Abstract
Key message
Retromer protein AtVPS29 upregulates the SLY1 protein and downregulates the RGA protein, positively stimulating the development of the root meristematic zone, which indicates an important role of AtVPS29 in gibberellin signaling.
Abstract
In plants, the large retromer complex is known to play roles in multiple development processes, including cell polarity, programmed cell death, and root hair growth in Arabidopsis. However, many of its roles in plant development remain unknown. Here, we show that Arabidopsis trimeric retromer protein AtVPS29 (vacuolar protein sorting 29) modulates gibberellin signaling. The SLEEPY1 (SLY1) protein, known as a positive regulator of gibberellic acid (GA) signaling, exhibited lower abundance in vps29-3 mutants compared to wild-type (WT) plants. Conversely, the DELLA repressor protein, targeted by the E3 ubiquitin ligase SCF (Skp, Cullin, F-box) complex and acting as a negative regulator of GA signaling, showed increased abundance in vps29-3 mutants compared to WT. The vps29-3 mutants exhibited decreased sensitivity to exogenous GA supply in contrast to WT, despite an upregulation in the expression of GA receptor genes within the vps29-3 mutants. In addition, the expression of the GA synthesis genes was downregulated in vps29-3 mutants, implying that the loss of AtVPS29 causes the downregulation of GA synthesis and signaling. Furthermore, vps29-3 mutants exhibited a reduced meristematic zone accompanied by a decreased cell number. Together, these data indicate that AtVPS29 positively regulates SLY1-mediated GA signaling and plant growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytohormone GAs regulate plant development and growth at various developmental stages, including germination. For example, defects in GA synthesis or signaling cause non-germination or poor germination, severe dwarfism, and delayed flowering (Daviere and Achard 2013).
The stability and activity of proteins involved in signal transduction mediated by GA are regulated by post-translational modifications. First, the stability of DELLA proteins is regulated through the ubiquitination by the activity of single polypeptide or SCF complex E3 ubiquitin ligases. The F-box protein of the SCF complex plays a role in selecting target proteins for polyubiquitination by the E3 ligase activity of the SCF complex (Hua and Vierstra 2011). The Arabidopsis SLY1 gene encodes the F-box subunit that acts as a bridge between Skp and DELLA proteins (McGinnis et al. 2003; Dill et al. 2004; Fu et al. 2004). DELLA proteins are transcriptional regulators that contain a conserved DELLA motif (Asp-Glu-Leu-Leu-Ala) in the N-terminal region and negatively regulate GA signaling. DELLA proteins directly interact with SLY1 and are rapidly degraded by the 26S proteasome complex after polyubiquitination. Proteins involved in GA signaling are also regulated by sumoylation pathway. SLY1 directly interacts with and is sumoylated by the E3 SUMO (Small Ubiquitin-related Modifier) ligase AtSIZ1 (Kim et al. 2015). The level of SLY1 is much lower in siz1-2 mutants than in wild-type plants, and the level of sumoylated SLY1 is greatly increased by exogenous GA supply. DELLA protein RGA (REPRESSOR of ga1-3) is also sumoylated, although its E3 SUMO ligase has not been identified yet (Conti et al. 2014). Furthermore, RGA is more highly accumulated in the SUMO protease ots1/ots2 mutants than in wild-type Arabidopsis, indicating that the SUMO-mediated pathway affects DELLA stability.
Retromers were initially characterized in yeast (Paravicini et al. 1992; Nothwehr and Hindes 1997; Seaman et al. 1997). They are protein complexes composed of a large subunit consisting of VPS26, VPS29, and VPS35 proteins and a small subunit consisting of sorting nexin dimers (Chandra et al. 2020). In yeast, this small subunit includes VPS5, while in animals and plants, it comprises SNX1/2 (Sorting Nexin 1/2) (Chandra et al. 2020). Retromers are responsible for transporting membrane proteins from the late endosome to the trans-Golgi network (Horazdovsky et al. 1997; Seaman et al. 1997; Tu and Seaman 2021). In plants, VPS26, VPS29, and VPS35 are localized to the prevacuolar compartment (Hashiguchi et al. 2010; Jha and Larson 2023) and exhibit potential interactions with each other (Jaillais et al. 2007; Zelazny et al. 2013; Jha and Larson 2023). These complexes are known to be involved in various plant developmental processes, including programmed cell death (Münch et al. 2015), oil body biogenesis and degradation during vegetative growth (Thazar-Poulot et al. 2015), and root hair growth (Jha et al. 2018; Jha and Larson 2023). Notably, VPS29 is implicated in the maturation of storage proteins 12S globulin and 2S albumin during seed development (Shimada et al. 2006), as well as in establishing cell polarity during plant organogenesis by influencing the subcellular trafficking of PIN proteins (Jaillais et al. 2007).
Accumulated data prove that SLY1 positively regulates growth through GA signaling. For instance, sly1 mutants show increased seed dormancy and hypersensitivity to the inhibition of seed germination by abscisic acid (Strader et al. 2004). In addition, sly1 plants display severe dwarfism and recover their phenotypes to the wild-type level in the absence of DELLA repressors (Dill et al. 2004; Fu et al. 2004; Olszewski et al. 2002). The vps29-3 mutant also exhibits a severe dwarf phenotype commonly found in GA signaling-defective plants (Jaillais et al. 2007; Jha and Larson 2023). Notably, it has been reported that GA targets the retromer complex for trafficking modulation (Salanenka et al. 2018). Low GA levels promote the vacuolar delivery and degradation of multiple cargos, including PIN proteins, while high GA levels promote their recycling to the plasma membrane (PM). All of these data imply that SLY1-mediated GA signaling can be connected with protein trafficking by retromer complex.
Therefore, we questioned whether AtVPS29 affects GA signaling in Arabidopsis. We found that the level of SLY1 was much lower in the vps29-3 mutant compared to the wild-type plants, while the DELLA protein RGA was highly accumulated in the vps29-3 mutant. Both shoot and root growth of vps29-3 mutants displayed reduced sensitivity to exogenous GA. In addition, vps29-3 mutants showed a shortened meristematic zone and a decreased cell number. Taken together, we present evidence that AtVPS29 plays a significant regulatory role in plant development through its involvement in GA signaling.
Materials and methods
Plant materials and growth conditions
The Arabidopsis thaliana Columbia-0 ecotype (wild-type) and the T-DNA insertion knock-out mutant vps29-3 were used in this study. The vps29-3 (Columbia accession, SALK010106) T-DNA mutant line was obtained from ABRC. For plants grown on plates, seeds were surface sterilized in commercial bleach containing 5% sodium hypochlorite and 0.1% Triton X-100 solution for 10 min, rinsed five times in sterilized water, and stratified at 4 °C for 2 days in the dark. Seeds were sown on agar plates containing Murashige and Skoog (MS) medium, 2% sucrose, and 0.8% agar, buffered to pH 5.7. For plants grown in soil, seeds were directly sown in sterile vermiculite. All plants, including seedlings, were grown at 22 °C under a 16 h light/8 h dark cycle in a growth chamber.
Production and purification of the anti-AtVPS29 antibody
AtVPS29-specific antiserum was generated at Abcam Inc. Briefly, His6-tagged C-terminal AtVPS29 (amino acids, 172–190) was expressed in E. coli BL21 (DE3) and purified by affinity chromatography using Ni2+-nitrilotriacetate (Ni2+-NTA) resins (Qiagen). Antiserum was obtained from the rabbit immunized with purified His6-AtVPS29 peptides. Anti-AtVPS29 immunoglobulins were affinity purified from the antiserum by absorption with AtVPS29 protein bound to PVDF membrane.
Examination of SLY1 and RGA levels in vps29-3 mutants
To examine relative levels of SLY1 and RGA proteins in WT and vps29-3 mutants, total proteins were extracted from the leaves of WT and vps29-3 mutants grown for 15 d on MS media. Following 11% SDS-PAGE, the levels of SLY1 and RGA proteins were assessed by western blotting using anti-SLY1 or anti-RGA antibodies. Tubulin levels were also examined by western blotting with anti-tubulin antibody (Santa Cruz Biotechnology). Western blotting was performed five times, and one of these results was presented.
Transcript levels of genes related to GA signaling in vps29-3 mutants
WT and vps29-3 mutants were grown on plates containing MS medium for 15 d. Total RNA was extracted from the leaves of both WT and vps29-3 mutants. The extracted RNA was then quantified and divided into equal amounts. First-strand cDNA was synthesized from 5 μg total RNA using an iScript cDNA Synthesis Kit (Bio-Rad). An equal volume of cDNA was amplified by real-time qRT-PCR (MyiQ, Bio-Rad), according to the manufacturer’s protocol. The specific primers and template cDNA were combined with 25 µl of iQ SYBR Green Super Mix (Bio-Rad), and the reactions were performed under the following thermal conditions: 50 °C for 2 min; 95 °C for 10 min; 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The CT values obtained for target genes were normalized to the CT value for tubulin, and the data were analyzed using iCycler IQ software (Bio-Rad). PCR primers were designed using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3.cgi), and their specificity was verified by cloning into the pGEM T-Easy vector (Promega) and sequencing using an ABI 3730xl DNA Analyzer (Applied Biosystems). The primers used for qRT-PCR were as follows: SLY1, 5′-TGAAACACGTCGACGCAAAG-3′ and 5′-TCGTCTTGTGCCGTTTTGTG-3′; RGA, 5′-AACCAAGCGATTCTCGAAGC-3′ and 5′-AAGTGCAGGCCATTGAAGAC-3′; GID1a, 5′-TGGGGTTTTCTCGTTCGATG-3′ and 5′-ACAATGTCGCCATCAACAGG-3′; GID1b, 5′-5′-TGCGGTGTTGTTGTTGTCTC-3′ and 5′-ATCGTCGTAAGCACAAGGGTAG-3′; GID1c, 5′-TTGGAGGGACCGAAAGAACG-3′ and 5′-TCACCCTCAGGAAGAAACGC-3′; GA20ox2, 5′-GCTCAGAGAAAACCCGGTGA-3′ and 5′-TCTTGAACGGTTCTCGAGCC-3′; GA3ox1, 5′-GATCTCCTCTTCTCCGCTGC-3′ and 5′-TTTGGAAGGCACCCCAAGTT-3′; GA3ox2, 5′-TAGATCGCATCCCATTCACA-3′ and 5′-TCAATGTCGTCGAGAAGTCG-3′; Tubulin, 5′-GTGAGCGAACAGTTCACAGC-3′ and 5′-TTATTGCTCCTCCTGCACTT -3′.
Shoot and root growth analysis of vps29-3 mutants: WT and vps29-3 mutants were germinated on 1/2 MS agar-solidified medium (1% agar for vertical growth) supplemented with 0, 10, and 20 µM GA3, respectively. After growth for 8 days on vertical plates, shoot sizes and root lengths were measured using the ImageJ software with scanned images of seedlings (Schindelin et al. 2012).
Analysis of root apical meristem size of vps29-3 mutants
Arabidopsis seedlings were germinated in 1/2 MS agar-solidified medium (1% agar for vertical growth) supplemented with 1% sucrose. To quantify the number of the cortex cells and measure cell lengths at the meristematic region, roots were stained with 10 μg/ml propidium iodide (PI, Sigma-Aldrich) for 2 min. Confocal images were obtained using a Leica TCS SP8 confocal microscope equipped with a 561-nm laser. The numbers and lengths of cortical cells between the quiescent center and the first elongated cell were measured using the ImageJ software (Schindelin et al. 2012).
Results
The level of SLY1 is influenced by retromer protein AtVPS29
Abnormal GA signaling caused by the loss of SLY1 function results in defective growth, leading to severe dwarfism and low fertility (McGinnis et al. 2003). Arabidopsis vps29-3 mutants have also been reported to exhibit severe dwarfism and abnormal seed development (Shimada et al. 2006; Jaillais et al. 2007). Furthermore, the regulation of PM protein accumulation by GA requires the presence of retromer complex components (Salanenka et al. 2018). These suggest that SLY1 activity may be changed in the vps29-3 mutant, leading to abnormal SLY1-mediated GA signaling in the vps29-3 mutant. To test this hypothesis, we first generated an anti-AtVPS29 antibody and examined its specificity through western blotting. We observed clear detection of the AtVPS29 protein in WT, while it was not detected in vps29-3 plants, indicating that the antibody specifically interacts with VPS29 (Fig. 1a). Based on this result, we proceeded to analyze the levels of SLY1 protein in WT and vps29-3 mutants using western blotting with an anti-SLY1 antibody. Interestingly, we found that the level of SLY1 protein was significantly lower in the vps29-3 mutants (Fig. 1b). The SLY1 concentration in vps29-3 mutants was approximately 5.56-fold lower than that in WT. To investigate whether the reduced level of SLY1 protein in the vps29-3 mutants is influenced by the transcript level of SLY1, we performed real-time qRT-PCR analysis of SLY1 transcripts in the same samples. Surprisingly, the results showed that the transcript level of SLY1 was actually higher in the vps29-3 mutants compared to WT (Fig. 1c).
AtVPS29 positively regulates SLY1 level but negatively regulates its expression. Total proteins were extracted from leaves of 15-day-old wild-type (WT) and vps29-3 mutants. After performing 11% SDS-PAGE, the levels of AtVPS29 and SLY1 were examined using western blotting with anti-AtVPS29 (a) or anti-SLY1 (b) antibodies. Numbers under the lanes indicate relative intensities. Tubulin was used as a loading control. c Total RNA was isolated from 15-day-old WT and vps29-3 mutant plants. Transcript levels were assessed using real-time qRT-PCR with SLY1-specific primers. Results are presented as means ± S.D. (n = 3). Asterisks indicate statistically significant differences in transcript levels (*p < 0.05; Student’s t test) between WT and vps29-3 mutants. SLY1, SLEEPY1
Expression of GA receptor and biosynthesis genes was changed in vps29-3 mutants
We have demonstrated that the level of SLY1 protein is low in vps29-3 mutants, suggesting that the defective growth observed in these mutants may be partially attributed to the reduced level and activity of SLY1. However, it is also plausible that the dwarfism observed in vps29-3 mutants is a consequence of altered gene expression. Therefore, we investigated the transcript levels of the GA receptor genes GA-INSENSITIVE DWARF 1a (GID1a), GID1b, and GID1c in vps29-3 mutants. To assess the expression of these genes, we performed real-time qRT-PCR using total RNA isolated from both WT and vps29-3 mutants. Notably, our results revealed that the expression of GID1a, GID1b, and GID1c genes was significantly increased in vps29-3 mutants compared to WT (Fig. 2).
Transcript levels of GA receptor genes in vps29-3 mutants. Total RNA was isolated from 15-day-old WT and vps29-3 mutants. Transcript levels were analyzed using real-time qRT-PCR with gene-specific primers. Results are presented as means ± S.D. (n = 3). Asterisks indicate statistically significant differences in transcript levels (*p < 0.05; ***p < 0.001; Student’s t test) between WT and vps29-3 mutants. Abbreviations: GID1a, GA-INSENSITIVE DWARF 1a; GID1b, GA-INSENSITIVE DWARF 1b; GID1c, GA-INSENSITIVE DWARF 1c
Next, we investigated the transcript levels of GA biosynthetic genes, namely GA20ox2, GA3ox1, and GA3ox2, under the same conditions in vps29-3 mutants. Interestingly, we observed a significant decrease in the levels of GA20ox2, GA3ox1, and GA3ox2 transcripts in vps29-3 mutants compared to WT (Fig. 3).
Transcript levels of GA-synthetic genes in vps29-3 mutants. Total RNA was isolated from 15-day-old WT and vps29-3 mutants. Transcript levels were analyzed using real-time qRT-PCR with gene-specific primers. Results are presented as means ± S.D. (n = 3). Asterisks indicate statistically significant differences in transcript levels (**p < 0.01; ***p < 0.001; Student’s t test) between WT and vps29-3 mutants. Abbreviations: GA20ox2, GIBBERELLIN 20-OXIDASE 2; GA3ox1, GIBBERELLIN 3-OXIDASE 1; GA3ox2, GIBBERELLIN 3-OXIDASE 2
The level of RGA protein is affected by AtVPS29
In Arabidopsis, the F-box protein SLY1 plays a crucial role in recognizing and targeting DELLA proteins, including RGA, for degradation by the 26S proteasome complex. Given the decreased level of SLY1 protein in vps29-3 mutants (Fig. 1b), we initially investigated the levels of RGA in both WT and vps29-3 mutants using western blotting with anti-RGA antibody. The results demonstrated a significant accumulation of RGA protein in the vps29-3 mutants compared to WT (Fig. 4a). The RGA concentration in vps29-3 mutants was approximately up to 5.43-fold higher than that in WT. Next, we examined the impact of RGA gene expression on the level of RGA protein. Transcript levels of the RGA gene were determined by real-time qRT-PCR using total RNA isolated from the same samples. Interestingly, we observed a lower transcript level of RGA in the vps29-3 mutants compared to WT (Fig. 4b).
AtVPS29 negatively regulates RGA level but positively regulates its expression. a Total proteins were extracted from leaves of 15-day-old WT and vps29-3 mutants. After subjecting them to 11% SDS-PAGE, the levels of RGA were analyzed by western blotting using anti-RGA antibody. Numbers under the lanes indicate relative intensities. Tubulin was used as a loading control. b Total RNA was isolated from 15-day-old WT and vps29-3 mutants. Transcript levels were determined using real-time qRT-PCR with RGA-specific primers. Results are expressed as means ± S.D. (n = 3). Asterisks indicate statistically significant differences in transcript levels (***p < 0.001; Student’s t test) between WT and vps29-3 mutants. RGA, REPRESSOR OF ga1-3
vps29-3 mutants are less sensitive to gibberellin
To assess the GA response of vps29-3 mutants, we germinated seeds from both WT and vps29-3 mutants on MS medium supplemented with 10 and 20 µM GA3. After 8 days, we measured leaf size and root length. When exposed to increasing concentrations of GA3 ranging from 10 to 20 μM, leaf growth and root elongation in WT exhibited a dose-dependent stimulation (Fig. 5a–c). In contrast, GA3 had minimal effects on leaf growth and root elongation in vps29-3 mutants. Specifically, leaf size and root length showed a 2.56-fold and 1.50-fold increase, respectively, in WT. Conversely, leaf size and root length exhibited only a 1.40-fold and 1.10-fold increase, respectively, in vps29-3 mutants.
vps29-3 mutants are less sensitive to exogenous GA. a Comparison of leaf size and root length between WT and vps29-3 mutants after 8 days of growth on 1/2 MS medium (mock) and 1/2 MS medium supplemented with 10 and 20 µM GA3, respectively. Scale bar 1 cm. b, c Statistical analysis of the differences in leaf size (b) and root length (c) between the genotypes shown in (a). Data are presented as means ± S.D (n > 10). Black and white circles indicate WT and vps29-3 mutants, respectively
The length of root meristematic zone was reduced in vps29-3 mutants
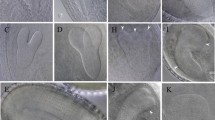
GA is an important regulator of Arabidopsis root growth (Fu and Harberd 2003; Griffiths et al. 2006; Willige et al. 2007; Ueguchi-Tanaka et al. 2007; Ubeda-Tomás et al. 2008). Reduction of endogenous GA levels by treating WT seedlings with paclobutrazol (PAC, an inhibitor of GA biosynthesis) results in a reduced root growth rate and also in a reduction in root meristem size (Ubeda-Tomás et al. 2009). Therefore, the decrease in SLY1 protein and the increase in RGA protein in vps29-3 mutants, along with reduced sensitivity to GA, strongly suggest that root growth is impaired in vps29-3 mutants. Consequently, we examined the size of the root meristematic zone in 5-day-old WT and vps29-3 mutants. Propidium iodide staining revealed that the length of the meristematic zone was much shorter in vps29-3 mutants compared to WT (Fig. 6a). This observation implies that either cell number or length is affected in vps29-3 mutants. Further analyses indicated that cell number was decreased in vps29-3 mutants (Fig. 6b), while cortex cell size remained unaffected (Fig. 6c).
vps29-3 mutants show reduced root meristem size. a Confocal images of root tips of WT and vps29-3 mutants. Roots from seedlings 7 days after germination were used for imaging. White arrowheads indicate the boundary between the meristematic and elongation zones of the root. Scale bars correspond to 25 μm. b Root meristem cell number in the roots of 7-day-old WT and vps29-3 mutants. Asterisks indicate statistically significant differences in transcript levels (***p < 0.001; Student’s t test) between WT and vps29-3 mutants. Values represent the mean of 15 measurements ± SD. c Average cortex cell sizes in the roots of 6-day-old WT and vps29-3 mutants. Each value represents measurements from at least 15 seedlings. The median, minimum, and maximum values are shown. NS, not significant
Discussion
This study emphasizes the positive regulatory role of retromer protein AtVPS29 activity in plant development through SLY1-mediated GA signaling.
GA-dependent developmental processes are influenced by DELLA proteins, which act as repressors and are regulated through phosphorylation and ubiquitination mediated by the E3 ligase activity of SCFSLY1. Previous studies have extensively investigated SLY1 and DELLA proteins to unravel the mechanisms of GA signaling. Notably, SLY1- and DELLA-mediated growth are also modulated by sumoylation.
The severe dark green dwarfism observed in Arabidopsis sly1 mutants is a prominent characteristic. Interestingly, a similar dwarf phenotype is observed in vps29-3 mutants. Hence, we aimed to investigate whether a VPS29 mutation affects GA signaling by disrupting SLY1-mediated degradation of DELLA proteins. Our results revealed lower levels of SLY1 protein in vps29-3 mutants compared to WT (Fig. 1b), despite higher transcript levels in vps29-3 mutants (Fig. 1c). This strongly suggests that AtVPS29 activity plays a role in stabilizing SLY1 protein post-translationally. GA signaling is primarily regulated by the negative activity of DELLA proteins, which undergo degradation by the 26S proteasome complex following polyubiquitination by SCFSLY1 (Dill et al. 2004). Our findings demonstrated that the level of SLY1 protein was positively regulated by AtVPS29 activity (Fig. 1b), implying that the abundance of DELLA proteins, including RGA, might be influenced in vps29-3 mutants. Remarkably, the level of RGA was significantly elevated in vps29-3 mutants compared to WT (Fig. 4a), while its transcript level was decreased in vps29-3 mutants (Fig. 4b). We found that the expression patterns of the SLY1 and RGA genes show no correlation with their protein levels. This implies that AtVPS29 loss might influence various signaling pathways, impacting not only the expression of SLY1 and RGA but also the post-translational modification systems. Several studies have indicated a lack of clear correlation between transcript and protein levels. Richter et al. (2010) demonstrated an absence of correlation between DELLA protein abundance and DELLA gene expression during late germination stages and following GA3 treatment. This supports the notion that feedback mechanisms likely regulate DELLA protein levels through a balance of synthesis and degradation. Li et al. (2015) observed reduced transcription levels of RGA genes in det1-1 mutants compared to the wild-type under darkness. Interestingly, the mutants exhibited higher abundance of the RGA protein, suggesting a potential post-translational role of De-etiolated 1 (DET1) in elevating RGA protein level during dark condition. Zia et al. (2019) found that although the expression of the IAA28 gene was induced by auxin treatment, its protein level decreased. This suggests a discordance between gene expression and protein abundance in response to auxin. Therefore, based on these findings, we cautiously speculate that similar mechanisms might regulate the expression of SLY1 and RGA genes, as well as the degradation of their respective proteins in vps29-3 mutants, although the exact mechanisms remain unclear. Moreover, all these results support the notion that AtVPS29 stimulates SLY1-mediated degradation of RGA. Therefore, these findings strongly indicate that the dwarfism observed in vps29-3 mutants is attributable to the loss of SLY1 activity.
However, it is also plausible that the growth defects in vps29-3 mutants may arise from alterations in the expression of genes involved in GA synthesis and response. The data revealed decreased transcript levels of genes encoding GA biosynthetic enzymes in vps29-3 mutants (Fig. 3), suggesting that the loss of AtVPS29 leads to the downregulation of GA synthesis. However, transcript levels of genes encoding GA receptors were increased in vps29-3 mutants (Fig. 2). Previous studies have demonstrated that GA20ox2, GA3ox1, GID1a, and GID1b, responsible for encoding GA biosynthetic enzymes and GA receptors, are downregulated by GA treatment and loss-of-function DELLA mutations (Hedden and Phillips 2000; Fleets and Sun 2005; Griffiths et al. 2006; Zentella et al. 2007), implying their expression is positively regulated by DELLA proteins. However, Zentella et al. (2007) also reported an intriguing finding: in a DEX-inducible transgenic system, they observed an increase in the transcript levels of GA3ox1, GA20ox2, GID1a, and GID1b genes upon RGA induction. This suggests that DELLA proteins not only act as repressors of GA signaling but also modulate GA homeostasis by potentially upregulating the expression of GA biosynthetic and GA receptor genes. Based on these findings, we cautiously speculate that the expression of downstream genes of RGA in the vps29-3 mutants could be regulated not only by a negative feedback loop through DELLA proteins but also by another potential alternative mechanism. In addition, despite the increased expression of genes encoding GA receptors in vps29-3 mutants, the responsiveness of vps29-3 mutants to GA in terms of shoot and root growth was substantially diminished compared to WT (Fig. 5a–c), suggesting reduced sensitivity to GA in vps29-3 mutants.
Plant growth involves the integration of various environmental and endogenous signals that, in conjunction with the intrinsic genetic program, determine plant size. At the cellular level, growth rate is governed by two interconnected processes: cell proliferation and expansion. GA plays a pivotal role in regulating growth and development in response to environmental fluctuations (Olszewski et al. 2002; Achard et al. 2006, 2008). Given the impairment of the SLY1-mediated signaling pathway (Figs. 1b and 4a), reduced GA response (Fig. 5a–c), and shortened root length in vps29-3 mutants (Fig. 5a), we, therefore, expanded our investigation to include the measurement of the root meristematic zone length and cell number and length within this zone. The results unequivocally demonstrated that vps29-3 mutants had a shortened meristematic zone (Fig. 6a), accompanied by a decline in cell count within the meristematic zone (Fig. 6b). This implies that the decrease in cell number within the meristematic zone of vps29-3 mutants resulted from impaired GA signaling, ultimately contributing to a reduction in the length of the meristematic zone.
SLY1 undergoes direct sumoylation by AtSIZ1 both in vitro and in vivo, and the level of SLY1 protein is reduced in siz1-2 mutants compared to WT (Kim et al. 2015). In addition, GA promotes the sumoylation of SLY1 by AtSIZ1 (Kim et al. 2015). Moreover, Arabidopsis siz1-2 mutants exhibit a severe dwarf phenotype (Catala et al. 2007) because the accumulation of DELLA repressors in the absence of SLY1 inhibits cell elongation and division (Achard et al. 2009; Peng et al. 1997; Dill et al. 2001). These data indicate that AtSIZ1 stabilizes SLY1 through its E3 ligase activity, positively regulating SLY1-mediated GA signaling and thereby influencing plant growth. In the present study, we observed a notable reduction in the level of SLY1 in vps29-3 mutants compared to WT (Fig. 1b). Furthermore, the level of RGA was considerably elevated in vps29-3 mutants in contrast to WT (Fig. 4a), implying a potential decrease in AtSIZ1 levels or activity in vps29-3 mutants. Therefore, these findings, together with previous our data, suggest the possibility that impaired SLY1-mediated GA signaling in vps29-3 mutants could be due to reduced SLY1 activity resulting from diminished AtSIZ1 activity (Fig. 7). Recently, several proteins associated with the regulation of retromer protein function have been identified in plants. These include the endosomal sorting complex required for transport (ESCRT)-associated protein apoptosis-linked gene-2 interacting protein X (ALIX) (Hu et al. 2022), BLISTER (Li et al. 2023), MoRab7, and the DEAH and RING domain-containing protein as FREE1 suppressor 1 (DRIF1) (Chen et al. 2023; Zhu et al. 2023). They play a role in regulating protein transport and signaling by interacting with retromer proteins. These findings suggest the involvement of various proteins in the regulation of the retromer complex’s function in plants.
Schematic representation of the possible mechanism of activation of GA signaling by AtVPS29. a In wild-type plants, SLY1 undergoes sumoylation via AtSIZ1 activity, which converts it into its active form. Sumoylated SLY1 forms the SCFSLY1−SUMO complex, which targets DELLA proteins, including RGA, for ubiquitination. Polyubiquitinated DELLA proteins are subsequently degraded by the 26S proteasome complex. This process promotes cell division and expansion, leading to growth and development. b Due to potentially reduced AtSIZ1 activity in vps29-3 mutants, SLY1 might not undergo sumoylation, leading to its inactivation and consequent accumulation of RGA. As a result, cell division and expansion are inhibited, leading to dwarfism
In conclusion, AtVPS29 exerts positive and negative regulatory effects on the levels of SLY1 and RGA proteins, respectively, primarily at the post-translation level. The depletion of AtVPS29 results in reduced responsiveness to exogenous GA, a shortened meristematic zone, and a decrease in cell numbers within meristematic zone. These findings provide strong evidence suggesting that AtVPS29, a component of the large retromer complex, plays a significant role in modulating GA signaling, consequently influencing plant growth.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 331:91–94
Achard P, Renou J-P, Berthomé R, Harberd NP, Genschik P (2008) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18:656–660
Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GT, Genschik P (2009) Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr Biol 19:1188–1193
Catala R, Ouyang J, Abreu IA, Hu Y, Seo H, Zhang X, Chua NH (2007) The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses. Plant Cell 19:2952–2966
Chandra M, Kendall AK, Jackson LP (2020) Unveiling the cryo-EM structure of retromer. Biochem Soc Trans 48(5):2261–2272
Chen X, Selvaraj P, Lin L, Fang W, Wu C, Yang P, Zhang J, Abubakar YS, Yang F, Lu G, Liu W, Wang Z, Naqvi NI, Zheng W (2023) Rab7/Retromer-based endolysosomal trafficking is essential for proper host invasion in rice blast. New Phytol 239(4):1384–1403
Conti L, Nelis S, Zhang C, Woodcock A, Swarup R, Galbiati M, Tonelli C, Napier R, Hedden P, Bennett M, Sadanandom A (2014) Small Ubiquitin-like Modifier protein SUMO enables plants to control growth independently of the phytohormone gibberellin. Dev Cell 28:102–110
Daviere J, Achard P (2013) Gibberellin signaling in plants. Development 140:1147–1151
Dill A, Jung HS, Sun TP (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98:14162–14167
Dill A, Thomas SG, Hu J, Steber CM, Sun TP (2004) The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. Plant Cell 16:1392–1405
Fleet CM, Sun TP (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8:77–85
Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421:740–743
Fu X, Richards DE, Fleck B, Xie D, Burton N, Harberd NP (2004) The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16:1406–1418
Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, Thomas SG (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414
Hashiguchi Y, Niihama M, Takahashi T, Saito C, Nakano A, Tasaka M, Morita MT (2010) Loss-of-function mutations of retromer large subunit genes suppress the phenotype of an Arabidopsis zig mutant that lacks Qb-SNARE VTI11. Plant Cell 22:159–172
Hedden P, Phillips AL (2000) Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci 5:523–530
Horazdovsky BF, Davies BA, Seaman MN, McLaughlin SA, Yoon S, Emr SD (1997) A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell 8:1529–1541
Hu S, Li B, Wu F, Zhu D, Zouhar J, Gao C, Shimada T, Rojo E, Hara-Nishimura I, Jiang L, Shen J (2022) Plant ESCRT protein ALIX coordinates with retromer complex in regulating receptor-mediated sorting of soluble vacuolar proteins. Proc Natl Acad Sci USA 119(20):e2200492119
Hua Z, Vierstra RD (2011) The cullin-RING ubiquitin-protein ligases. Annu Rev Plant Biol 62:299–334
Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miège C, Gaude T (2007) The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130:1057–1070
Jha SG, Larson ER (2023) Diversity of retromer-mediated vesicular trafficking pathways in plants. Front Plant Sci 4:1184047
Jha SG, Larson ER, Humble J, Domozych DS, Barrington DS, Tierney ML (2018) Vacuolar Protein Sorting 26C encodes an evolutionarily conserved large retromer subunit in eukaryotes that is important for root hair growth in Arabidopsis thaliana. Plant J 94(4):595–611
Kim SI, Park BS, Kim DY, Yeu SY, Song SI, Song JT, Seo HS (2015) E3 SUMO ligase AtSIZ1 positively regulates SLY1-mediated GA signalling and plant development. Biochem J 469(2):299–314
Li K, Gao Z, He H, Terzaghi W, Fan LM, Deng XW, Chen H (2015) Arabidopsis DET1 represses photomorphogenesis in part by negatively regulating DELLA protein abundance in darkness. Mol Plants 8(4):622–630
Li H, Huang R, Liao Y, Yang S, Feng B, Qin H, Zhou J, Zeng Y, Shen J, Zhuang X, Jiang L, Otegui MS, Zhang S, Gao C (2023) A plant-unique protein BLISTER coordinates with core retromer to modulate endosomal sorting of plasma membrane and vacuolar proteins. Proc Natl Acad Sci USA 120(1):e2211258120
McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15:1120–1130
Münch D, Teh OK, Malinovsky FG, Liu Q, Vetukuri RR, Kasmi FE, Brodersen P, Hara-Nishimura I, Dangle JL, Petersen M, Mundy J, Hofius D (2015) Retromer contributes to immunity-associated cell death in Arabidopsis. Plant Cell 27:463–479
Nothwehr SF, Hindes AE (1997) The yeast VPS5/GRD2 gene encodes a sorting nexin-1-like protein required for localizing membrane proteins to the late Golgi. J Cell Sci 110:1063–1072
Olszewski N, Sun TP, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14(Suppl):S61-80
Paravicini G, Horazdovsky BF, Emr SD (1992) Alternative pathways for the sorting of soluble vacuolar proteins in yeast: a vps35 null mutant missorts and secretes only a subset of vacuolar hydrolases. Mol Biol Cell 3:415–427
Peng J, Caro P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11:3194–3205
Richter R, Behringer C, Müller IK, Schwechheimer C (2010) The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Dev 24(18):2093–2104
Salanenka Y, Verstraeten I, Löfke C, Tabata K, Naramoto S, Glanc M, Friml J (2018) Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane. Proc Natl Acad Sci USA 115(14):3716–3721
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Seaman MN, Marcusson EG, Cereghino JL, Emr SD (1997) Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol 137:79–92
Shimada T, Koumoto Y, Li L, Yamazaki M, Kondo M, Nishimura M, Hara-Nishimura I (2006) AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol 47(9):1187–1194
Strader LC, Ritchie S, Soule JD, McGinnis KM, Steber CM (2004) Recessive-interfering mutations in the gibberellin signaling gene SLEEPY1 are rescued by overexpression of its homologue, SNEEZY. Proc Natl Acad Sci USA 101:12771–12776
Thazar-Poulot N, Miquel M, Fobis-Loisy I, Gaude T (2015) Peroxisome extensions deliver the Arabidopsis SDP1 lipase to oil bodies. Proc Natl Acad Sci USA 112:4158–4163
Tu Y, Seaman MNJ (2021) Navigating the controversies of retromer-mediated endosomal protein sorting. Front Cell Dev Biol 9:658741
Ubeda-Tomás S, Swarup R, Coates J, Swarup K, Laplaze L, Beemster GT, Hedden P, Bhalerao R, Bennett MJ (2008) Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nat Cell Biol 10:625–628
Ubeda-Tomás S, Federici F, Casimiro I, Beemster GTS, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett MJ (2009) Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr Biol 19:1194–1199
Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I, Matsuoka M (2007) Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 19:2140–2155
Willige BC, Ghosh S, Nill C, Zourelidou M, Dohmann EM, Maier A, Schwechheimer C (2007) The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19:1209–1220
Zelazny E, Santambrogio M, Pourcher M, Chambrier P, Berne-Dedieu A, Fobis-Loisy I, Miege C, Jaillais Y, Gaude T (2013) Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis. J Biol Chem 288:8815–8825
Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, Sun TP (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19:3037–3057
Zhu Y, Zhao Q, Cao W, Huang S, Ji C, Zhang W, Trujillo M, Shen J, Jiang L (2023) The plant-unique protein DRIF1 coordinates with SNX1 to regulate membrane protein homeostasis. Plant Cell:koad227
Zia SF, Berkowitz O, Bedon F, Whelan J, Franks AE, Plummer KM (2019) Direct comparison of Arabidopsis gene expression reveals different responses to melatonin versus auxin. BMC Plant Biol 19:567
Funding
Open Access funding enabled and organized by Seoul National University. This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (Project No. 2021R1A2C1003446).
Author information
Authors and Affiliations
Contributions
WKM and DHK performed the experiments for research. HSS designed the research. HSS wrote the manuscript. WKM, DHK, JTS, and HSS evaluated the results and the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Ralf Welsch.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Min, W.K., Kwon, D.H., Song, J.T. et al. Arabidopsis retromer subunit AtVPS29 is involved in SLY1-mediated gibberellin signaling. Plant Cell Rep 43, 53 (2024). https://doi.org/10.1007/s00299-024-03144-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00299-024-03144-8