Abstract
Key message
The small-molecule glucosyltransferase loss-of-function mutant ugt76b1 exhibits both SID2- or NPR1-dependent and independent facets of enhanced plant immunity, whereupon FMO1 is required for the SID2 and NPR1 independence.
Abstract
The small-molecule glucosyltransferase UGT76B1 inactivates salicylic acid (SA), isoleucic acid (ILA), and N-hydroxypipecolic acid (NHP). ugt76b1 loss-of-function plants manifest an enhanced defense status. Thus, we were interested how UGT76B1 genetically integrates in defense pathways and whether all impacts depend on SA and NHP. We study the integration of UGT76B1 by transcriptome analyses of ugt76b1. The comparison of transcripts altered by the loss of UGT76B1 with public transcriptome data reveals both SA-responsive, ISOCHORISMATE SYNTHASE 1/SALICYLIC ACID INDUCTION DEFICIENT 2 (ICS1/SID2)- and NON EXPRESSOR OF PR GENES 1 (NPR1)-dependent, consistent with the role of UGT76B1 in glucosylating SA, and SA-non-responsive, SID2/NPR1-independent genes. We also discovered that UGT76B1 impacts on a group of genes showing non-SA-responsiveness and regulation by infections independent from SID2/NPR1. Enhanced resistance of ugt76b1 against Pseudomonas syringae is partially independent from SID2 and NPR1. In contrast, the ugt76b1-activated resistance is completely dependent on FMO1 encoding the NHP-synthesizing FLAVIN-DEPENDENT MONOOXYGENASE 1). Moreover, FMO1 ranks top among the ugt76b1-induced SID2- and NPR1-independent pathogen responsive genes, suggesting that FMO1 determines the SID2- and NPR1-independent effect of ugt76b1. Furthermore, the genetic study revealed that FMO1, ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1), SID2, and NPR1 are required for the SA–JA crosstalk and senescence development of ugt76b1, indicating that EDS1 and FMO1 have a similar effect like stress-induced SA biosynthesis (SID2) or the key SA signaling regulator NPR1. Thus, UGT76B1 influences both SID2/NPR1-dependent and independent plant immunity, and the SID2/NPR1 independence is relying on FMO1 and its product NHP, another substrate of UGT76B1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salicylic acid (SA) and N-hydroxypipecolic (NHP) acid play a central and concerted role in establishing Arabidopsis pathogen defense mainly against (hemi)-biotrophic pathogens such as Pseudomonas syringae. They coordinately affect both local immunity and systemic acquired resistance (SAR). Thereby, NHP is indispensable for triggering of SAR, whereas SA is required for a fully established local and systemic defense (Ding and Ding 2020; Vlot et al. 2009). Their biosynthesis is also highly interconnected. ICS1/SID2 is responsible for synthesizing SA in Arabidopsis thaliana (Dewdney et al. 2000; Nawrath and Metraux 1999). FMO1 converts pipecolic acid into NHP and orchestrates defense via both SID2- (SA-)-dependent and independent pathways during SAR (Bernsdorff et al. 2016; Hartmann and Zeier 2018; Hartmann et al. 2018; Zeier 2021). Several players organizing the interplay of SA and NHP biosynthesis and signaling have been identified. A cascade of both positive and negative transcription factors channels immune perception to enhance transcription of biosynthetic genes and the immune regulators EDS1 and PHYTOALEXIN-DEFICIENT 4 (PAD4), which are required for both SA and NHP formation (Bartsch et al. 2006; Huang et al. 2020; Zeier 2021; Shields et al. 2022). NPR1 is a shared, key downstream regulator of SA- and NHP-mediated local and systemic responses (Ding and Ding 2020; Ding et al. 2020; Vlot et al. 2009; Zheng and Dong 2013). While SA and NHP act mostly synergistic, the SA pathway usually exerts an antagonistic effect on the JA pathway. This antagonism requires NPR1 (Vlot et al. 2009). However, NPR1-independent and also SID2- (SA-) independent regulation of pathogen defense plays a vital function in regulating defense as well. An NPR1-independent defense response was found in several mutants, such as ssi1, ssi2, cpr5, cpr6, acd6, and cdd1 (Bowling et al. 1997; Clarke et al. 1998; Rate et al. 1999; Shah et al. 1999, 2001; Swain et al. 2011, 2015). The lesion-mimic Arabidopsis mutant syp121 syp122 suggested that some SA-independent signals are mediated by FMO1 (Zhang et al. 2008). Furthermore, the activity and mutual enhancement of SA and NHP is also regulated at the metabolic level. Four independent studies suggested that the small-molecule glucosyltransferase UGT76B1 can conjugate and inactivate SA and NHP in a competitive manner, in fact in concert with another immune-stimulating compound, isoleucic acid (ILA) (Bauer et al. 2021; Cai et al. 2021; Holmes et al. 2021; Mohnike et al. 2021). Thereby, UGT76B1 plays a decisive role in the interplay of SA, NHP, and ILA balancing a low-level defense status in naïve, non-infected plants, whereas it attenuates defense upon infection (Bauer et al. 2021; Holmes et al. 2021; Mohnike et al. 2021). Consequently, UGT76B1 had been shown to suppress defense against Pseudomonas syringae accompanied by downregulation of SA marker genes such as PR1 and upregulation of the mostly antagonistic JA pathway markers such as VSP2 and to delay senescence (von Saint Paul et al. 2011). To integrate the action of UGT76B1 into these defense pathways, we compared a non-targeted gene expression analysis of ugt76b1 with public expression data revealing both SA- and non-SA-responsive genes. Further comparison with public data revealed many SID2- and also NPR1-independently regulated genes among non-SA responsive group of ugt76b1, with FMO1 ranking top. Consistently, the resistance of ugt76b1 against Pseudomonas syringae is partially independent from SID2 and NPR1, whereas the resistance against Pseudomonas is known to completely rely on FMO1 (Bauer et al. 2021). Further genetic analyses showed that the induction of the SA marker PR1, the suppression of the JA marker VSP2 by ugt76b1, and the senescence phenotype of ugt76b1 are mainly dependent on SID2, NPR1, EDS1, and FMO1. Thus, the impact of ugt76b1 may be mediated through the upregulation of SA (SID2) and NHP (FMO1) pathways. The SID2- and NPR1-dependent mode is consistent with the role of UGT76B1 in glucosylating SA, while the SID2- and NPR1-independent regulation is mediated through FMO1 and its product NHP in accordance with the dual action of UGT76B1 to glucosylate and inactivate SA and NHP.
Materials and methods
Plant material and growth condition
Arabidopsis thaliana plants (Col-0 accession) were grown in soil under a regime of 14 h light (45–60 µmol m−2 s−1) and 20 °C; temperature was reduced to 18 °C in the dark phase with 75% relative humidity. Mutant Arabidopsis lines were obtained from the Arabidopsis stock center (fmo1, SALK_026163; ora59, GK_061A12; ugt76b1, SAIL 1171A11) (Bartsch et al. 2006; Scholl et al. 2000; von Saint Paul et al. 2011) and from colleagues (eds1-2, Corina Vlot-Schuster, Bayreuth; jin1, Susanne Becker, Würzburg; npr1-1, Corina Vlot-Schuster, Bayreuth; sid2-1, Christiane Nawrath, Lausanne) (Bartsch et al. 2006; Berger et al. 1996; Cao et al. 1994; Nawrath and Metraux 1999). All double mutants were generated by genetic crossing and then selected by PCR-based genotyping or CAPS polymorphisms (Cao et al. 1994; Nawrath and Metraux 1999; Rosso et al. 2003; Sessions et al. 2002).
Pseudomonas infection
The biotrophic pathogen Pseudomonas syringae pv tomato DC3000 (Ps-vir) was used in this project. Bacteria were streaked out onto fresh solid King’s B medium containing 50 μg mL−1 kanamycin and grown for 2 days at 28 °C. A single colony was picked and grown overnight in liquid King’s B medium with antibiotic at 28 °C at a shaker speed of 170 rpm. When bacteria reached late log phase of growth (OD600 = 0.6–1.0), they were diluted to 5 × 105 cfu mL−1 in 10 mM MgCl2 for the inoculation of plants. An OD600 = 0.001 corresponds to 5 × 105 colony-forming units mL−1. Four leaves of 5- to 6-week-old Arabidopsis (6th–11th leaves) were labeled by a marker pen and infiltrated with the diluted bacteria using a 1 mL syringe. Control plants were infiltrated with 10 mM MgCl2 as mock treatment. Bacteria (cfu cm−2) were quantified 0 and 3 days after inoculation. To determine the bacteria number after inoculation, leaf discs with an area of 0.20 cm2 were cut using the lid of a 0.5 mL Eppendorf tube. Two leaf discs from each individually infected plant were harvested. Six leaf discs from three individual plants were pooled as one biological replicate. In total, at least four independent biological replicates were analyzed. Bacterial numbers were calculated according to Katagiri et al. (2002).
Real-time PCR
Plants were grown on soil employing 16 h light/8 h darkness regime. Total RNA was extracted from about 60 mg of rosette leaf powder using RNeasy Plant Mini kit (Qiagen, Germany) and dissolved in 30 µL of RNase/DNase free water. Quality and concentration were analyzed using the Nanodrop ND-1000 spectrophotometer (Kisker-Biotech, Germany). Primers for RT-qPCR were designed using the Primer Express 3.0 software (Applied Biosystems, Germany) according to the reference mRNA sequences (Supplementary Table 6). The first-strand cDNA was transcribed from 1 µg total RNA using QuantiTect Reverse Transcription Kit (Qiagen, Germany). The Applied Biosystems (Germany) 7500 real-time PCR system was used for quantitative PCR recording SYBR Green fluorescence (Thermo Scientific or Bioline, Germany). Each sample was repeated with two technical replicates. UBQ5 (At3g62250) and S16 (At5g18380, At2g09990) were chosen as two reference genes to normalize the relative abundance of the genes of interest according to GeNorm analysis (Vandesompele et al. 2002). Arithmetic means and standard errors from log10-transformed data of RT-qPCR data from more than three independent experiments were statistically assessed by an “R” software package employing two-way analysis of variance (ANOVA; linear mixed effect models) followed by post hoc Tukey’s HSD test correction.
Untargeted microarray analysis and data analysis
Arabidopsis plants were grown under a 14 h light/10 h dark regime at 45–60 µmol m−2 s−1 fluorescent light. The transcriptome analysis of ugt76b1-1, UGT76B1-OE-7 and wild type (accession Columbia) was performed using A. thaliana Agilent At8×60 K one-color microarrays (Design ID: 29132, A-GEOD-16892) (Agilent, Germany) according to the manufacturer’s instructions. The assays were done as previously described (Georgii et al. 2017). Three biological replicates of each genotype were analyzed. Leaves from eight 4-week-old Arabidopsis plants were harvested to be pooled as one replicate. The “One-color Microarray-Based Gene Expression Analysis-Low Input Quick Amp Labeling” according to Agilent G4140-90040 was employed. The fluorescent signals from the arrays were analyzed by the Agilent Feature Extraction Software (Agilent, Germany). Probes were mapped to AGI loci using TAIR10 (Berardini et al. 2015). The R software package Limma was used to perform quantile normalization and compute differential gene expression. Transcripts with more than twofold changes compared to the control (Col) and a significant change based on corrected p values smaller than 0.05 were chosen for further analysis. BioMaps (www.virtualplant.org) version 1.3 was used for functional analysis of gene lists. Over-representation of Gene Ontology terms (https://www.arabidopsis.org/tools/go_term_enrichment.jsp) was assessed using binomial-test p values. A corrected p value (with Bonferroni correction) smaller than 0.016 was considered to indicate a significant over-representation. Genevestigator (https://www.genevestigator.com/gv/) was used to compare the expression pattern of genes of interest with public data.
Results
UGT76B1 expression negatively regulates defense-responsive genes
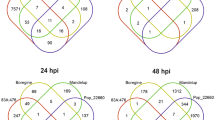
UGT76B1 has been shown to glucosylate and inactivate the three immune-modulatory ILA, SA, and NHP (Bauer et al. 2021; Holmes et al. 2021; Mohnike et al. 2021). To identify genes and pathways that are specifically affected by the action of UGT76B1, we compared differential gene expression pattern of the loss-of-function mutant ugt76b1-1 and a constitutively UGT76B1-overexpressing line (UGT76B1-OE) relative to the wild-type Columbia (Col) by a non-targeted analysis employing the Agilent G4140-90040 A. thaliana microarray based on the TAIR10 annotation. The expression of 1164 genes was altered at least twofold (adjusted P ≤ 0.05) by ugt76b1-1 compared to Col. Among these genes, 807 genes were upregulated and 357 genes were downregulated (Fig. 1). The constitutive overexpression of UGT76B1 (UGT76B1-OE) led to a change in the expressions of 398 genes in comparison to wild type (at least twofold change, P ≤ 0.05). Among these 398 genes, 129 genes were induced, whereas 269 genes were suppressed (Fig. 1). According to TAIR gene ontology (GO) function analysis, genes induced by ugt76b1-1 are enriched in the group of “response to salicylic acid”, “response to chitin”, “systemic acquired resistance”, “protein phosphorylation”, “response to molecule of bacterial origin”, “ER unfolded protein response”, “response to molecule of fungal origin”, “response to abscisic acid”, and “response to salt stress” sorted by adjusted P value from smallest to largest (P ≤ 0.012) (Fig. 2a). However, only the groups of “response to hormone” and “response to abiotic stimulus” have been shown to be enriched by ugt76b1-suppressed genes (P ≤ 0.01) (Fig. 2b). In addition, the groups of “response to wounding”, “response to other organism”, “response to jasmonic acid”, “jasmonic acid metabolic process”, and “response to osmotic stress” have been shown to be over-represented by UGT76B1 overexpression-induced genes sorted by adjusted P value from smallest to largest (P ≤ 0.01) (Fig. 2c). Moreover, genes suppressed by overexpression of UGT76B1 are related to “response to bacterium”, “response to oomycetes”, “defense response to fungus”, “systemic acquired resistance”, “signal transduction”, “protein phosphorylation”, and “cellular response to salicylic acid stimulus” sorted by P value from smallest to largest (P ≤ 0.01) (Fig. 2d). A Venn diagram indicates that 127 genes were oppositely regulated by the loss-of-function vs. the ectopic expression of UGT76B1. The vast majority, 119 genes, were upregulated by the ugt76b1 knockout and downregulated by the UGT76B1 overexpression (Fig. 1; Supplementary Table 7). Only eight genes were induced by overexpression of UGT76B1 but suppressed by the loss-of-function of UGT76B1 (Fig. 1; Supplementary Table 8). The common 119 ugt76b1 up- and UGT76B1-OE downregulated genes are enriched in the groups of “response to salicylic acid”, “defense response to bacterium”, “systemic acquired resistance”, “defense response to fungus”, “response to molecule of bacterial origin”, “response to oomycetes”, “signal transduction”, “cellular response to oxygen-containing compound”, “protein phosphorylation”, “response to lipid”, and “response to inorganic substance” sorted by P value from smallest to largest (P ≤ 0.01) (Supplementary Fig. 1). Among the common eight genes suppressed by ugt76b1, however, induced by UGT76B1-OE, At4g23600 encoding a tyrosine transaminase family protein is responsible for regulating the JA pathway (Lopukhina et al. 2001) (Supplementary Table 8), consistent with the suppression of the JA pathway by ugt76b1 and its upregulation by UGT76B1-OE. This strongly suggests that UGT76B1’s function mainly leads to suppression of a set of defense-responsive genes.
Transcriptional reprogramming of genes by UGT76B1 expression. Microarray analysis was performed using A. thaliana Agilent At8×60 K one-color microarrays. Gene expression was compared among ugt7b1-1, UGT76B1-OE-7, and wild type (accession Columbia). Differentially expressed genes by loss-of-function of UGT76B1 and constitutive overexpression of UGT76B1 are indicated. Genes induced or suppressed more than twofold are indicated as red and blue arrow, respectively. The Venn diagrams display the overlaps between genes oppositely regulated by ugt76b1 and UGT76B1-OE (colour figure online)
GO term-based functional analysis of genes deregulated by the altered expression of UGT76B1. a Eight hundred and seven genes induced more than twofold and b three hundred and fifty-seven genes suppressed by loss-of-function of UGT76B1 were grouped according to GO term analysis. c One hundred and twenty-nine genes induced more than twofold, and d two hundred and sixty-nine genes suppressed more than twofold by UGT76B1 overexpression were grouped according to GO term analysis. GO biological process assignments by Panther at TAIR (https://www.arabidopsis.org/tools/go_term_enrichment.jsp) were used to calculate the P value of over-representation by a binomial test (with Bonferroni correction) with a cutoff of 0.016 for a and c and a cutoff of 0.010 for b and d
SA-responsive and non-responsive genes of ugt76b1 show both SID2- and NPR1-dependent and -independent regulation
UGT76B1 attenuates basal and induced defense responses, glucosylating SA, NHP, and ILA. NHP and possibly its biosynthetic precursor pipecolic acid (Pip) amplify the biosynthesis of SA and regulate both SID2-dependent and independent responses (Bernsdorff et al. 2016; Hartmann and Zeier 2018; Hartmann et al. 2018; Bauer et al. 2021). ILA application can increase Pip and NHP abundance (Bauer et al. 2021) suggesting the existence of both SID2-dependent and independent regulations, as well. We therefore hypothesize that genes induced by ugt76b1 may be classified as SA-responsive and non-responsive. Accordingly, differential gene expression of ugt76b1 was compared with public expression data involving SA responses. The genes induced by ugt76b1 were compared to Affymetrix microarray-based public data deposited at Genevestigator (https://www.genevestigator.com/gv/plant.jsp; Zimmermann et al. 2005). These experiments comprise the response to exogenous SA or benzothiadiazol (BTH, a functional analogue of SA) treatment, to infection by different strains of Pseudomonas syringae (P. syringae pv. tomato DC3000, P. syringae pv. tomato DC3000 avrRptm1, P. syringae pv. phaseolicola, and P. syringae pv. maculicola) of wild type (vs. mock), to infection of sid2 by P. syringae pv. tomato avirulent strain DC3000 avrB, and to P. syringae pv. maculicola infection of npr1-1 or sid2. One thousand and six Affymetrix features matched the 1164 genes identified in our study. They were first classified into three groups according to the strength in response to SA and BTH treatment. A group of 494 genes was induced or suppressed by SA and BTH more than twofold (384 induced and 110 suppressed transcripts; log2FC ≥ 1.0), and 152 genes showed an intermediate change of 1.5-to-2-fold (117 induced and 45 suppressed transcripts; 0.58 ≤ log2FC < 1.0) indicating a potential regulation by SA or BTH, whereas 350 genes were altered by a factor of less than 1.5 (219 induced and 131 suppressed features; log2FC < 0.58). This latter group was, therefore, classified as non-SA responsive. The genes were further sorted according to induction to pathogen infections. The majority of the SA-responsive genes are related to pathogen defense. Among the SA-responsive group, the 368 defense-responsive genes account for 93% of 384 ugt76b1-induced genes, and 92 defense-responsive genes overlap with more than 80% of 110 ugt76b1-suppressed genes (Table 1). Among the potentially SA-responsive group, 84% of ugt76b1-induced and 58% of ugt76b1-suppressed genes were categorized as defense-related genes (Table 1). A much lower frequency of defense-related transcripts was observed among the non-SA responsive genes. However, still more than half, i.e., 57% of the 219 ugt76b1-induced, non-SA-responsive genes, were associated with pathogen defense, whereas only 20% of the 131 ugt76b1-suppressed genes classified to this category (Table 1). The altered transcription in response to various Pseudomonas infection experiments, yet lacking response to SA or BTH treatment further supports the association with defense in a non-SA responsive group (Table 1). Thus, UGT76B1 suppresses a set of defense-responsive genes even among the non-SA responsive group.
SA responses are critically dependent on the biosynthetic function of SID2 and the signaling node of NPR1 (Ding and Ding 2020; Vlot et al. 2009). SID2-dependent and independent regulations of defense responses had been revealed for the function of Pip and FMO1 (Bernsdorff et al. 2016; Hartmann and Zeier 2018; Hartmann et al. 2018). To explore the dependence of the ugt76b1 mutants on SID2 or NPR1 in regulating defense, SID2 and NPR1 dependence was classified among both SA-responsive and non-SA responsive based on transcriptional responses of Pseudomonas syringae pv. maculicola infected npr1-1 or sid2 (vs. infected Col) or of P. syringae pv. tomato avirulent strain DC3000 avrB infection of sid2 (vs. infected Col or non-infected sid2) or the non-infected sid2 mutants vs. non-infected Col (https://www.genevestigator.com/gv/plant.jsp: date; Zimmermann et al. 2005). Most of the SA-responsive genes showed SID2 and NPR1 dependence when infected with Pseudomonas syringae pv. maculicola, especially in the SA-inducible group (Supplementary Table 1). Thus, UGT76B1 has a major role in suppressing a set of SA-responsive genes regulated via SID2 and NPR1.
Among the non-SA responsive groups of ugt76b1-induced genes, most genes showed independence from SID2 or NPR1 (Supplementary Table 2). Seventy-one genes showing SID2- and NPR1-independent pathogen responses after comparison with public data were extracted (Table 2; Supplementary Table 2). Indeed, many studies confirm that FMO1, WRKY55, KTI1, CRK20, SRG1, CYP71A12, RABA4C, PUB23, MYB15, PICBP, TPS4, and MLO6 are involved in the defense response (Table 2) (Attaran et al. 2008; Chezem et al. 2017; Cui et al. 2021; Ederli et al. 2011; Ellinger et al. 2014; Gruner et al. 2018; Lemarie et al. 2015; Li et al. 2008; Mishina and Zeier 2006; Reddy et al. 2003; Stegmann et al. 2012; Wang et al. 2020). The ethylene signaling responsive proteins ERF1 and ERF13 were also induced by ugt76b1 (Table 2) (Onate-Sanchez and Singh 2002; Solano et al. 1998). CSAP is ABA-responsive and positively regulates dark induced senescence (Table 2) (So et al. 2020). JUL1 participates in the ABA-mediated microtubule disorganization, stomatal closure, and tolerance to drought stress (Yu et al. 2020). This strongly suggests that several defense-related genes altered by ugt76b1 are linked to aspects other than the SID2/NPR1-regulated SA pathway.
The enhanced resistance of ugt76b1 against Pseudomonas syringae DC3000 is partially mediated through NPR1 and SID2
The ugt76b1 loss-of-function mutant showed activated defense against Pseudomonas syringae pv tomato DC3000 (von Saint Paul et al. 2011), which was attributed to the glucosylation and inactivation of the immune-stimulatory ILA, SA, and NHP by UGT76B1 (Bauer et al. 2021; Holmes et al. 2021; Mohnike et al. 2021). Pip mediates both SID2-dependent and independent defenses via FMO1 encoding the NHP-synthesizing enzyme (Bernsdorff et al. 2016; Vlot et al. 2021). Finally, NPR1, downstream of stress-induced SA biosynthesis, is the master regulator of the SA defense pathway. To exam the roles of SID2 and NPR1 in ugt76b1-activated immunity, we compared P. syringae infection of ugt76b1-1 npr1 and ugt76b1-1 sid2 double mutants with wild type and the corresponding npr1 and sid2 single mutants. Both ugt76b1 npr1 and ugt76b1 sid2 showed enhanced bacterial growth compared to Col plants, indicating that the higher resistance of ugt76b1 is positively regulated by and dependent on both NPR1 and SID2 (Fig. 3). However, when compared to the npr1 single mutant, ugt76b1 npr1 showed reduced bacterial proliferation, suggesting a partially NPR1-independent enhancement of resistance due to the loss of UGT76B1. When compared to the sid2 mutant, further resistance gained by ugt76b1 sid2 indicated a partial SID2-independent regulation as well (Fig. 3).
The impact of UGT76B1 on susceptibility towards Pseudomonas syringae DC3000 infection has an NPR1- and SID2-dependent component. Bacterial growth in inoculated Arabidopsis leaves of 4-week-old plants was quantified. Arithmetic means and standard errors from log10-transformed data of at least four independent replicates from five separate experiments are displayed. A linear mixed effect model was used to account for random effects from the experiment. For each time point, Tukey post hoc tests were performed to compare all pairs of groups (only specific comparisons of single and matched double mutants are shown). Computations were done in R using the packages nlme and multcomp; ***P value ≤0.001; **P value ≤0.01. No significances were observed among T0
The antagonistic impact of ugt76b1 on the SA and JA pathways depends on EDS1, NPR1, and FMO1
The loss of UGT76B1 results in the antagonistic repression of the SA pathway and activation of the JA pathway (von Saint Paul et al. 2011). ugt76b1 sid2-1, ugt76b1 npr1, ugt76b1 eds1, and ugt76b1 fmo1 double mutants were employed to test whether the impact of ugt76b1 on the SA–JA crosstalk is influenced by NPR1, EDS1, and FMO1. The induction of SA marker PR1 and suppression of JA marker VSP2 of ugt76b1 were relying on SID2 (von Saint Paul et al. 2011). The enhanced expression of PR1 and SAG13 and the suppression of VSP2, a marker of MYC2/JIN1-mediated branch by ugt76b1 are completely dependent on NPR1 (Fig. 4). Similarly, the loss of EDS1 and FMO1 abolishes both the induction of PR1 and SAG13 and the suppression of VSP2 (Fig. 4), although there is a not significant tendency that PR1 can be further induced in fmo1 by introgressing ugt76b1 (Fig. 4). Thus, the activation of the SA pathway and the suppression of the JA pathway by the loss of UGT76B1 is dependent on SA and NHP biosynthesis and NPR1 signaling.
Marker gene expression in ugt76b1 knockout after introgression of npr1, fmo1, and eds1. Gene expression of PR1, SAG13, and VSP2 in four-week-old ugt76b1-1 and ugt76b1 double mutants with npr1, fmo1, and eds1 was measured by RT-qPCR. Expression levels were normalized to UBIQUITIN5 and S16 transcripts; levels relative to Col wild-type plants are displayed. Arithmetic means and standard errors from log10-transformed data of three independent replicates from two separate experiments are displayed. The dashed, horizontal lines indicate a twofold change. Statistical analysis was performed by the software R using two-way analysis of variance (ANOVA; linear mixed effect models) followed by post hoc Tukey’s HSD test correction. ***P value ≤0.001
The early senescence upon loss of UGT76B1 relies on EDS1, FMO1, and NPR1
The early senescence of ugt76b1 requires basal SA level (von Saint Paul et al. 2011). The master regulator NPR1 was reported to positively influence senescence (Yoshimoto et al. 2009; Zheng and Dong 2013). EDS1 regulates plant immunity via both SID2-mediated SA synthesis and an SID2-independent manner upstream of FMO1 (Bartsch et al. 2006). FMO1 controls SAR in both SA-dependent and independent manners (Bernsdorff et al. 2016; Hartmann and Zeier 2018; Hartmann et al. 2018). Therefore, to explore the dependence of the senescence phenotype of ugt76b1 on NPR1, EDS1, and FMO1 aging was observed for ugt76b1 npr1, ugt76b1 eds1, and ugt76b1 fmo1 double mutants. The early senescence of ugt76b1 is completely relying on NPR1, EDS1, and FMO1 (Fig. 5).
The impact of UGT76B1 expression on the onset of senescence is dependent on NPR1, FMO1, and EDS1. Six-week-old wild type (Col), ugt76b1, npr1, ugt76b1 npr1, fmo1, ugt76b1 fmo1, eds1, and ugt76b1 eds1. Senescence is indicated by yellowing of leaves of ugt76b1 (arrows), which is eliminated by the introgression of npr1, fmo1, or eds1. Similar results were observed in independent growth campaigns. Bar 1 cm
Discussion
UGT76B1 competitively glucosylates and inactivates the immune-stimulating SA, ILA, and NHP and thereby keeps defense in check in naïve, uninfected plants. SA and NHP accumulate after infection and their abundance positively correlates with the resistance to pathogens. Thus, the enhanced SAR-like defense status of ugt76b1 was primarily linked to a higher level of SA and NHP (Bauer et al. 2021; Cai et al. 2021; Holmes et al. 2021; Mohnike et al. 2021; von Saint Paul et al. 2011). To explore the dependence of the activated immunity of ugt76b1 on SA or NHP and to discover potential SA-unrelated effects, genes altered by ugt76b1 were first classified as SA-responsive or non-SA responsive according to the responsiveness to exogenous SA and the SA analogue BTH. Most of the SA-responsive genes of ugt76b1 show SID2 and NPR1 dependence based on the responsiveness of npr1 and sid2 to pathogen infections (Supplementary Table 1). Thus, UGT76B1 has a key role in suppressing a set of SA-responsive genes, which are mainly regulated via SID2 and NPR1. The important role of UGT76B1 in suppressing the SA-responsive group is consistent with the function of UGT76B1 to glucosylate SA. NPR1 independence within the SA signaling pathway may require the WHIRLY (WHY) transcription factor family (Desveaux et al. 2004, 2005; Vlot et al. 2009). However, very few genes of the SA-responsive group showed SID2 dependence, yet NPR1 independence in response to pathogen infections, thereby suggesting the existence of an independent link (Supplementary Table 1; Fig. 6: factor Y).
UGT76B1’s impact on defense pathways. NHP and SA are synthesized by FMO1 and SID2, respectively, and controlled by a common regulator EDS1. UGT76B1 glucosylates NHP and SA and thereby inhibits their immune-activating action. Thus, the loss of UGT76B1 activates SID2- and NPR1-dependent SA signaling. Within the SA pathway, some genes are regulated dependent on SID2, however independent from NPR1, suggesting the existent of an additional path or factor (“Y”). Furthermore, transcriptome analysis of ugt76b1 revealed another group of non-SA responsive genes appearing to be SID2- and NPR1-independent, this group also includes ABA-regulated genes. The non-SA responses may regulate ABA-related abiotic stresses for instance salt stress as well. Non-SA responsive, however SID2- and NPR1-independently induced genes such as WRKY55 may regulate SA biosynthesis. Compared with Hartmann et al. (2018), 71 ugt76b1-upregulated genes overlapped with SAR-induced genes, which are completely dependent on FMO1. Furthermore, many genes within non-SA responsive group showed regulation at least partially relying on functional NPR1. The suppression of the JA marker VSP2 in the ugt76b1 mutant scenario requires EDS1, FMO1, and NPR1 probably due to the repressive effect of SA pathway. Both SID2-dependent and independent defense responses and senescence development of ugt76b1 rely on FMO1. The dashed lines indicate hypothetical relations
Moreover, many genes among the SA-responsive group can be regulated by pathogen infections independent from both SID2 and NPR1 (Supplementary Table 1 and 11), suggesting the existence of an independent signaling pathway that can target the same genes as SA. Since both SA and NHP accumulated to a higher level in ugt76b1, NHP may be the relevant signal. Indeed, 47 among 51 SA-responsive, but independently from SID2 and NPR1 ugt76b1-upregulated genes and 39 out of 53 ugt76b1-suppressed genes overlapped with SAR-induced genes (Hartmann et al. 2018; Supplementary Table 11). All these SAR-regulated genes are controlled by FMO1, which is responsible for producing NHP, suggesting that NHP itself is involved in this signaling pathway. FMO1 and its product NHP are known to regulate SA biosynthesis to enhance plant defense and SA is required for fully realizing the NHP-triggered defense (Vlot et al. 2021; Yildiz et al. 2021) (Fig. 6). However, NHP also can regulate defense responses independent from SA biosynthesis, i.e., it can still induce plant immunity in sid2 (Bernsdorff et al. 2016; Yildiz et al. 2021; Zeier 2021). Moreover, NHP accumulation is independent from SID2, i.e., SA biosynthesis (Bauer et al. 2021; Hartmann and Zeier 2018; Hartmann et al. 2018; Zeier 2021). Thus, the enhanced level of NHP of ugt76b1 plants may point to the existence of an SID2-independent defense regulation apart from the immediate effect of the missing NHP glucosylation by UGT76B1 (Bauer et al. 2021; Holmes et al. 2021; Mohnike et al. 2021). Consistently, many genes of the non-SA responsive group of ugt76b1 are highly responsive to pathogen infections independent from SID2 (Table 2 and Supplementary Table 2). SAR triggerd by exogenous application of NHP requires functional NPR1 (Yildiz et al. 2021). Among the non-SA-responsive group regulated by ugt76b1, many genes indeed showed NPR1-dependent; however, SID2-independent upregulation responding to pathogen infections, suggesting that ugt76b1-triggered non-SA responsive plant defense is caused by NHP accumulation and at least partially relies on NPR1 (Fig. 6). However, the extent of intercellular hyphae development and oospore formation was significantly reduced by NHP when infecting npr1 plants with the oomycete Hyaloperonospora arabidopsidis, suggesting the residual NPR1-independent defense response induced by NHP (Yildiz et al. 2021). Seventy non-SA responsive genes of ugt76b1-regulated genes showed both SID2- and NPR1-independent pathogen responses. FMO1 ranks top among the SID2/NPR1-independent defense genes upregulated by ugt76b1 (Table 2). However, FMO1 induction by SAR also shows SID2 independence. The SID2-independent defense regulation in SAR is completely relying on FMO1 and its product NHP (Bernsdorff et al. 2016; Gruner et al. 2013; Hartmann and Zeier 2018; Hartmann et al. 2018). Consistently, the resistance of ugt76b1 against Pseudomonas syringae is partially dependent on both SID2 and NPR1 (Fig. 3), however, completely relying on FMO1 (Bauer et al. 2021). Seventy non-SA responsive genes showing SID2/NPR1-independent pathogen responses (Table 2) overlap with SAR-upregulated genes, which are completely dependent on FMO1 (Supplementary Table 9) (Hartmann et al. 2018). Moreover, there is a close coexpression between UGT76B1 and FMO1 (Supplementary Fig. 2). Thus, except the influence caused by the lost ability to glucosylate SA in ugt76b1, the impact of ugt76b1 on plant defense has also an SID2/NPR1-independent component regulated via FMO1 (Fig. 6), since its product NHP can be competitively glucosylated by UGT76B1 as well. FMO1 and its product NHP could be responsible for the SID2/NPR1-independent pathogen resistance of ugt76b1 (Fig. 6).
Besides FMO1, 70 further, ugt76b1-upregulated genes can be induced by pathogen infections independent from SID2 and NPR1 (Table 2). NHP is known to activate SA biosynthesis genes (Vlot et al. 2021; Yildiz et al. 2021).These genes (Fig. 6: X factor) may be the downstream targets of FMO1 and NHP, which can further amplify the defense response for instance to regulate SA biosynthesis. For instance, WKRY55 mediates defense response and senescence development through manipulating SA biosynthesis (Wang et al. 2020). SRG1, together with SRG2 and SRG3, are positive regulators of SA-controlling plant immunity (Cui et al. 2021). Apart from manpulating SA signaling, some other X genes regulated by ugt76b1 may impact defense by different mechanisms. For instance, KTI1 inhibits cell death to result in the enhanced susceptibility towards pathogens (Li et al. 2008), whereas CRK20 mediates the favorable apoplastic conditions to promote pathogen proliferation (Ederli et al. 2011). The camalexin biosynthesis-regulating gene CYP71A12 favors the resistance by increasing the accumulation of camalexin (Lemarie et al. 2015), and overexpression of RABA4C causes resistance against pathogens by promoting the deposition of callose (Ellinger et al. 2014). The transcription factor MYB15 encodes a positive regulator inducing lignin accumulation to fight against pathogens (Chezem et al. 2017). The gene PUB22 ubiquitinates and degrades a positive regulator of PAMP-triggered immunity (Stegmann et al. 2012). The pathogen-induced CAM-binding protein-encoding gene PICBP is highly induced after pathogen infections (Reddy et al. 2003). The terpene synthase TPS4 contributes to the resistance against pathogens by terpene production (Attaran et al. 2008). These examples suggest that the loss of UGT76B1 triggers a broad activation of immunity including many aspects. Furthermore, two other ABA-related genes, CSAP and JUL1, were found to participate in ABA-mediated senescence and tolerance to drought stress, respectively (So et al. 2020; Yu et al. 2020). By now, their roles in immunity are not confirmed yet. This suggests that X factors may include ABA-mediated genes and X factors may regulate ABA-mediates responses such as salt stress (Fig. 6). Indeed, many ABA-responsive genes are regulated by SAR, however, still dependent on FMO1 to be induced (Gruner et al. 2013). In agreement with this, the GO term enrichment also indicates that ugt76b1-upregulated genes are over-represented in genes related to “response to abscisic acid” and “response to salt stress” categories (Fig. 2a). Therefore, the enhanced immunity status of ugt76b1 may be partially due to ABA-mediated responses as well and /or indicates an link of UGT76B1 to abiotic stresses (Fig. 6), ABA-mediated abiotic stresses such as salt stresses require to be explored in ugt76b1 mutants in future studies. Since the enhanced resistance of ugt76b1 is fully determined by FMO1 (Bauer et al. 2021) and all these 70 genes are overlapping with FMO1-dependent SAR-induced genes, the SID2- and NPR1-independent regulation is likely to be controlled by FMO1 and its product NHP. In the future, more signaling components downstream of FMO1 should be explored as well.
Antagonism between SA and JA pathways is extensively studied and conserved in many different species (Pieterse et al. 2012). Treatment with SA or pathogen infection suppresses JA-regulated VSP2 expression in Arabidopsis (Koornneef et al. 2008; Leon-Reyes et al. 2009), which also requires NPR1 (Spoel et al. 2003). SID2 is necessary for regulating SA–JA crosstalk, including PR1 regulation, enhanced senescence, and suppression of VSP2 by ugt76b1 (von Saint Paul et al. 2011). Similar to SID2, NPR1 is required for ugt76b1 to suppress the JA pathway, e.g., VSP2 expression (Figs. 4 and 6). Moreover, EDS1, upstream of both SA and NHP biosynthesis, and FMO1, known to regulate stress-induced SA biosynthesis upstream of SID2 (Mishina and Zeier 2006; Vlot et al. 2021) are required for the induction of SA response and suppression of the JA response in ugt76b1 as well (Figs. 4 and 5). Therefore, the need of EDS1 and FMO1 for ugt76b1 to influence SA–JA crosstalk may be related to the impact of ugt76b1 on SA biosynthesis. Furthermore, Yan et al. (2014) showed that MeJA treatment of Arabidopsis seedlings suppresses ALD1 expression and Pip levels, suggesting a suppression of Pip biosynthesis by JA. In turn, Pip (or NHP) may confer direct suppression on JA pathway as well. Nevertheless, it cannot be excluded that FMO1 and NHP may directly suppress the JA pathway of ugt76b1, independent from SA. The requirement of NPR1, EDS1, and FMO1 in developing early senescence of ugt76b1 may be due to the need of integrate SA pathway.
Together, UGT76B1 impacts plant immunity by both SID2- and NPR1-dependent and independent regulation. The SID2- and NPR1-dependent regulation is mainly due to the lost ability of UGT76B1 to glucosylate SA, whereas the SID2- and NPR1-independent regulation is relying on FMO1 and its product NHP. The identified SID2- and NPR1-independent defense genes among the non-SA-responsive group of ugt76b1-regulated genes illustrate the importance of an additional regulation not associated with SA signaling which is controlled by UGT76B1 via manipulation of NHP abundance.
Data availability
The raw data of the microarray expression are availabale at https://www.ebi.ac.uk/biostudies/arrayexpress/studies/ with ther code E-MTAB-13784.
References
Attaran E, Rostás M, Zeier J (2008) Pseudomonas syringae elicits emission of the terpenoid (E, E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene in Arabidopsis leaves via jasmonate signaling and expression of the terpene synthase TPS4. Mol Plant Microbe Interact 21:1482–1497. https://doi.org/10.1094/MPMI-21-11-1482
Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18:1038–1051. https://doi.org/10.1105/tpc.105.039982
Bauer S, Mekonnen DW, Hartmann M, Yildiz I, Janowski R, Lange B, Geist B, Zeier J, Schäffner AR (2021) UGT76B1, a promiscuous hub of small molecule-based immune signaling, glucosylates N-hydroxypipecolic acid, and balances plant immunity. Plant Cell 33:714–734. https://doi.org/10.1093/plcell/koaa044
Berardini TZ, Reiser L, Li D, Mezheritsky Y, Muller R, Strait E, Eva H (2015) The Arabidopsis information resource: making and mining the “gold standard” annotated reference plant genome. Genesis 53:474–485. https://doi.org/10.1002/dvg.22877
Berger S, Bell E, Mullet JE (1996) Two methyl jasmonate-insensitive mutants show altered expression of AtVsp in response to methyl jasmonate and wounding. Plant Physiol 111:525–531. https://doi.org/10.1104/pp.111.2.525
Bernsdorff F, Doring AC, Gruner K, Schuck S, Bräutigam A, Zeier J (2016) Pipecolic acid orchestrates plant systemic acquired resistance and defense priming via salicylic acid-dependent and -independent pathways. Plant Cell 28:102–129. https://doi.org/10.1105/tpc.15.00496
Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X (1997) The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 9:1573–1584. https://doi.org/10.1105/tpc.9.9.1573
Cai J, Jozwiak A, Holoidovsky L, Meijler MM, Meir S, Rogachev I, Aharoni A (2021) Glycosylation of N-hydroxy-pipecolic acid equilibrates between systemic acquired resistance response and plant growth. Mol Plant 14:440–455. https://doi.org/10.1016/j.molp.2020.12.018
Cao H, Bowling SA, Gordon AS, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6:1583–1592. https://doi.org/10.1105/tpc.6.11.1583
Chezem WR, Memon A, Li FS, Weng JK, Clay NK (2017) SG2-type R2R3-MYB transcription factor MYB15 controls defense-induced lignification and basal immunity in Arabidopsis. Plant Cell 29:1907–1926. https://doi.org/10.1105/tpc.16.00954
Clarke JD, Liu Y, Klessig DF, Dong X (1998) Uncoupling PR gene expression from NPR1 and bacterial resistance: characterization of the dominant Arabidopsis cpr6-1 mutant. Plant Cell 10:557–569. https://doi.org/10.1105/tpc.10.4.557
Cui B, Xu S, Li Y, Umbreen S, Frederickson D, Yuan B, Jiang J, Liu F, Pan Q, Loake GJ (2021) The Arabidopsis zinc finger proteins SRG2 and SRG3 are positive regulators of plant immunity and are differentially regulated by nitric oxide. New Phytol 230:259–274. https://doi.org/10.1111/nph.16993
Desveaux D, Subramaniam R, Despres C, Mess JN, Levesque C, Fobert PR, Dangl JL, Brisson N (2004) A “Whirly” transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Dev Cell 6:229–240. https://doi.org/10.1016/S1534-5807(04)00028-0
Desveaux D, Marechal A, Brisson N (2005) Whirly transcription factors: defense gene regulation and beyond. Trends Plant Sci 10:95–102. https://doi.org/10.1016/j.tplants.2004.12.008
Dewdney J, Reuber TL, Wildermuth MC, Devoto A, Cui J, Stutius LM, Drummond EP, Ausubel FM (2000) Three unique mutants of Arabidopsis identify eds loci required for limiting growth of a biotrophic fungal pathogen. Plant J 24:205–218. https://doi.org/10.1046/j.1365-313x.2000.00870.x
Ding P, Ding Y (2020) Stories of salicylic acid: a plant defense hormone. Trends Plant Sci 25:549–565. https://doi.org/10.1016/j.tplants.2020.01.004
Ding Y, Dommel MR, Wang C, Li Q, Zhao Q, Zhang X, Dai S, Mou Z (2020) Differential quantitative requirements for NPR1 between basal immunity and systemic acquired resistance in Arabidopsis thaliana. Front Plant Sci 11:570–422. https://doi.org/10.3389/fpls.2020.570422
Ederli L, Madeo L, Calderini O, Gehring C, Moretti C, Buonaurio R, Paolocci F, Pasqualini S (2011) The Arabidopsis thaliana cysteine-rich receptor-like kinase CRK20 modulates host responses to Pseudomonas syringae pv. tomato DC3000 infection. J Plant Physiol 168:1784–1794. https://doi.org/10.1016/j.jplph.2011.05.018
Ellinger D, Glockner A, Koch J, Naumann M, Sturtz V, Schutt K, Manisseri C, Somerville SC, Voigt CA (2014) Interaction of the Arabidopsis GTPase RabA4c with its effector PMR4 results in complete penetration resistance to powdery mildew. Plant Cell 26:3185–3200. https://doi.org/10.1105/tpc.114.127779
Georgii E, Jin M, Zhao J, Basem K, Philippe S, Andreas A, Barbro W, Anton RS (2017) Relationships between drought, heat and air humidity responses revealed by transcriptome-metabolome co-analysis. BMC Plant Biol 17:120. https://doi.org/10.1186/s12870-017-1062-y
Gruner K, Griebel T, Navarova H, Attaran E, Zeier J (2013) Reprogramming of plants during systemic acquired resistance. Front Plant Sci 4:252. https://doi.org/10.3389/fpls.2013.00252
Gruner K, Zeier T, Aretz C, Zeier J (2018) A critical role for Arabidopsis MILDEW RESISTANCE LOCUS O2 in systemic acquired resistance. Plant J 94:1064–1082. https://doi.org/10.1111/tpj.13920
Hartmann M, Zeier J (2018) l-lysine metabolism to N-hydroxypipecolic acid: an integral immune-activating pathway in plants. Plant J 96:5–21. https://doi.org/10.1111/tpj.14037
Hartmann M, Zeier T, Bernsdorff F, Reichel-Deland V, Kim D, Hohmann M, Scholten N, Schuck S, Bräutigam A, Holzel T, Ganter C, Zeier J (2018) Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173:456–469. https://doi.org/10.1016/j.cell.2018.02.049
Holmes EC, Chen YC, Mudgett MB, Sattely ES (2021) Arabidopsis UGT76B1 glycosylates N-hydroxy-pipecolic acid and inactivates systemic acquired resistance in tomato. Plant Cell 33:750–765. https://doi.org/10.1093/plcell/koaa052
Huang W, Wang Y, Li X, Zhang Y (2020) Biosynthesis and regulation of salicylic acid and N-hydrooxypipecolic acid in plant immunity. Mol Plant 13:31–41. https://doi.org/10.1016/j.molp.2019.12.008
Katagiri F, Thilmony R, He SY (2002) The Arabidopsis thaliana-Pseudomonas syringae interaction. Arabidopsis Book 1:e0039. https://doi.org/10.1199/tab.0039
Koornneef A, Leon-Reyes A, Ritsema T, Verhage A, Den Otter FC, Van Loon LC, Pieterse CM (2008) Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol 147:1358–1368. https://doi.org/10.1104/pp.108.121392
Lemarie S, Robert-Seilaniantz A, Lariagon C, Lemoine J, Marnet N, Levrel A, Jubault M, Manzanares-Dauleux MJ, Gravot A (2015) Camalexin contributes to the partial resistance of Arabidopsis thaliana to the biotrophic soilborne protist Plasmodiophora brassicae. Front Plant Sci 6:539. https://doi.org/10.3389/fpls.2015.00539
Leon-Reyes A, Spoel SH, De Lange ES, Abe H, Kobayashi M, Tsuda S, Millenaar FF, Welschen RA, Ritsema T, Pieterse CM (2009) Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol 149:1797–1809. https://doi.org/10.1104/pp.108.133926
Li J, Brader G, Palva ET (2008) Kunitz trypsin inhibitor: an antagonist of cell death triggered by phytopathogens and fumonisin b1 in Arabidopsis. Mol Plant 1:482–495. https://doi.org/10.1093/mp/ssn013
Lopukhina A, Dettenberg M, Weiler EW, Hollander-Czytko H (2001) Cloning and characterization of a coronatine-regulated tyrosine aminotransferase from Arabidopsis. Plant Physiol 126:1678–1687. https://doi.org/10.1104/pp.126.4.1678
Mishina TE, Zeier J (2006) The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiol 141:1666–1675. https://doi.org/10.1104/pp.106.081257
Mohnike L, Rekhter D, Huang W, Feussner K, Tian H, Herrfurth C, Zhang Y, Feussner I (2021) The glycosyltransferase UGT76B1 modulates N-hydroxy-pipecolic acid homeostasis and plant immunity. Plant Cell 33:735–749. https://doi.org/10.1093/plcell/koaa045
Nawrath C, Metraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11:1393–1404. https://doi.org/10.1105/tpc.11.8.1393
Onate-Sanchez L, Singh KB (2002) Identification of Arabidopsis ethylene-responsive element binding factors with distinct induction kinetics after pathogen infection. Plant Physiol 128:1313–1322. https://doi.org/10.1104/pp.010862
Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521. https://doi.org/10.1146/annurev-cellbio-092910-154055
Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT (1999) The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11:1695–1708. https://doi.org/10.1105/tpc.11.9.1695
Reddy VS, Ali GS, Reddy ASN (2003) Characterization of a pathogen-induced calmodulin-binding protein: mapping of four Ca2+-dependent calmodulin-binding domains. Plant Mol Biol 52:143–159. https://doi.org/10.1023/A:1023993713849
Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B (2003) An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol 53:247–259. https://doi.org/10.1023/B:PLAN.0000009297.37235.4a
Scholl RL, May ST, Ware DH (2000) Seed and molecular resources for Arabidopsis. Plant Physiol 124:1477–1480. https://doi.org/10.1104/pp.124.4.1477
Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, Clarke JD, Cotton D, Bullis D, Snell J, Miguel T, Hutchison D, Kimmerly B, Mitzel T, Katagiri F, Glazebrook J, Law M, Goff SA (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14:2985–2994. https://doi.org/10.1105/tpc.004630
Shah J, Kachroo P, Klessig DF (1999) The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defensin gene expression salicylic acid dependent. Plant Cell 11:191–206. https://doi.org/10.1105/tpc.11.2.191
Shah J, Kachroo P, Nandi A, Klessig DF (2001) A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J 25:563–574. https://doi.org/10.1046/j.1365-313x.2001.00992.x
Shields A, Shivnauth V, Castroverde CDM (2022) Salicylic acid and N-Hydroxypipecolic acid at the fulcrum of the plant immunity-growth equilibrium. Front Plant Sci 13:841688. https://doi.org/10.3389/fpls.2022.841688
So WM, Kim SY, Hyoung S, Shin JS (2020) The novel protein CSAP accelerates leaf senescence and is negatively regulated by SAUL1 in the dark. Plant Cell Rep 39:325–334. https://doi.org/10.1007/s00299-019-02493-z
Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12:3703–3714. https://doi.org/10.1101/gad.12.23.3703
Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Metraux JP, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CM (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15:760–770. https://doi.org/10.1105/tpc.009159
Stegmann M, Anderson RG, Ichimura K, Pecenkova T, Reuter P, Zarsky V, McDowell JM, Shirasu K, Trujillo M (2012) The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 24:4703–4716. https://doi.org/10.1105/tpc.112.104463
Swain S, Roy S, Shah J, Van Wees S, Pieterse CM, Nandi AK (2011) Arabidopsis thaliana cdd1 mutant uncouples the constitutive activation of salicylic acid signalling from growth defects. Mol Plant Pathol 12:855–865. https://doi.org/10.1111/j.1364-3703.2011.00717.x
Swain S, Singh N, Nandi AK (2015) Identification of plant defence regulators through transcriptional profiling of Arabidopsis thaliana cdd1 mutant. J Biosci Bioeng 40:137–146. https://doi.org/10.1007/s12038-014-9498-9
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:00341–003411. https://doi.org/10.1186/gb-2002-3-7-research0034
Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206. https://doi.org/10.1146/annurev.phyto.050908.135202
Vlot AC, Sales JH, Lenk M, Bauer K, Brambilla A, Sommer A, Chen Y, Wenig M, Nayem S (2021) Systemic propagation of immunity in plants. New Phytol 229:1234–1250. https://doi.org/10.1111/nph.16953
von Saint Paul V, Zhang W, Kanawati B, Geist B, Faus-Kessler T, Schmitt-Kopplin P, Schäffner AR (2011) The Arabidopsis glucosyltransferase UGT76B1 conjugates isoleucic acid and modulates plant defense and senescence. Plant Cell 23:4124–4145. https://doi.org/10.1105/tpc.111.088443
Wang Y, Cui X, Yang B, Xu S, Wei X, Zhao P, Niu F, Sun M, Wang C, Cheng H, Jiang YQ (2020) WRKY55 transcription factor positively regulates leaf senescence and the defense response by modulating the transcription of genes implicated in the biosynthesis of reactive oxygen species and salicylic acid in Arabidopsis. Co Biol 147:dev189647. https://doi.org/10.1242/dev.189647
Yan H, Yoo MJ, Koh J, Liu L, Chen Y, Acikgoz D, Wang Q, Chen S (2014) Molecular reprogramming of Arabidopsis in response to perturbation of jasmonate signaling. J Proteome Res 13:5751–5766. https://doi.org/10.1021/pr500739v
Yildiz I, Mantz M, Hartmann M, Zeier T, Kessel J, Thurow C, Gatz C, Petzsch P, Kohrer K, Zeier J (2021) The mobile SAR signal N-hydroxypipecolic acid induces NPR1-dependent transcriptional reprogramming and immune priming. Plant Physiol 186:1679–1705. https://doi.org/10.1093/plphys/kiab166
Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K (2009) Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21:2914–2927. https://doi.org/10.1105/tpc.109.068635
Yu SG, Kim JH, Cho NH, Oh TR, Kim WT (2020) Arabidopsis RING E3 ubiquitin ligase JUL1 participates in ABA-mediated microtubule depolymerization, stomatal closure, and tolerance response to drought stress. Plant J 103(2):824–842. https://doi.org/10.1111/tpj.14775
Zeier J (2021) Metabolic regulation of systemic acquired resistance. Curr Opin Plant Biol 62:102050. https://doi.org/10.1016/j.pbi.2021.102050
Zhang Z, Lenk A, Andersson MX, Gjetting T, Pedersen C, Nielsen ME, Newman MA, Hou BH, Somerville SC, Thordal-Christensen H (2008) A lesion-mimic syntaxin double mutant in Arabidopsis reveals novel complexity of pathogen defense signaling. Mol Plant 1:510–527. https://doi.org/10.1093/mp/ssn011
Zheng Q, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64:839–863. https://doi.org/10.1146/annurev-arplant-042811-105606
Zimmermann P, Hennig L, Gruissem W (2005) Gene-expression analysis and network discovery usingGenevestigator. Trends Plant Sci 10(9):407–9. https://doi.org/10.1016/j.tplants.2005.07.003
Acknowledgements
The authors are grateful to Sibylle Bauer, A. Corina Vlot-Schuster and Jörg Durner for discussions.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work of Wei Zhang was supported by the National Natural Science Foundation of China (NSFC Grant Nos. 31600993) and Scholarship from China Scholarship Council and research grants from JiangSu University [Grant number 21JDG073].
Author information
Authors and Affiliations
Contributions
ARS and WZ conceived and designed the project. RM helped with the pathogen infection experiments. EG contributed to the analysis of real-time PCR and microarray data. BG provided expertise technical assistance. WZ performed all other experiments. WZ evaluated the data and wrote the manuscript. ARS and WZ finally edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Chun-Hai Dong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, W., Maksym, R., Georgii, E. et al. SA and NHP glucosyltransferase UGT76B1 affects plant defense in both SID2- and NPR1-dependent and independent manner. Plant Cell Rep 43, 149 (2024). https://doi.org/10.1007/s00299-024-03228-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00299-024-03228-5