Abstract
Key message
Overexpression of the Aux/IAA protein TaIAA15-1A from wheat improves drought tolerance by regulating the ABA signalling pathway in transgenic Brachypodium.
Abstract
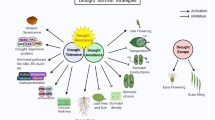
Drought is a major abiotic stress that causes severe crop yield loss. Aux/IAA genes have been shown to be involved in drought stress responses. However, to the best of our knowledge, there has been little research on the molecular mechanism of the wheat Aux/IAA gene in the context of drought tolerance. In this study, we found that expression of the wheat Aux/IAA gene TaIAA15-1A was upregulated by PEG6000, NaCl, SA, JA, IAA and ABA. Transgenic plants overexpressing TaIAA15-1A showed higher drought tolerance than wild-type (WT) plants. The physiological analyses showed that the transgenic lines exhibited a higher survival rate, shoot length, and relative water content than the WT plants. The activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) were enhanced in transgenic lines, causing a reduction in the hydrogen peroxide (H2O2) and superoxide anion radical (O2−) contents. Transcriptome analysis showed that TaIAA15-1A overexpression alters the expression of these genes involved in the auxin signalling pathway, ABA signalling pathway, phenolamides and antioxidant pathways. The results of exogenous ABA treatment suggested that TaIAA15-1A overexpression increased sensitivity to ABA at the germination and postgermination stages compared to WT plants. These results indicate that TaIAA15-1A plays a positive role in plant drought tolerance by regulating ABA-related genes and improving antioxidative stress ability and has potential application in genetically modified crops.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Awan SA, Khan I, Rizwan M et al (2021) Exogenous abscisic acid and Jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol Plant 172(2):809–819
Bates LS, Waldren PR, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Baxter A, Mittler R, Suzuki NN (2013) ROS as key players in plant stress signaling. J Exp Bot 65:1229–1240
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chen D, Richardson T, Chai S et al (2016) Drought-up-regulated TaNAC69-1 is a transcriptional repressor of TaSHY2 and TaIAA7, and enhances root length and biomass in wheat. Plant Cell Physiol 57(10):2076–2090
Dhindsa RA, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 126:93–101
Du H, Liu H, Xiong L (2013) Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci 4:397
Gay C, Collins J, Gebicki JM (1999) Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem 273:149–155
He Y, Liu Y, Li M et al (2021) The Arabidopsis SMALL AUXIN UP RNA32 protein regulates ABA-mediated responses to drought stress. Front Plant Sci 12:625493
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Jakoby M, Weisshaar B, Dröge-Laser W et al (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7(3):106–111
Jung H, Lee DK, Choi YD et al (2015) OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci 236:304–312
Li X, Yu B, Wu Q et al (2021) OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice. PLoS Genet 17(8):e1009699
Liu J, Ma XL, Shang XW et al (2009) Regulation of exogenous auxin IAA on drought and salt stress during seedling stage of spring wheat (cv. Xihan No. 2). J Gansu Agric Univ 2(44):47–51
Mao H, Jian C, Cheng X et al (2022) The wheat ABA receptor gene TaPYL1-1B contributes to drought tolerance and grain yield by increasing water-use efficiency. Plant Biotechnol J 20(5):846–861
Maraschin Fdos S, Memelink J, Offringa R (2009) Auxin-induced, SCF(TIR1)-mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J 59(1):100–109
Mega R, Abe F, Kim JS et al (2019) Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat Plants 5(2):153–159
Mutka AM, Fawley S, Tsao T et al (2013) Auxin promotes susceptibility to Pseudomonas syringae via a mechanism independent of suppression of salicylic acid-mediated defenses. Plant J 74:746–754
Pérez-Alonso MM, Ortiz-García P, Moya-Cuevas J et al (2021) Endogenous indole-3-acetamide levels contribute to the crosstalk between auxin and abscisic acid, and trigger plant stress responses in Arabidopsis. J Exp Bot 72(2):459–475
Robles P, Navarro-Cartagena S, Ferrández-Ayela A et al (2018) The characterization of arabidopsis mterf6 mutants reveals a new role for mterf6 in tolerance to abiotic stress. Int J Mol Sci 19(8):2388
Salehin M, Li B, Tang M et al (2019) Auxin-sensitive Aux/IAA proteins mediate drought tolerance in arabidopsis by regulating glucosinolate levels. Nat Commun 10(1):4021
Sallam A, Alqudah AM, Dawood MFA et al (2019) Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int J Mol Sci 20(13):3137
Shi H, Chen L, Ye T et al (2014) Modulation of auxin content in arabidopsis confers improved drought stress resistance. Plant Physiol Biochem 82:209–217
Spiro RG (1966) Analysis of sugars found in glycoprotein. Method Enzymol 8:3–26
Tian F, Gong J, Zhang J et al (2013) Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J Exp Bot 64:1509–1520
Vain P, Worland B, Thole V et al (2008) Agrobacterium-mediated transformation of the temperate grass Brachypodium distachyon (genotype Bd21) for T-DNA insertional mutagenesis. Plant Biotechnol J 6(3):236–245
Varet H, Brillet-Guéguen L, Coppée JY et al (2016) SARTools: A DESeq2- and EdgeR-Based R pipeline for comprehensive differential analysis of RNA-Seq Data. PLoS ONE 11(6):0157022
Wang F, Niu H, Xin D et al (2021) OsIAA18, an Aux/IAA transcription factor gene, is involved in salt and drought tolerance in rice. Front Plant Sci 12:738660
Wolters H, Jürgens G (2009) Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet 10(5):305–317
Xiao HM, Cai WJ, Ye TT, Ding J, Feng YQ (2018) Spatio-temporal profiling of abscisic acid, indoleacetic acid and jasmonic acid in single rice seed during seed germination. Anal Chim Acta 1031:119–127
Yuan HM, Liu WC, Jin Y et al (2013) Role of ROS and auxin in plant response to metal-mediated stress. Plant Signal Behav 8(7):24671
Zhang N, Yin Y, Liu X et al (2017) The E3 ligase TaSAP5 alters drought stress responses by promoting the degradation of DRIP proteins. Plant Physiol 175(4):1878–1892
Zhang H, Liu D, Yang B et al (2020) Arabidopsis CPK6 positively regulates ABA signaling and drought tolerance through phosphorylating ABA-responsive element-binding factors. J Exp Bot 71(1):188–203
Zhang A, Yang X, Lu J et al (2021) OsIAA20, an Aux/IAA protein, mediates abiotic stress tolerance in rice through an ABA pathway. Plant Sci 308:110903
Zhou SM, Sun XD, Yin SH et al (2014) The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol Biochem 84:213–223
Zulfiqar A, Samara SS, Ihsan K et al (2016) Functions of plant’s bzip transcription factors. Pak J Agric Sci 53:303–314
Acknowledgements
This work was funded by Natural Science Foundation of Shandong Province (ZR2022QC129); Doctoral research start-up funds, Liaocheng University (318052018); State Key Laboratory of Crop Biology, Shandong Agricultural University (2021KF03).
Author information
Authors and Affiliations
Contributions
PS and SG conceived and designed the experiments; PS performed most experiments; CS performed exogenous ABA treatments; YJL performed expression analysis. KW, HS, SHW, XQL performed physiological and biochemical indice measures; PS wrote and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Zheng-Yi Xu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


299_2022_2965_MOESM2_ESM.jpg
Supplementary Fig S2. The expression levels of ABA biosynthesis and signal transduction pathway genes in TaIAA15-1A transgenic lines and WT plants file2 (JPG 2078 KB)

299_2022_2965_MOESM3_ESM.jpg
Supplementary Fig. S3. Effect overview of TaIAA15-1A overexpression on bZIP transcription factors. (A) Heatmap of bZIP transcription factors based on RNA‐seq. (B) Expression levels of bZIP transcription factors in TaIAA15-1A transgenic lines and WT plants file3 (JPG 58 KB)

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, P., Sui, C., Li, J. et al. The Aux/IAA protein TaIAA15-1A confers drought tolerance in Brachypodium by regulating abscisic acid signal pathway. Plant Cell Rep 42, 385–394 (2023). https://doi.org/10.1007/s00299-022-02965-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-022-02965-9