550.x
MVSP (2002) Multivariate statistical package. Kovach Computering Services
Newsham KK (2010) The biology and ecology of the liverwort Cephaloziella varians in Antarctica. Antarct Sci 2:131–143. doi:10.1017/S0954102009990630
Nguyen-Viet H, Gilbert D, Mitchell EAD, Badot PM, Bernard N (2007) Effects of experimental lead pollution on the microbial communities associated with Sphagnum fallax (Bryophyta). Microb Ecol 54:232–241. doi:10.1007/s00248-006-9192-z
Odgen CG, Hedley RH (1980) An atlas of freshwater testate amoebae. British Museum of Natural History and Oxford University Press, London and Oxford
Opravilová V, Hájek M (2006) The variation of testacean assemblages (Rhizopoda) along the complete base-richness gradient in fens: a case study from the western Carpathians. Acta Protozool 45:191–204
Payne RJ, Mitchell EAD (2007) Ecology of testate amoebae from mires in the central Rhodope Mountains, Greece and development of a transfer function for palaeohydrological reconstruction. Protist 158:159–171. doi:10.1016/j.protis.2006.11.003
Petz W (1997) Ecology of the active soil microfauna (Protozoa, Metazoa) of Wilkes Land, east Antarctica. Polar Biol 18:33–44. doi:10.1007/s003000050156
Searles PS, Kropp BR, Flint SD, Caldwell MM (2001) Influences of solar UV-B radiation on peatland microbial communities of southern Argentina. New Phytol 152:213–221. doi:10.1046/j.0028-646X.2001.00254.x
Smith HG (1992) Distribution and ecology of the testate rhizopod fauna in continental Antarctic zone. Polar Biol 12:629–634. doi:10.1007/BF00236985
Smith HG, Wilkinson DM (1987) Biogeography of testate rhizopods in the southern temperate and Antarctic zoes. In: Colloque sur les ecosystems terrestres subantarctique. Paimpont 1986. CNFRA 58:83–96
Ter Braak CJF (1988) CANOCO: a FORTRAN program for canonical community ordination by [partial] [detrended] [canonical] correspondence analysis, principal components analysis, and redundancy analysis (version 2.1). Report LWA-88-02, Wagenigen: Agricultural Mathematics Group. 95
Ter Braak CJF (1994) Canonical community ordination: part I: basic theory and linear methods. Ecoscience 1:127–140
Todorov M, Golemansky V (1996) Notes on testate amoebae from Livingston Island, South Shetland Islands, Antarctic. Bulg Antarct Res Life Sci 1:70–81
Tolonen K, Warner BG, Vasander H (1992) Ecology of testaceans (Protozoa: Rhizopoda) in mires in southern Finland: I. Autecology. Arch Protistenkd 142:119–138. doi:10.1016/S0003-9365(11)80076-X
Tolonen K, Warner BG, Vasander H (1994) Ecology of testaceans (Protozoa, Rhizopoda) in mires in Southern Finland. 2. multivariate-analysis. Arch Protistenkd 144:97–112. doi:10.1016/S0003-9365(11)80230-7
Tsyganov AN, Nijs I, Beyens L (2011) Does climate warming stimulate or inhibit soil protist communities? A test on testate amoebae in high-arctic tundra with free-air temperature increase. Protist 162:237–248. doi:10.1016/j.protis.2010.04.006
Tsyganov AN, Aerts R, Nijs I, Cornelissen JHC, Beyens L (2012) Sphagnum-dwelling testate amoebae in subarctic bogs and more sensitive to soil warming in the growing season than in winter: the results of 8-year field climate manipulations. Protist 163:400–414. doi:10.1016/j.protis.2011.07.005
Utermölh H (1958) Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt Internat Verein de Limnol 9:1–38
Vincke S, Gremmen N, Beyens L, van de Vijver B (2004a) The moss dwelling testacean fauna of Île de Possession. Polar Biol 27:753–766. doi:10.1007/s00300-004-0655-8
Vincke S, Ledeganck P, Beyens L, van de Vijver B (2004b) Soil testate amoebae from sub-Antarctic Iles Crozet. Antarct Sci 16:165–174. doi:10.1017/S0954102004001993
Vincke S, van de Vijver B, Gremmen N, Beyens L (2006) The moss dwelling testacean fauna of the Stromness Bay (South Georgia). Acta Protozool 45:65–75
Warner BG (1987) Abundance and diversity of testate amoebae (Rhizopoda, Testacea) in Sphagnum peatlands in south-western Ontario, Canada. Arch Protistenkd 133:270–275. doi:10.1016/S0003-9365(87)80051-9
Wilkinson DM (2001) What is the upper size limit for cosmopolitan distribution in free-living microorganisms? J Biogeogr 28:258–291. doi:10.1046/j.1365-2699.2001.00518.x
Acknowledgments
We thank our colleagues Marta Nieckarz and Adam Latusek for their invaluable help and technical assistance during collection of the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mieczan, T., Adamczuk, M. Ecology of testate amoebae (Protists) in mosses: distribution and relation of species assemblages with environmental parameters (King George Island, Antarctica). Polar Biol 38, 221–230 (2015). https://doi.org/10.1007/s00300-014-1580-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1580-0