Abstract
Global warming will lead to increased nitrogen supply in tundra ecosystems. How increased N supply affected leaf production, leaf turnover and dead leaf N input into the soil of Empetrum nigrum and Andromeda polifolia (evergreens), Eriophorum vaginatum (graminoid) and Betula nana (deciduous) in a sub-arctic tundra in northern Sweden between 2003 and 2007 was experimentally investigated. There was considerable interspecific variation in the response of leaf production to N addition, varying from negative, no response to a positive response. Nitrogen addition effects on leaf turnover also showed considerable variation among species, varying from no effect to increased leaf turnover (up to 27% in Eriophorum). Nitrogen addition resulted in a four to fivefold increase in N content in the dead leaves of both evergreens and a 65% increase in Eriophorum. Surprisingly, there was no increase in Betula. The response of dead leaf P contents to N addition was rather species specific. There was no response in Empetrum, whereas there were significant increases in Andromeda (+214%) and Eriophorum (+32%), and a decrease of 47% in Betula. As an overall result of the changes in leaf production, leaf turnover and dead leaf N and P contents, nitrogen addition increased in all species except Betula the amount of N and, for Andromeda and Eriophorum the amount of P transferred to the soil due to leaf litter inputs. However, the way in which this was achieved differed substantially among species due to interspecific differences in the response of the component processes (leaf production, leaf turnover, dead leaf nutrient content).
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Northern tundra ecosystems are generally characterized by low nutrient mineralization rates as a consequence of low temperature, low pH and low quality of soil organic matter (Robinson 2002). Climate scenarios for the twenty first century predict substantial climate warming and this will be most manifest at northern high-latitude sites, with the exception of Greenland, with an estimated mean annual increase between 1.0 and 7.5°C (ACIA 2004). Temperature increases may affect a wide range of abiotic and biotic processes in high-latitude ecosystems. For the abiotic processes, this may include increased soil nitrogen availability. Rustad et al. (2001) performed meta-analysis on the results of warming studies at 32 research sites representing four broadly defined biomes, including high-latitude or -altitude tundra, low tundra, grassland and forest. They found considerable variation among sites in response to warming. For this meta-analysis, N mineralization data were available for nine tundra sites. Using the data reported by Rustad et al. (2001) I calculated that for these tundra sites, heating during at least 3 years increased net N-mineralization from 0.32 ± 0.31 (SE) g N m−2 year−1 in the controls to 0.53 ± 0.31 (SE) g N m−2 year−1 in the heated plots, an increase of about 70% (Aerts et al. 2006a). These results show that warming does indeed lead to higher N availability in high-latitude tundra sites, but the variability is substantial.
The warming-induced changes in soil nutrient availability have a potentially strong impact on ecosystem functioning as plant growth in most high-latitude sites is nutrient limited (Shaver and Chapin 1995; Shaver et al. 2001; Van Heerwaarden et al. 2003). Changes in soil nutrient availability may lead to direct responses due to phenotypic responses of the standing vegetation, but also to long-term responses as a result of changes in the species composition of the vegetation. At the community level, nutrient addition usually leads to the replacement of slow growing evergreens with low nutrient loss rates by fast-growing deciduous species and graminoids with high nutrient loss rates (Aerts 1999; Walker et al. 2006). Both these phenotypic responses and the interspecific differences in productivity and nutritional characteristics of plant species provide an important feedback on soil nutrient availability (Aerts 1999). In nutrient-poor environments, species produce relatively small amounts of litter due to the long lifespans of the various tissues. This litter generally has low nutrient concentrations and high concentrations of secondary compounds such as lignin and phenolics. Litter decomposition rates are positively related to the N concentration in the litter and negatively related to the lignin/N ratio (Aerts 1997). Thus, species from nutrient-poor environments produce litter that decomposes slowly and from which only low amounts of nutrients are released. The opposite holds for species from fertile environments. Due to their high tissue turnover rates they produce relatively large amounts of litter. Moreover, this litter contains relatively high concentrations of mineral nutrients and low concentrations of secondary compounds (Aerts 1999). As a result, this litter decomposes relatively quickly and releases large amounts of nutrients.
At the phenotypic level, similar patterns have been found. Increased N supply leads to higher leaf production rates (Aerts et al. 1992; Thornton and Millard 1993; Poorter and Nagel 2000; Zak et al. 2000), whereas the life expectancy of leaves of single species is usually reduced in response to increased N supply (Reader 1980; Shaver 1983; Aerts 1989; Cordell et al. 2001; Reich et al. 2004). However, in some other studies leaf life expectancy was higher at high than at low N supply (Turner and Olson 1976; Bazzaz and Harper 1977) or there was no effect at all (Aerts and De Caluwe 1995). In general, N addition also increases N contents of senesced leaves (Kemp et al. 1994; Shaver and Mellilo 1984; Vitousek 1998; Van Heerwaarden et al. 2003). As a result, it is to be expected that increased N supply increases the amount of N returned to the soil as a result of leaf litter production.
These observations raise the question how the increased nitrogen availability induced by climate change will affect leaf production and turnover of tundra species and how changed leaf dynamics feedback on nutrient input into the soil as a result of leaf litter production. I hypothesize that higher N supply leads to higher leaf production, higher rates of leaf turnover (lower leaf life expectancy) and higher N contents in senesced leaves, thus causing higher N inputs into the soil. I tested this hypothesis in a 4-year study on the effects of experimentally increased N supply on leaf production and leaf turnover of four dominant plant species in sub-arctic tundra in northern Sweden. These species were: E. hermaphroditum and Andromeda polifolia (woody evergreens), Betula nana (woody deciduous) and Eriophorum vaginatum (graminoid). The major growth-forms in high-latitude sites usually differ in their responsiveness to environmental change. In general, responsiveness tends to increase in the order: woody evergreen < woody deciduous < graminoid (Quested et al. 2003; Van Wijk et al. 2004; Cornelissen et al. 2004). If this was also true with respect to leaf dynamics and nutrient inputs into the soil, then growth-form specific responses to N availability would affect the relative impact of these growth-forms on soil nutrient inputs.
Study site and methods
Study site and plot design
The study was conducted at the Stordalen mire in northern Sweden (68°20′N, 19°00′E), 10 km east of the Abisko Scientific Research Station. Annual precipitation in this area amounts to 320 mm per year with a mean summer temperature of 7°C and a mean winter temperature of −6°C. The length of the growth season is 130 days. I conducted this study in the elevated ombrotrophic (rain-fed) part of the bog in the E. hermaphroditum–Vaccinium microcarpum association.
To mimic climate change-induced increase in soil N availability we started a long-term N fertilization experiment that has been running now since 1998 at the Stordalen mire. In May 1998 we laid out 16 plots measuring 1.5 by 1.5 m on relatively dry hummocks. The plots were separated by buffer zones of at least 1 m. Eight plots served as controls, whereas the other eight were fertilized with N. Each plot contained at least some individuals of the following species: E. hermaphroditum Hagerup (woody evergreen), Andromeda polifolia L. (woody evergreen), B. nana L. (woody deciduous), V. uliginosum L. (woody deciduous), E. vaginatum L. (graminoid) and Rubus chamaemorus L. (perennial herb). Total vascular plant cover in the plots was about 80% (Karlsson, unpublished data). Further details about this experiment can be found in Van Heerwaarden et al. (2003) and Aerts et al. (2006b).
During 1998–2002, so prior to the present experiment, the N-plots received 10 g N m−2 year−1, given as NH4Cl. This amount corresponds to about 2% of the amount of N present in the upper 10 cm of the peat soil. We chose this relatively high amount, as the microbial community in (sub-) arctic soils usually is a strong sink for added nutrients and nutrient addition effects on plants are initially masked by this process (Jonasson et al. 1999). During the present experiment (2003–2007) the supplied amount of N was lowered to 5 g N m−2 year−1. During 1998–2002, N was supplied in three equal portions in mid-June, mid-July and mid-August dissolved in 5 l of lake water. The control plots received the same amount of water. During the present experiment the N was supplied in two equal portions in July dissolved in 5 l of lake water.
Methods
Leaf production and leaf survival
The study focused on the leaf dynamics of four dominant species in the plots: E. hermaphroditum and A. polifolia (woody evergreens), B. nana (woody deciduous) and E. vaginatum (graminoid). Sampling was started in the end of July 2003 and continued 4 years with annual intervals until July 2007. As the species involved different growth forms, the methods that I used to determine annual leaf production and leaf survival differed among growth forms. For both evergreen species I collected during each sampling occasion three randomly chosen branches in each plot. Due to the closely packed leaf whorls at the transition between years, the branches could be separated into annual leaf cohorts. As the leaves leave a scar on the branch when they are shed, I could determine the amount of leaves produced in each leaf cohort and the percentage remaining leaves (cf. Karlsson 1992). By repeating this procedure for 4 years, I could determine the leaf survival of the leaf cohorts 2003 and 2004. For Eriophorum I tagged both in July 2003 and in July 2004 three tussocks in each plot. The second youngest leaf was marked with a tiny aluminium ring. During each consecutive sampling occasion I counted the amount of living and dead leaves on the tagged plant. In this way I could determine leaf production and leaf survival of the 2003 and 2004 leaf cohorts. As B. nana is deciduous it was not feasible to determine leaf survival. In each plot I tagged three randomly selected branches and counted the number of produced leaves on each sampling occasion.
Dead leaf N and P contents
The nutrient input due to leaf turnover is not only determined by the rate of leaf turnover, but also by the nutrient content of the shed leaves. In the year when the experiment was started, about 25 recently senesced leaves of each species were collected in each plot in the beginning of September, counted and dry weights were determined (48 h, 70°C). These leaves were random collections of all age classes (except for Betula as this is a deciduous species) and thus represent the ‘average’ dead leaf input into the soil. The leaves were grinded and N and P concentrations were determined with standard chemical methods. Using data on mean leaf weight and leaf N and P concentrations, the N and P contents per leaf were calculated.
Calculations and statistical analysis
For each plot mean leaf production and percentage leaf survival were calculated using the averaged data of the three separate measurements per plot. In some cases, rings or tags were lost and the mean values per plot were than based on two (or in 2 cases 1) individual measurements per plot.
The annual data on leaf survival were used to determine leaf life expectancy (e x). Maximum life span of the leaves of the perennials I investigated was 3 years (in a few exceptional cases 4 years). As life expectancy measurements for the perennials therefore require at least 3 years of measurements, this parameter was calculated for the leaf cohorts of 2003 and 2004 only. Life expectancy of these cohorts was calculated using standard demographic techniques (Pielou 1977). For the calculations I assumed linear leaf loss rates between sampling intervals. As the study was terminated in 2007, I determined for the 2005 and 2006 leaf cohorts only leaf production and 1st year (2005 and 2006 cohorts) and 2nd year (2005 cohort) leaf survival.
Annual leaf production and life expectancy data were analysed with two-way ANOVA with N supply and year as independent variables. In addition, the effect of N supply on leaf production and, when applicable, on leaf life expectancy of annual cohorts were determined with one-way ANOVA.
Results
Leaf production and leaf survival
Leaf production of E. hermaphroditum was relatively constant among years, except for a lower production in 2006 (Tables 1, 2). The effect of N addition on the number of produced leaves was rather variable and the overall effect was not significant. However, there was a significant N supply × year interaction. No effects of N supply were observed in 2003 and 2006. However, in 2004 there was an 11% increase whereas in 2005 leaf production was decreased by 9% in response to N supply. Nitrogen supply increased leaf survival after 1 year for the 2003 and 2006 leaf cohorts by 14% for both years and 2-year survival was increased by 73 and 66% for the 2004 and 2005 cohorts, respectively. Maximum leaf life span was 3 years. Life expectancies of the 2003 and 2004 leaf cohorts were similar and were not affected by N supply (Tables 1, 2).
As was the case for Empetrum, leaf production of the other evergreen species, Andromeda polifolia, was relatively constant among years, except for a lower leaf production in 2006 (Tables 1, 3). However, in contrast to Empetrum, N addition resulted in lower leaf survival rates after 1 year for the 2004 (−18%) and 2005 (−21%) cohorts and 29% lower leaf survival for the 2004 cohort after 2 years. Maximum leaf life span was 3 years. Life expectancy for the 2004 leaf cohort was considerably higher than that of the 2003 cohort and was reduced by 14% in response to enhanced N supply (Tables 1, 3).
Nitrogen supply reduced leaf production of the graminoid E. vaginatum for the 2003 cohort by 30%, but had no effect for the 2004 cohort (Tables 1, 4). Nitrogen addition did not significantly reduce leaf survival, except for a 14% reduction of leaf survival after 9 months for the 2003 cohort, but the overall pattern resulted in a reduced life expectancy for both the 2003 (−27%) and 2004 (−26%) leaf cohorts (Tables 1, 4). Maximum life span was slightly over 3 years. In the deciduous dwarf shrub B. nana, N addition resulted in a consistent reduction of twig length varying from 21 to 31% (Table 5). However, the number of produced leaves per twig was not affected (Tables 1, 5). It is striking to see that leaf production in 2005 and 2006 was 70–80% higher than in 2003 and 2004. Nitrogen addition resulted in a lower number of inflorescences, except in 2004.
Leaf N and P contents
For obvious reasons, dead leaf N and P contents (expressed per leaf) differed strongly among species (Table 6). The amounts of N and P were very low in the very small leaves of E. hermaphroditum and substantially higher in Eriophorum and Betula. The other evergreen, A. polifolia, had intermediate N and P contents.
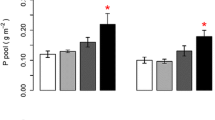
Nitrogen addition resulted in a four to fivefold increase in N content in the dead leaves of both evergreens and a 65% increase in Eriophorum. Surprisingly, there was no increase in Betula. The response of P contents to N addition was rather species specific. There was no response in Empetrum, whereas there were significant increases in Andromeda (+214%) and Eriophorum (+32%), and a decrease of 47% in Betula.
Discussion
Leaf production and turnover in relation to N supply
Considering all four species, leaf production varied both in response to N addition and among years. However, contrary to my hypothesis, there was no consistent N addition effect (Tables 1, 2, 3, 4, and 5) as I found both positive effects, negative effects and no effect, even within one species among years (Empetrum). Actually, the variation among years (up to 88% in Betula) mostly exceeded the N addition effect (up to 30% in Eriophorum). On the one hand this emphasizes the need for multi-year studies, but on the other hand this raises the question what the underlying cause of this large inter-annual variability might be. The most likely explanation is the temperature sensitivity of photosynthetic production in tundra plants (Arft et al. 1999; Rustad et al. 2001; Aerts et al. 2006a). Thus, NPP in tundra ecosystems is not only N-limited (see “Introduction”), but also temperature limited. This is most apparent from the experimental warming studies that have been performed in tundra ecosystems. Walker et al. (2006) performed a meta-analysis on the results of the experimental warming studies that are embedded in the International Tundra Experiment (ITEX). They found that already after two seasons of experimental warming height and cover of deciduous shrubs and graminoids were increased. This indicates that inter-annual variability in summer temperatures might partly explain the interannual differences in leaf production that I found. The changes in leaf production that we found for most species do indeed correlate with the interannual variability of summer temperatures as measured at the weather station in Abisko (10 km from our site). However, given the different interannual responses of Betula compared to the other species, it is clear that other factors also play a role. Currently, it is not clear what these factors might be.
It is striking to see that N addition resulted in a strong reduction of twig length in Betula (Table 5). A possible reason is that increased N supply resulted in decreased auxin production in this species, leading to the loss of apical dominance and thus reduced twig length (cf. Lambers et al. 2006). I have observed similar effects in a fertilization experiment with ericaceous species (Aerts 1994).
Leaf life expectancy in the controls showed little variation among the species with a perennial canopy and varied from slightly over 1 year in Eriophorum to 1.5 years in Empetrum and Andromeda. For the deciduous Betula it was about 0.3 years. In general these data are similar to those found in related studies. For Empetrum this compares well with data from Karlsson (1992) for the same region and with data from Aerts (1989) for the cool temperate ericoid species Erica tetralix and Calluna vulgaris. Leaf survival rates of annual cohorts, and thus life expectancy, of Andromeda determined in this study resemble very closely those of Flower-Ellis (1980) determined in 1974 at the same location. Shaver and Chapin (1995) found slightly higher life expectancy (1.5 years) for the graminoids (mostly E. vaginatum) in Alaskan tussock tundra than I found for Eriophorum.
As was the case for leaf production, I did not find the hypothesized lower leaf life expectancy in response to N addition. Instead I found variable effects (Tables 1, 2, 3, and 4), varying from no effect in Empetrum, to a negative effect for one leaf cohort of Andromeda, to a consistent negative effect in both cohorts of Eriophorum. These variable responses to the increased N supply of leaf life expectancy in evergreens have been reported before. As was the case for Empetrum in this study, no effect of increased N supply was found by Aerts (1989) with the evergreen shrub Calluna vulgaris, and by Reader (1980) with the evergreen shrubs Kalmia polifolia and Ledum groenlandicum. Decreasing leaf lifespan upon enhanced nutrient supply was also found by Reader (1980) who studied the evergreen shrub Chamaedaphne calyculata, by Aerts (1989) for the evergreen shrub E. tetralix, and by Shaver (1983) for the evergreen shrub Ledum palustre. The response I found in the graminoid Eriophorum vaginatum contrasted with the results of Shaver and Laundre (1997) who found no effect of increased NPK supply on leaf turnover of E. vaginatum in Alaskan tussock tundra. This is in line with a study on leaf lifespan with four temperate Carex species from fens differing in nutrient availability. Aerts and De Caluwe (1995) found that leaf lifespan was not significantly affected by enhanced N supply, except in C. diandra, where leaf lifespan decreased upon enhanced N supply. A possible explanation for these variable responses is given by Oikawa et al. (2006). They showed that the mechanism by which leaf lifespan is determined depends on the availability of the resource (nutrients vs. light) that is most limiting to plant growth. In dense stands, where light intensity deeper in the canopy is very low, leaf life expectancy is decreased. In more open stands this is very often not the case. Although this may explain the variation in responses in the studies cited above, it is questionable if this explanation also holds for the variable responses I found in this study. The vegetation in my experimental plots was very low statured (<10 cm) and light penetration to the soil surface was high, both in the controls and in the N-fertilized plots. Alternatively, it has been suggested that the N nutrition status of plants partly determines the activity of the phytohormones abscisic acid (ABA) and cytokinins (Lambers et al. 2006). Cytokinins delay leaf senescence, whereas ABA can induce leaf senescence. Thus, the phytohormone synthesis of plant species may respond differently to N supply, thereby bringing about differences in leaf life span. Unfortunately, no information on these processes is available for the species that I studied.
Plant–soil feedbacks
Nitrogen addition did in general result in a strong increase in the N and P content of senesced leaves (Table 6). However, the responses were very species specific. In Empetrum and Andromeda litter N contents increased four to fivefold, whereas the increase in Eriophorum was about 60% and there was no increase in Betula. Thus, these species respond very differently to increased N supply, but contrary to general ecological belief I found that both evergreen species showed the strongest response. Phosphorus contents also changed in response to increased N supply (Table 6). Normally one would expect lower P contents due to dilution effects. However, this was only the case for Betula. Both in Andromeda and Eriophorum P contents increased. A possible reason might be that due to increased N supply the root mass of these species increased, thereby facilitating the spatial foraging for the immobile phosphate. This effect is probably the strongest if the larger investment in root mass is mainly directed to fine roots, as these are most efficient in P acquisition. Such a mechanism has been found before with species from heathlands and fens (Aerts 1994).
The patterns in leaf production, leaf turnover and leaf litter N contents have important implications for the nutritional feedback of plant responses to soil N and P availability (Aerts and Chapin 2000). Higher leaf production and higher leaf turnover in response to N supply increase the amount of N going to the soil compartment. This effect is reinforced when litter N contents also increase in response to higher N supply. From the qualitatively summarized responses for the four studied species (Table 7) two main conclusions can be drawn. First of all, it showed that increased N supply increased for three out of four species the amount of N and for two species the amount of P transferred to the soil due to leaf litter inputs. However, the mechanism by which this is achieved differs substantially among species. For example in Empetrum this is the result of strongly increased litter N contents whereas in Andromeda it is the result of both higher leaf production, higher leaf turnover and higher leaf litter N contents. Thus, although the amount of N and P transferred to the soil increased for most species, the mechanism by which this is achieved differed substantially due to interspecific differences in the response of the component processes (leaf production, leaf turnover, dead leaf N and P contents). In addition, it must be emphasized that, although I generally found indications for higher leaf litter N and P inputs into the soil upon enhanced N supply, this does not mean that the high N and P input is readily available for plant uptake. In an earlier study (Aerts et al. 2006b) we studied in the same experiment that the effect of N addition on litter decomposition rates of Empetrum, Eriophorum, Betula and Rubus chamaemorus (thus Andromeda was not included in that study). In the controls of this decomposition experiment, we measured even after three years no or only slight net N mineralization. In the N-fertilized treatments we even found strong net N immobilization during 3 years, especially in Eriophorum and Betula. Thus, although N inputs into the soil increased, this N was for at least 3 years occluded in organic soil N pools. Nevertheless, it might be expected that this N is released over longer periods of time, but long-term (>3 years) decomposition studies are needed to substantiate this claim.
References
ACIA (2004) Impacts of a warming arctic. Cambridge University Press, London, p 139
Aerts R (1989) The effect of increased nutrient availability on leaf turnover and aboveground productivity of two evergreen ericaceous shrubs. Oecologia 78:115–120
Aerts R (1994) The effect of nitrogen supply on the partitioning of biomass and nitrogen in plant species from heathlands and fens: alternatives in plant functioning and scientific approach. In: Roy J, Garnier E (eds) A whole-plant perspective on carbon–nitrogen interactions. SPB Academic Publishing, The Hague, pp 247–265
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Aerts R (1999) Interspecific competition in natural plant communities: mechanisms, trade-offs, and plant-soil feedbacks. J Exp Bot 50:29–37
Aerts R, De Caluwe H (1995) Interspecific and intraspecific differences in shoot and leaf lifespan of four Carex species which differ in maximum dry matter production. Oecologia 102:467–477
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Aerts R, De Caluwe H, Konings H (1992) Seasonal allocation of biomass and nitrogen in four Carex species from mesotrophic and eutrophic fens as affected by nitrogen supply. J Ecol 80:653–664
Aerts R, Cornelissen JHC, Dorrepaal E (2006a) Plant performance in a warmer world: general responses of plants from cold biomes and the importance of winter and spring events. Plant Ecol 182:65–77
Aerts R, van Logtestijn RSP, Karlsson PS (2006b) Nitrogen supply differentially affects litter decomposition rates and nitrogen dynamics of sub-arctic bog species. Oecologia 146:652–658
Arft AM, Walker MD, Gurevitch J et al (1999) Response patterns of tundra plant species to experimental warming: a meta-analysis of the International Tundra Experiment. Ecol Monogr 69:491–511
Bazzaz FA, Harper JL (1977) Demographic analysis of the growth of Linum usitatissimum. New Phytol 78:193–208
Cordell S, Goldstein G, Meinzer FC, Vitousek PM (2001) Regulation of leaf life-span and nutrient-use efficiency of Metrosideros polymorpha trees at two extremes of a long chronosequence in Hawaii. Oecologia 127:198–206
Cornelissen JHC, Quested HM, Gwynn-Jones D, van Logtestijn RSP, De Beus MAH, Kondratchuk A, Callaghan TV, Aerts R (2004) Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Funct Ecol 18:779–786
Flower-Ellis JGK (1980) Diurnal dry weight variation and dry matter allocation of some tundra plants. 1. Andromeda polifolia L. In: Sonesson M (ed) Ecology of a Subarctic Mire. Ecol Bull 30:139–162
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV (1999) Responses in microbes and plants to changed temperature, nutrient, and light regimes in the Arctic. Ecology 80:1828–1843
Karlsson PS (1992) Leaf longevity in evergreen shrubs: variation within and among European species. Oecologia 91:346–349
Kemp PR, Waldecker DG, Owensby CE, Reynolds JF, Virginia RA (1994) Effects of elevated CO2 and nitrogen fertilization pretreatments on decomposition of tallgrass prairie leaf litter. Plant Soil 165:115–127
Lambers H, Chapin FS, Pons TL (2006) Plant physiological ecology. Springer, New York
Oikawa S, Hikosaka K, Hirose T (2006) Leaf lifespan and lifetime carbon balance of individual leaves in a stand of an annual herb, Xanthium canadense. New Phytol 172:104–116
Pielou EC (1977) Mathematical ecology. Wiley, New York
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
Quested HM, Cornelissen JHC, Press MC, Callaghan TV, Aerts R, Trosien F, Riemann P, Gwynn-Jones D, Kondratchuk A, Jonasson S (2003) Decomposition of sub-arctic plants with differing nitrogen economies: a functional role for hemiparasites. Ecology 84:3209–3221
Reader RJ (1980) Effects of nitrogen fertilizer, shade and removal of new growth on longevity of overwintering bog ericad leaves. Can J Bot 58:1737–1743
Reich PB, Uhl C, Walters MB, Progh L, Ellsworth DS (2004) Leaf demography and phenology in Amazonian rain forest: a census of 40000 leaves of 23 tree species. Ecol Monogr 62:365–392
Robinson CH (2002) Controls on decomposition and soil nirtogen availability at high latitudes. Plant Soil 242:65–81
Rustad LE, Campbell JL, Marion GM et al (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Shaver GR (1983) Mineral nutrition and leaf longevity in Ledum palustre: the role of individual nutrients and the timing of leaf mortality. Oecologia 56:160–165
Shaver GR, Chapin FS (1995) Long-term responses to factorial NPK fertilizer treatment by Alaskan wet and moist tundra sedge species. Ecography 18:259–275
Shaver GR, Mellilo J (1984) Nutrient budgets of marsh plants: efficiency concepts and relation to availability. Ecology 65:1491–1510
Shaver GR, Laundre J (1997) Exsertion, elongation, and senescence of leaves of Eriophorum vaginatum and Carex bigelowii in Northern Alaska. Global Change Biol 3(Suppl 1):146–157
Shaver GR, Bret-Harte MS, Jones MH, Johnstone J, Gough L, Laundre J, Chapin FS (2001) Species composition interacts with fertilizer to control long-term vegetation change in tundra productivity. Ecology 82:3163–3181
Thornton B, Millard P (1993) The effects of nitrogen supply and defoliation on the seasonal internal cycling of nitrogen in Molinia caerulea. J Exp Bot 44:531–536
Turner J, Olson PR (1976) Nitrogen relations in a Douglas-fir plantation. Ann Bot 4:1185–1193
Van Heerwaarden LM, Toet S, Aerts R (2003) Nitrogen and phosphorus resorption efficiency and proficiency in six sub-arctic bog species after 4 years of nitrogen fertilization. J Ecol 91:1060–1070
Van Wijk M, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FSIII, Cornelissen JHC, Gough L, Hobbie SE, Jonasson S, Lee JA, Michelsen A, Press MC, Richardson SJ, Rueth H (2004) Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalisations and differences in ecosystem and plant type responses to global change. Global Change Biol 10:105–123
Vitousek PM (1998) Foliar and litter nutrients, nutrient resorption, and decomposition in Hawaiian Metrosideros polymorpha. Ecosystems 1:401–407
Walker MD, Wahren CH, Hollister RD et al (2006) Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci USA 103:1342–1346
Zak DR, Pregitzer KS, Curtis PS, Vogel CS, Holmes WE, Lussenhop J (2000) Atmospheric CO2, soil-N availability, and allocation of biomass and nitrogen by Populus tremuloides. Ecol Appl 10:34–46
Acknowledgments
We gratefully acknowledge the director and staff of the Abisko Scientific Research Station for facilitating this study. Thanks are also due to Richard van Logtestijn and Ellen Dorrepaal who did part of the nutrient additions for us. This study was financially supported by USF grant 98/24.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Aerts, R. Nitrogen supply effects on leaf dynamics and nutrient input into the soil of plant species in a sub-arctic tundra ecosystem. Polar Biol 32, 207–214 (2009). https://doi.org/10.1007/s00300-008-0521-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0521-1