Abstract
A growing body of evidence suggests that ocean acidification acting synergistically with ocean warming alters carbonate biomineralization in a variety of marine biota. Magnesium often substitutes for Ca in the calcite skeletons of marine invertebrates, increasing their solubility. The spatio-environmental distribution of Mg in marine invertebrates has seldom been studied, despite its importance for assessing vulnerabilities to ocean acidification. Because pH decreases with water depth, it is predicted that levels of Mg in calcite skeletons should also decrease to counteract dissolution. Such a pattern has been suggested by evidence from echinoderms. Data on magnesium content and depth in Arctic bryozoans (52 species, 103 individuals, 150 samples) are here used to test this prediction, aided by comparison with six conceptual models explaining all possible scenarios. Analyses were based on a uniform dataset spanning more than 200 m of coastal water depth. No significant relationship was found between depth and Mg content; indeed, the highest Mg content among the analyzed taxa (8.7 % mol MgCO3) was recorded from the deepest settings (>200 m). Our findings contrast with previously published results from echinoderms in which Mg was found to decrease with depth. The bryozoan results suggest that ocean acidification may have less impact on the studied bryozoans than is generally assumed. In the broad context, our study exemplifies quantitative testing of spatial patterns of skeletal geochemistry for predicting the biological effects of environmental change in the oceans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocean acidification is a decrease in pH in the oceans caused by the uptake of anthropogenic CO2 from the atmosphere (e.g., Orr et al. 2005) and is recognized to have negative effects on many groups of planktonic and benthic marine invertebrates with calcareous skeletons (for reviews see Hoegh-Guldberg et al. 2007; Fabry 2008; Pörtner 2008; Fabry et al. 2008; Rost et al. 2008; Doney et al. 2009; Pelejero et al. 2010). It may have a biotic impact at various levels, from cells to larvae, mature individuals, populations and entire ecosystems/regions. These effects may be expressed as decreases or increases in calcification rate, skeletal thinning and dissolution, decrease in abundance, recruitment and survival rates, and alteration in reproductive, metabolic, growth or physiological traits (e.g., Kleypas et al. 1999; Riebesell et al. 2000; Gazeau et al. 2007; Dupont et al. 2008, 2010; Kurihara 2008; Kuffner et al. 2008; Wood et al. 2008, 2011; Clark et al. 2009; de Moel et al. 2009; Findlay et al. 2010a).
Ruttimann (2006) predicted that a decline in coccolithophore production caused by ocean acidification could affect climate, contributing to global climate warming by decreasing the Earth’s albedo. There are indications that the processes of ocean acidification and ocean warming are closely linked and even synergistic (e.g., Rodolfo-Metalpa et al. 2010). However, the effect of elevated CO2 on marine biota is still being assessed and debated (e.g., Martin et al. 2008; Nienhuis et al. 2010). A number of studies using short-term experimental conditions to simulate long-term effects have lacked acclimatization time and multigenerational adaptations to the environment (as has recently been shown to occur in a species of coccolithophore, Lohbeck et al. 2012), causing ‘shock responses’ that may not apply in nature. Therefore, it is necessary to search for ‘natural proxies’ to test predictions related to ocean acidification and warming.
Most previous studies of the effects of ocean acidification on invertebrates have concerned corals, molluscs, echinoderms or crustaceans, with relatively few focusing on bryozoans (cf. Martin et al. 2008; Smith 2009; Rodolfo-Metalpa et al. 2010; Lombardi et al. 2011a, b; Loxton et al. 2013). Like echinoderms, corals and pteropods, bryozoans have calcareous skeletons potentially vulnerable to dissolution (e.g., Kukliński and Taylor 2009; Smith 2009; and literature cited therein). Their diversity (at least 6,000 species), abundance, habitat-creating abilities and significant contribution to the carbonate budget since the Ordovician (e.g., Taylor and Allison 1998) underline the importance of bryozoans among benthic organisms (Gordon et al. 2009).
Magnesium in the form of magnesium carbonate (magnesite) is a common component of many marine invertebrates producing shells and skeletons of calcite, including echinoderms, corals, molluscs and bryozoans (e.g., Lowenstam and Weiner 1989; Ries 2004; Stolarski et al. 2007; Lombardi et al. 2008; Ries et al. 2009; Taylor et al. 2009). The solubility of calcite increases with increase in mol% MgCO3 (e.g., Morse et al. 2006; Andersson et al. 2009), to the extent that calcite containing a high proportion of MgCO3 is even more soluble than aragonite (e.g., Andersson et al. 2009), the other common calcium carbonate biomineral which is generally regarded as being especially prone to dissolution. Quantitative spatial patterns of Mg contents in calcium carbonate biomineralizers, including bryozoans, have seldom been investigated (but see, for example, Smith et al. 2006; Kukliński and Taylor 2008, 2009).
With respect to carbonate minerals, seawater saturation state is lower in polar than in temperate and tropical latitudes (e.g., Andersson et al. 2008) because calcium carbonate is more soluble in cold water. It is also recognized worldwide that pH and CO2 levels change with water depth (e.g., Bathurst 1975; Palmer et al. 1998; Palmer 2009, fig. P25). For example, Anderson et al. (2010) noted a decrease in pH of about 0.4 to a minimum of 7.6 in the upper 200 m of Arctic shelf depths, which is equivalent to the global changes in oceanic pH projected by the year 2100 (Caldeira and Wickett 2003, fig. 1), and undersaturation with respect to aragonite at greater depths. Thus, depth gradients in skeletal magnesium content might be anticipated, with Mg declining with depth in order to counteract the threat of dissolution. By comparing skeletal magnesium content and depth, it should be possible to investigate mineralogical responses of organisms living in different pH regimes.
Among echinoderms, a bathymetric gradient has been recognized in crinoids that matched the prediction of declining Mg with depth (Roux et al. 1995; Kroh and Nebelsick 2010, fig. 9), while Sewell and Hofmann (2011) examined the effect of depth-related processes on the skeletal magnesium content of echinoids and their test thickness. Although the echinoid study focused only on the deep environments of the Antarctic region, the crinoid data came from two sources (Clarke and Wheeler 1922; Roux et al. 1995), both showing a trend of decreasing Mg with depth down to over 1,000 m. Additionally, Lowenstam and Rossman (1975) found a decrease in silica and phosphate content with water depth in holothurian dermal sclerites, clearly marked even at shelf depths. Amini and Rao (1998) found a decrease in Mg content within carbonate bulk samples along a depth gradient on the Tasmanian temperate shelf. On the other hand, Ponder and Glendinning (1974) suggested that one foraminifera species responded to depth increase by slightly decreasing the Mg content of its test, whereas others showed no trend.
Sea ice retreat in the central Arctic Ocean due to warming will lead to higher pCO2 values in the surface waters and reduced CaCO3 saturation states on the shelf zone (see Fabry et al. 2009). Therefore, we here focus on the shelf biota of the Arctic, which is particularly susceptible to rising atmospheric CO2. We evaluate the potential vulnerability of Arctic marine benthic communities to acidification and warming using the carbonate skeletons of bryozoans as a model. Bryozoans represent one of the best model groups in the Arctic due to their richness, high abundance, wide distribution and diverse spectrum of mineralogical and geochemical compositions. Our main goals are to (1) test the prediction that Mg content in bryozoan skeletons decreases along a depth gradient, (2) propose a conceptual model of the relationship between depth and Mg content and (3) demonstrate the utility of statistical treatments of biogeochemical and biomineralogical data for investigating important issues of ocean change.
Materials and methods
Models
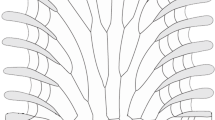
Six models summarizing possible relationships between magnesium content and depth (Fig. 1) were developed. These are similar in concept to the models proposed by Fabry (2008) and Ries (2011) and are inspired by some evolutionary models (e.g., Jablonski 1997; Trammer and Kaim 1999). The models show the following patterns: in model A, Mg remains constant with depth; model B predicts variations in Mg at each depth; model C suggests constant Mg across depths; model D assumes Mg varying in parallel with depth; model E considers Mg decreasing with depth; and model F demonstrates Mg increasing with depth.
Data
The data used to test the relationship between depth and Mg content in bryozoan skeletons were obtained from Kukliński and Taylor (2009). There are several advantages of this dataset: (1) taxonomic identifications were made by one scientist, (2) uniform analytical methods of magnesium content were obtained using standard XRD methods (for details see Kukliński and Taylor 2009), (3) precise depth values and high sampling resolution were available, (4) replicates of the same species were analyzed, (5) a large number of samples (>100) were available, (6) the environmental context and ecological distributional patterns of Arctic bryozoans are quite well known, (7) the quality of the specimens (e.g., for contaminant epibionts) was carefully controlled and (8) sampling was essentially random. Other published datasets, for example on bryozoans (e.g., Borisenko and Gontar 1991; Smith et al. 2006; Kukliński and Taylor 2008; Smith and Clark 2010), lack sampling depth data, focus on areas other than the Arctic, employ a smaller number of samples or used destructive mineralogical/geochemical analysis techniques excluding further specimen verification causing taxonomic inconsistencies. The bryozoans studied by Kukliński and Taylor (2009) came from numerous localities (see Fig. 2), ranging from northern Norway to Greenland, Spitsbergen and the Siberian coast (Laptev Sea), and can be regarded as fairly representative for the Arctic as a whole. Bryozoan classification followed the Bryozoa Home Page (2010).
Map of bryozoan localities (black dots) from this study of the relationship between skeletal Mg content and depth. The map was generated using Ocean Data View Software (Schlitzer 2012) based on sampling localities of Kukliński and Taylor (2009). Note that due to map scale a single dot may represent several neighboring localities
A total of 150 analyses were undertaken of 76 bryozoan species exhibiting various colony forms. Mg content was expressed as mole percentage (% mol) of magnesite (MgCO3; here referred to simply as magnesium; see also Tucker and Wright 1990). The error associated with this method is estimated to be 2 % (see Kukliński and Taylor 2009). Mean values were calculated for species with more than one analysis, such as some erect species where Mg was measured at the tips and bases of the colonies. Exclusion of analyses from samples for which no depth information was available reduced the dataset to 103 points. This comprised 52 species from 40 genera classified in 29 families within two bryozoan orders (see Table 1). When sample collection depth was expressed as a range (e.g., ‘11–36 m’), median depth was used.
Applied indexes
Few publications have studied statistical patterns in the mineralogy and geochemistry of skeletons (e.g., Ries 2004; Stanley et al. 2005; Kukliński and Taylor 2009; and literature cited therein). Thus, there are no established standard protocols for data analysis and interpretation. Therefore, we here propose several additional metrics to supplement those previously used for analytical purposes, for example, minimum, maximum or average Mg content values. For each depth bin, we recorded maximum, minimum and mean values of MgCO3, maximum variance of MgCO3 in a single species within the bin, ABC index, magnesium ratio, presence/absence of MgCO3 bearing taxa within the bin and number of overlaps (see Table 1). The ABC (aragonite, bimineralic and calcite) index is a measure of the ratio of bryozoan taxa of particular mineralogy within each bin and is expressed as number of samples per bin and also as number of genera per bin (as a proxy for species). The presence or absence of species with skeletons containing MgCO3 was also determined. Finally, a ‘number of overlaps’ metric was calculated to provide an index of similar Mg contents in taxa from the same depth. Species with calcitic skeletons were divided into low-Mg calcite (<4 mol% MgCO3), intermediate-Mg calcite (4–8 mol% MgCO3) and high-Mg calcite (>8 mol% MgCO3) categories, following Rucker and Carver (1969).
Statistical analyses
Linear regression was used to explore the relationship between depth and magnesium content. Statistical analyses were performed using mean values for given depth in cases of two, three or more Mg content replicates from the same depth. We performed analyses on datasets that included zero values as well as those without zero values. Based on depth distributions of Arctic bryozoan communities (for details see Kuklinski et al. 2005), samples were divided into three depth bins: <50, ~100 and ≥200 m. In addition, data were also analysed at higher sampling resolutions based on depth points available in our dataset (see Fig. 3). Although all data were transformed, this did not improve normality; therefore, differences in Mg content between depth bins were identified using one-way nonparametric Kruskal–Wallis tests. The same procedure was performed on all available Arctic data and separately only on data obtained from Spitsbergen. This allowed us to test whether the broad pattern of Mg distribution in bryozoan skeletons from the Arctic is similar to that observed locally, helping when drawing conclusions about the influence of environmental factors and scale on observed patterns. All statistical analyses were performed using Statistica 8 software.
Mean (circles), minimum and maximum (whiskers) values of magnesium content in Arctic bryozoans along a depth gradient. Note that only a single data point is available from a depth of 50 m. Original data from Kukliński and Taylor (2009, appendix)
Results
Maximum values of Mg in our dataset were unexpectedly found in the bryozoans from the deepest depth bin (below 200 m), while the lowest values were found in the shallowest bin (<50 m) (Table 1). Mean values of Mg increased with water depth, as did their variance (Table 1). However, there was no significant relationship between depth and skeletal Mg content for all samples (R 2 = 0.02, p = 0.121; without zero values R 2 = 0.04, p = 0.037), or for mean values from given depths (R 2 = 0.03, p = 0.591 see also Fig. 3; without zero values R 2 = 0.08, p = 0.390). A Kruskal–Wallis test showed no differences among the three depth bins (H (2,103) = 4.58, p = 0.101).
Similar patterns were found when data from Spitsbergen were analyzed separately: no significant relationship was found between depth and Mg for the full data or for mean values (R 2 = 0.01, p = 0.284; R 2 = 0.09, p = 0.547, respectively). A one-way Kruskal–Wallis test revealed no difference between depth bins (H (2,71) = 2.81, p = 0.244). The fact that there is a lack of pattern locally as well as for the entire area covered by this study demonstrates that local differences in environmental conditions (e.g., within-region temperature differences observed within the Arctic) have no appreciable influence on the pattern of skeletal Mg with depth.
Analysis of mean values (Fig. 3) for given depths revealed some trends in skeletal Mg between certain depths: Mg decreased between 1 and 24 m depth, and again between 25 and 44 m, but increased between 100 and 210 m depth. However, there was very weak or no statistical relationship within these bins between depth and skeletal Mg content (1–24 m: R 2 = 0.29, p < 0.001; 25–44 m: R 2 = 0.05, p = 0.467; 100–210 m: R 2 = 0.01, p = 0.504).
Discussion
Our findings do not support the prediction based on solubility that Mg should decrease with depth in bryozoan skeletons. The results from bryozoans contrast with those from crinoids summarized by Kroh and Nebelsick (2010), one of the few published studies dealing with skeletal Mg content in relation to depth. Using data from two literature sources, Kroh and Nebelsick found a linear relationship between skeletal Mg and depth (see also Roux et al. 1995). The contrast between the bryozoan and crinoid patterns may reflect a different degree of biological control (Lowenstam and Weiner 1989) of skeletal chemistry in these two groups. In addition, our bryozoan data were gathered only from the Arctic, whereas the crinoid data of Kroh and Nebelsick (2010) came from all around the world and included tropical sites and fewer polar sites. Borremans et al. (2009) among others have noted that patterns of ocean chemistry are spatially homogenous, and geographical factors can be ruled out as the cause of the observed differences. However, the crinoid data ranged down to depths of more than 2,000 m, an order of magnitude greater than our bryozoan data. This is congruent with the study of Schlager and James (1978) which demonstrated that a large depth gradient might be responsible for decreasing Mg content in abiogenic sea floor precipitates such as cold water calcite cements. However, for the depth range covered by our bryozoan analysis, crinoids show a clear pattern of decreasing Mg, contrasting with the lack of correlation in the bryozoans. Salinity should also be considered as a potential factor explaining the difference between the crinoid and bryozoan patterns, due to the known influence of salinity on magnesium content in skeletons such as those of echinoids (e.g., Borremans et al. 2009). In the case of the analyzed bryozoans, salinity is rather unimportant because our material came from a region where salinity showed little geographical variation (e.g., Karnovsky et al. 2003; Walkusz et al. 2009), while at the global scale of the crinoid data salinity is expected to vary more greatly.
Following the study of Chave (1954), temperature-related variations in Mg content in skeletal carbonate have been documented in various groups, for example, molluscs (Taylor and Reid 1990) and bryozoans (Kukliński and Taylor 2009; Taylor et al. 2009). More recently, Findlay et al. (2010b) showed a change in the Mg content of barnacle shells with latitude (and thus temperature), comparing temperate and Arctic representatives. In higher latitudes (colder waters), the rate of temperature change with water depth is generally lower than close to the equator. For example, Kwaśniewski et al. (2010, fig. 2) showed for Spitsbergen that temperature declines by only 2–3 °C, maximally 4 °C, over 150 m of depth. These and other authors (e.g., Karnovsky et al. 2003; Walkusz et al. 2009) have also noted that temperatures here are spatially heterogeneous, but differ only by up to a few degrees over distances of several kilometers. Such slight variability in temperature may make any changes related to Mg level undetectable above the ‘noise’ of background factors.
Kukliński et al. (2005) identified several depth-specific bryozoan assemblages and dominants clustered as ‘shallow’ or ‘deeper’ over the coastal range encompassed by this study. They found that a major change in assemblages occurs at depths between 35 and 50 m. In contrast, the current study found no patterns in Mg content correlated with this ecological boundary (see also Fig. 3).
Variance of Mg content in single species within a bin is highest in the shallowest depth bin, lowest in the intermediate bin and of intermediate level in the deepest bin. The ABC ratio on the other hand shows that the diversity of bryozoan mineralogies lacks a relationship with depth. The number of genera decreases with depth but this is not associated with changes in the number of genera with particular mineralogies and is not the result of a sampling artifact (see Table 1). Kukliński et al. (2005) found, for example, a higher bryozoan diversity in deeper than shallower assemblages. In contrast to mineralogical diversity, the magnesium ratio index shows significant changes between depths (see Table 1). In the deepest depth bin, bryozoans lacking magnesium are restricted to a single genus, while in the shallowest depth bin skeletons lacking a detectable Mg content occur in three species distributed between three genera and two families (more sensitive analytical methods will be required in future to check whether these bryozoans, unlike previously reported calcitic skeletons, really possess no trace of Mg in their skeletons). This pattern contrasts with the expected increase in taxa lacking magnesium with water depth due to the greater solubility of Mg-rich calcite with depth.
We may predict that with respect to skeletal mineralogy, bryozoans will be adapted, preadapted or ecophenotypically responsive for inhabiting particular depths, leading to a strong Mg signature for each depth bin. For example, Kukliński and Taylor (2009) explained latitudinal patterns of bryozoan mineralogy in terms of ecophenotypic responses and genetic adaptations/preadaptations. The present study on patterns of Mg with depth has failed to find support for a correlation of Mg with depth, possibly reflecting the fact that Arctic communities may be too young to have developed genetic adaptations/preadaptations in skeletal mineralogy. This hypothesis could be tested using mineralogical data from other groups, and by more thorough sampling, ideally in polar, temperate and tropical regions.
The lack of any depth-related changes in skeletal Mg could indicate a high degree of biological control of skeletal mineralogy in Arctic bryozoans. The observed pattern of skeletal Mg seems to fit best with the ‘disequilibrium’ model (Fig. 2), with Mg varying irregularly at particular depths without any predictive linear trends. Our results may imply that bryozoans will show some resilience to the effects of ocean acidification and warming, at least with respect to the Mg content of their calcitic skeletons. However, this issue will require further investigations, ideally utilizing other groups of Mg calcite calcifiers as well as focusing on geographical areas other than the Arctic.
References
Amini ZZ, Rao CP (1998) Depth and latitudinal characteristics of sedimentological and geochemical variables in temperate shelf carbonates, eastern Tasmania, Australia. Carbonates Evaporites 13:145–156
Anderson LG, Tanhua T, Björk G, Hjalmarsson S, Jones EP, Jutterström S, Rudels B, Swift JH, Wåhlstöm I (2010) Arctic Ocean shelf-basin interaction: an active continental shelf CO2 pump and its impact on the degree of calcium carbonate solubility. Deep Sea Res Pt I 57:869–879
Andersson AJ, Mackenzie FT, Bates NR (2008) Life on the margin: implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar Ecol Prog Ser 373:265–273
Andersson AJ, Kuffner IB, Mackenzie FT, Jokiel PL, Rodgers KS, Tan A (2009) Net loss of CaCO3 from a subtropical calcifying community due to seawater acidification: mesocosm-scale experimental evidence. Biogeosciences 6:1811–1823
Bathurst RGC (1975) Carbonate sediments and their diagenesis. Dev Sedimentol 12:1–658
Borisenko YA, Gontar VI (1991) Skeletal composition of cold-water bryozoans. Biol Morya 1:80–90 (in Russian)
Borremans C, Hermans J, Baillon S, André L, Dubois P (2009) Salinity effects on the Mg/Ca and Sr/Ca in starfish skeletons and the echinoderm relevance for paleoenvironmental reconstructions. Geology 37:351–354
Bryozoa Home Page (2010) Indexes to bryozoan taxa edited by P. Bock. Available from: http://bryozoa.net
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Chave KE (1954) Aspects of the biogeochemistry of magnesium 1. Calcareous marine organisms. J Geol 62:266–283
Clark D, Lamare M, Barker M (2009) Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among a tropical, temperate, and a polar species. Mar Biol 156:1125–1137
Clarke FW, Wheeler WC (1922) The inorganic constituents of marine invertebrates. US Geol Surv Prof Pap 124:1–62
de Moel H, Ganssen GM, Peeters FJC, Jung SJA, Kroon D, Brummer GJA, Zeebe RE (2009) Planktic foraminiferal shell thinning in the Arabian Sea due to anthropogenic ocean acidification? Biogeosciences 6:1917–1925
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1:169–192
Dupont S, Havenhand J, Thorndyke W, Peck L, Thorndyke M (2008) Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis. Mar Ecol Prog Ser 373:285–294
Dupont S, Ortega-Martinez O, Thorndyke M (2010) Impact of near-future ocean acidification on echinoderms. Ecotoxicol 19:449–463
Fabry VJ (2008) Marine calcifiers in a high-CO2 ocean. Science 320:1020–1022
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. J Mar Sci 65:414–432
Fabry VJ, McClintock JB, Mathis JT, Grebmeier JM (2009) Ocean acidification at high latitudes: the bellwether. Oceanography 22:160–171
Findlay HS, Kendall MA, Spicer JI, Widdicombe S (2010a) Post-larval development of two intertidal barnacles at elevated CO2 and temperature. Mar Biol 157:725–735
Findlay HS, Kendall MA, Spicer JI, Widdicombe S (2010b) Relative influences of ocean acidification and temperature on intertidal barnacle post-larvae at the northern edge of their geographic distribution. Estuar Coast Shelf Sci 86:675–682
Gazeau F, Quiblier C, Jansen JM, Gattuso JP, Middelburg JJ, Heip CHR (2007) Impact of elevated CO2 on shellfish calcification. Geophys Res Lett 34:L07603
Gordon DP, Taylor PD, Bigey FP (2009) Phylum Bryozoa. Moss animals, seamats, lace corals. In: Gordon DP (ed) New Zealand inventory of biodiversity, vol 1. Kingdom Animalia. Radiata, Lophotrochozoa, Deuterostomia. Canterbury University Press, New Zealand, pp 271–297
Hoegh-Guldberg O, Mumby PJ, Hooten AJ et al (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Jablonski D (1997) Body-size evolution in Cretaceous molluscs and the status of Cope’s rule. Nature 385:250–252
Karnovsky NJ, Kwasniewski S, Weslawski JM, Walkusz W, Beszczynska-Möller A (2003) Foraging behavior of little auks in a heterogeneous environment. Mar Ecol Prog Ser 253:289–303
Kleypas JA, Buddemeier RW, Archer D, Gattuso JP, Langdon C, Opdyke BN (1999) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118–120
Kroh A, Nebelsick JH (2010) Echinoderms and Oligo–Miocene carbonate systems: potential applications in sedimentology and environmental reconstruction. Int Assoc Sediment Spec Pub 42:201–228
Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1:114–117
Kukliński P, Taylor PD (2008) Are bryozoans adapted for living in the Arctic? Va Mus Nat Hist Spec Publ 15:101–110
Kukliński P, Taylor PD (2009) Mineralogy of Arctic bryozoan skeletons in a global context. Facies 55:489–500
Kukliński P, Gulliksen B, Lonne OJ, Węsławski JM (2005) Composition of bryozoan assemblages related to depth in Svalbard fjords and sounds. Polar Biol 28:619–630
Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser 373:275–284
Kwasniewski S, Gluchowska M, Wojczulanis-Jakubas K, Jakubas D, Blachowiak-Samolyk K, Cisek M, Stempniewicz L (2010) The impact of the divergent hydrographic conditions and zooplankton communities on provisioning Little Auks along the West coast of Spitsbergen. Prog Oceanogr 87:72–82
Lohbeck KT, Riebesell U, Reusch TBH (2012) Adaptive evolution of a key phytoplankton species to ocean acidification. Nat Geosci 5:346–351
Lombardi C, Cocito S, Hiscock K, Occhipinti-Ambrogi A, Setti M, Taylor PD (2008) Influence of seawater temperature on growth bands, mineralogy and carbonate production in a bioconstructional bryozoans. Facies 54:333–342
Lombardi C, Rodolfo-Metalpa R, Cocito S, Gambi MC, Taylor PD (2011a) Structural and geochemical alterations in the Mg calcite bryozoan Myriapora truncata under elevated seawater pCO2 simulating ocean acidification. Mar Ecol 32:211–221
Lombardi C, Gambi MC, Vasapollo C, Taylor PD, Cocito S (2011b) Skeletal alterations and polymorphism in a Mediterranean bryozoan at natural CO2 vents. Zoomorphology 130:135–145
Lowenstam HA, Rossman GR (1975) Amorphous, hydrous, ferric phosphatic dermal granules in Molpadia (Holothuroidea): physical and chemical characterization and ecologic implications of the bioinorganic fraction. Chem Geol 15:15–51
Lowenstam HA, Weiner S (1989) On biomineralization. Oxford University Press, Oxford
Loxton J, Kuklinski P, Mair JM, Spencer Jones M, Porter JS (2013) Patterns of magnesium-calcite distribution in the skeleton of some polar bryozoan species. In: Bryozoan studies 2010, Lecture Notes in Earth System Sciences, vol 143. Springer, Berlin, pp 169–185
Martin S, Rodolfo-Metalpa R, Ransome E, Rowley S, Buia MC, Gattuso JP, Hall-Spencer J (2008) Effects of naturally acidified seawater on seagrass calcareous epibionts. Biol Lett 4:689–692
Morse JW, Andersson AJ, MacKenzie F (2006) Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and ‘ocean acidification’: role of high Mg-calcite. Geochem Cosmochem Acta 70:5814–5830
Nienhuis S, Palmer AR, Harley CDG (2010) Elevated CO2 affects shell dissolution rate but not calcification rate in a marine snail. Proc R Soc B 277:2553–2558
Orr JC, Fabry VJ, Aumont O et al (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Palmer MR (2009) Paleo-ocean pH. Encycl Earth Sci Ser 15:743–746
Palmer MR, Pearson PN, Cobb SJ (1998) Reconstructing past ocean pH-depth profiles. Science 282:1468–1471
Pelejero C, Calvo E, Hoegh-Guldberg O (2010) Paleo-perspectives on ocean acidification. Trends Ecol Evol 25:332–344
Ponder RW, Glendinning IG (1974) The magnesium content of some miliolacean foraminifera in relation to their ecology and classification. Palaeogeogr Palaeoclimatol Palaeoecol 15:29–32
Pörtner HO (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser 373:203–217
Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FMM (2000) Reduced calcification in marine plankton in response to increased atmospheric CO2. Nature 407:634–637
Ries JB (2004) The effect of ambient Mg/Ca on Mg fractionation in calcareous marine invertebrates: a record of phanerozoic Mg/Ca in seawater. Geology 32:981–984
Ries JB (2011) A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim Cosmochim Acta 75:4053–4064
Ries JB, Cohen A, McCorkle D (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–1134
Rodolfo-Metalpa R, Lombardi C, Cocito S, Hall-Spencer JM, Gambi MC (2010) Effects of ocean acidification and high temperatures on the bryozoan Myriapora truncata at natural CO2 vents. Mar Ecol 31:447–456
Rost B, Zondervan I, Wolf-Gladrow D (2008) Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar Ecol Prog Ser 373:227–237
Roux M, Renard M, Améziane N, Emmanuel L (1995) Zoobathymetrie et composition chimique de la calcite des ossicules du pedoncule des crinoides. C R Acad Sci Paris Sér IIa 321:675–680
Rucker JB, Carver RE (1969) A survey of the carbonate mineralogy of cheilostome Bryozoa. J Paleontol 43:791–799
Ruttimann J (2006) Sick seas. Nature 442:978–980
Schlager W, James NP (1978) Low-magnesian calcite limestones forming at the deep-sea floor, tongue of the Ocean, Bahamas. Sedimentology 25:675–702
Schlitzer R (2012) Ocean data view, vol 4. http://odv.awi.de
Sewell MA, Hofmann GE (2011) Antarctic echinoids and climate change: a major impact on the brooding forms. Glob Change Biol 17:734–744
Smith AM (2009) Bryozoans as southern sentinels of ocean acidification: a major role for a minor phylum. Mar Freshwat Res 60:475–482
Smith AM, Clark DE (2010) Skeletal carbonate mineralogy of bryozoans from Chile: an independent check of phylogenetic patterns. Palaios 25:229–233
Smith AM, Key MM Jr, Gordon DP (2006) Skeletal mineralogy of bryozoans: taxonomic and temporal patterns. Earth Sci Rev 78:287–306
Stanley SM, Ries JB, Hardie LA (2005) Seawater chemistry, coccolithophore population growth, and the origin of Cretaceous chalk. Geology 33:593–596
Stolarski J, Meibom A, Przeniosło R, Mazur M (2007) A Cretaceous scleractinian coral with a calcitic skeleton. Science 318:92–94
Taylor JD, Reid DG (1990) Shell microstructure and mineralogy of the Littorinidae: ecological and evolutionary significance. Hydrobiologia 193:199–215
Taylor PD, Allison PA (1998) Bryozoan carbonates through time and space. Geology 26:459–462
Taylor PD, James NP, Bone Y, Kukliński P, Kyser KT (2009) Evolving mineralogy of the cheilostome bryozoans. Palaios 24:440–452
Trammer J, Kaim A (1999) Active trends, passive trends, Cope’s rule and temporal scaling: new categorization of cladogenetic body size changes. Hist Biol 13:113–125
Tucker ME, Wright VP (1990) Carbonate sedimentology. Blackwell, Oxford
Walkusz W, Kwasniewski S, Falk-Petersen S, Hop H, Tverberg V, Wieczorek P, Weslawski JM (2009) Seasonal and spatial changes in zooplankton composition in the glacially influenced Kongsfjorden, Svalbard. Polar Res 28:254–281
Wood HL, Spicer JI, Widdicombe S (2008) Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B 275:1767–1773
Wood HL, Spicer JI, Kendall MA, Lowe DM, Widdicombe S (2011) Ocean warming and acidification; implications for the Arctic brittlestar Ophiocten sericeum. Polar Biol 34:1033–1044
Acknowledgments
We would like to thank Caroline Kirk and Gordon Cressey (both formerly of the Department of Mineralogy, NHM, London) for help with previous XRD and mineralogical data analyses. TB is grateful to Dr Grażyna Bzowska, Tomasz Krzykawski M.Sc. (both University of Silesia, Sosnowiec) and Przemysław Gorzelak M.Sc. (Institute of Paleobiology, Warszawa) for fruitful discussions about invertebrate skeletal mineralogy and geochemistry. The journal Editor, Prof. Dieter Piepenburg, and his assistants, as well as Prof. Abigail Smith and four anonymous reviewers provided helpful suggestions on earlier versions of this paper. We are grateful to Prof. Noel P. James (Queen’s University, Ontario) for help with the literature and Emilia Trudnowska (Institute of Oceanology, Sopot) for assistance with map preparation. This study was completed thanks to a grant from the Polish Ministry of Science and Higher Education (No. N N304 404038).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Borszcz, T., Kukliński, P. & Taylor, P.D. Patterns of magnesium content in Arctic bryozoan skeletons along a depth gradient. Polar Biol 36, 193–200 (2013). https://doi.org/10.1007/s00300-012-1250-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1250-z