Abstract
Key message
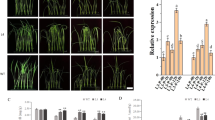
A Sec14-like protein, ZmSEC14p , from maize was structurally analyzed and functionally tested. Overexpression of ZmSEC14p in transgenic Arabidopsis conferred tolerance to cold stress.
Abstract
Sec14-like proteins are involved in essential biological processes, such as phospholipid metabolism, signal transduction, membrane trafficking, and stress response. Here, we reported a phosphatidylinositol transfer-associated protein, ZmSEC14p (accession no. KT932998), isolated from a cold-tolerant maize inbred line using the cDNA-AFLP approach and RACE-PCR method. Full-length cDNA that consisted of a single open reading frame (ORF) encoded a putative polypeptide of 295 amino acids. The ZmSEC14p protein was mainly localized in the nucleus, and its transcript was induced by cold, salt stresses, and abscisic acid (ABA) treatment in maize leaves and roots. Overexpression of ZmSEC14p in transgenic Arabidopsis conferred tolerance to cold stress. This tolerance was primarily displayed by the increased germination rate, root length, plant survival rate, accumulation of proline, activities of antioxidant enzymes, and the reduction of oxidative damage by reactive oxygen species (ROS). ZmSEC14p overexpression regulated the expression of phosphoinositide-specific phospholipase C, which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) and generates second messengers (inositol 1,4,5-trisphosphate and 1,2-diacylglycerol) in the phosphoinositide signal transduction pathways. Moreover, up-regulation of some stress-responsive genes such as CBF3, COR6.6, and RD29B in transgenic plants under cold stress could be a possible mechanism for enhancing cold tolerance. Taken together, this study strongly suggests that ZmSEC14p plays an important role in plant tolerance to cold stress.











Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- ROS:
-
Reactive oxygen species
- PIP2:
-
Phosphatidylinositol 4,5-bisphosphate
- IP3:
-
Inositol 1,4,5-trisphosphate
- DAG:
-
1,2-Diacylglycerol
- COR:
-
Cold regulated
- KIN:
-
Cold induced
- RD:
-
Responsive to dehydration
- CBFs:
-
CRT/DRE binding factor
- DREBs:
-
DRE binding protein
- DRE/CRT:
-
Dehydration-responsive element/C-repeat
- CAMTA:
-
Calmodulin binding transcription activator
- PIPTs:
-
Phosphatidylinositol transfer proteins
- PtdIns:
-
Phosphatidylinositol
- PtdCho:
-
Phosphatidylcholine
- cDNA-AFLP:
-
cDNA-amplified fragment length polymorphism
- RACE:
-
Rapid amplification of cDNA ends
- WT:
-
Wild type
- MS:
-
Murashige and Skoog
- NBT:
-
Nitroblue tetrazolium
- DAB:
-
3,3′-Diaminobenzidine
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- O2 − :
-
Superoxide anions
- H2O2 :
-
Hydrogen peroxide
- PLC:
-
Phospholipases C
- PtdInsP:
-
Phosphatidylinositol monophosphate
- PA:
-
Phosphatidic acid
- PIPs:
-
Phosphoinositides
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Agarwal PK, Agarwal P, Reddy MK, Sopory SK (2006) Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep 25:1263–1274
Aravind L, Neuwald AF, Ponting CP (1999) Sec14p-like domains in NF1 and Dbl-like proteins indicate lipid regulation of Ras and Rho signaling. Curr Biol 9:R195–R197
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Browse J, Xin Z (2001) Temperature sensing and cold acclimation. Curr Opin Plant Biol 4:241–246
Catalá R, Santos E, Alonso JM, Ecker JR, Martinez-Zapater JM, Salinas J (2003) Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold acclimation response in Arabidopsis. Plant Cell 15:2940–2951
Cha MK, Hong SK, Oh YM, Kim IH (2003) The protein interaction of Saccharomyces cerevisiae cytoplasmic thiol peroxidase II with Sfh2p and its in vivo function. J Biol Chem 278: 34952–34958
Cheng LB, Li SY, Hussain J, Xu XY, Yin JJ, Zhang Y, Chen XH, Li LJ (2013) Isolation and functional characterization of a salt responsive transcription factor, LrbZIP from lotus root (Nelumbo nucifera Gaertn). Mol Biol Rep 40:4033–4045
Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17:1043–1054
Chinnusamy V, Zhu JH, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12:444–451
Cleves AE, McGee T, Bankaitis VA (1991) Phospholipid transfer proteins: a biological debut. Trends Cell Bio 1:31–34
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
DeWald DB, Torabinejad J, Jones CA, Shope JC, Cangelosi AR, Thompson JE, Prestwich GD, Hama H (2001) Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed Arabidopsis. Plant Physiol 126:759–769
Doherty GJ, Van Buskirk HA, Myers SJ, Thomashow MF (2009) Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 21:972–984
Dong CH, Zolman BK, Bartel B, Lee BH, Stevenson B, Agarwal M, Zhu JK (2009) Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signaling. Mol Plant 2:59–72
Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH (1997) Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390(6656):187–192
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt-and cold-responsive gene expression. Plant J 33:751–763
Fursova OV, Pogorelko GV, Tarasov VA (2009) Identification of ICE2, a gene involved in cold acclimation which determines freezing tolerance in Arabidopsis thaliana. Gene 429:98–103
Gelli M, Duo YC, Konda AR, Zhang C, Holding D, Dweikat I (2014) Identification of differentially expressed genes between sorghum genotypes with contrasting nitrogen stress tolerance by genome-wide transcriptional profiling. BMC Genom 15:179
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gilmour SJ, Fowler SG, Thomashow MF (2004) Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol 54:767–781
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102
Huang JG, Yang M, Liu P, Yang GD, Wu CA, Zheng CC (2009) GhDREB1 enhances abiotic stress tolerance, delays GA-mediated development and represses cytokinin signalling in transgenic Arabidopsis. Plant Cell Environ 32:1132–1145
Huang GT, Ma SL, Bai LP, Zhang L, Ma H, Jia P, Liu J, Zhong M, Guo ZF (2012) Signal transduction during cold, salt, and drought stresses in Plants. Mol Biol Rep 39:969–987
Keogh MR (2009) New insights into phospholipid metabolism and signaling in plants. Dissertation, NC State University
Kiba A, Nakano M, Vincent-Pope P, Takahashi H, Sawasaki T, Endo Y, Ohnishi K, Yoshioka H, Hikichi Y (2012) A novel Sec14 phospholipid transfer protein from Nicotiana benthamiana is up-regulated in response to Ralstonia solanacearum infection, pathogen associated molecular patterns and effector molecules and involved in plant immunity. J Plant Physiol 169:1017–1022
Kiba A, Galis I, Hojo Y, Ohnishi K, Yoshioka H, Hikichi Y (2014) SEC14 phospholipid transfer protein is involved in lipid signaling-mediated plant immune responses in Nicotiana benthamiana. PLoS ONE. doi:10.1371/journal.pone.0098150
Kim YS, Park S, Gilmour SJ, Thomashow MF (2013) Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J 75:364–376
Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a charge in calcium signature after acclimation. Plant Cell 8:489–503
Krugman T, Chagué V, Peleg Z, Balzergue S, Just J, Korol AB, Nevo E, Saranga Y, Chalhoub B, Fahima T (2010) Multilevel regulation and signaling processes associated with adaptation to terminal drought in wild emmer wheat. Funct Integr Genomics 10:167–186
Kurioka S, Matsuda M (1976) Phospholipase C assay using p-nitrophenylphosphorylcholine together with sorbitol and its application to studying the metal and detergent requirement of the enzyme. Anal Biochem 75:281–289
Lang P, Zhang C, Ebel R, Dane F, Dozier W (2005) Identification of cold acclimated genes in leaves of Citrus unshiu by mRNA differential display. Gene 359:111–118
Lee J, Lim YP, Han CT, Nou IS, Hur Y (2013) Genome-wide expression profiles of contrasting inbred lines of Chinese cabbage, Chiifu and Kenshin under temperature stress. Genes Genom 35:273–288
Manickavelu A, Kambara K, Mishina K, Koba T (2007) An efficient method for purifying high quality RNA from wheat pistils. Colloids Surf B Biointerfaces 54:254–258
Medina J, Catalá R, Salinas J (2011) The CBFs:three arabidopsis transcription factors to cold acclimate. Plant Sci 180:3–11
Monks DE, Aghoram K, Courtney PD, DeWald DB, Dewey RE (2001) Hyperosmotic stress induces the rapid phosphorylation of a soybean phosphatidylinositol transfer protein homolog through activation of the protein kinases SPK1 and SPK2. Plant Cell 13:1205–1219
Monroy AF, Dhindsa RS (1995) Low-temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25 degrees C. Plant Cell 7:321–331
Mukhopadhyay A, Vij S, Tyagi AK (2004) Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc Natl Acad Sci USA 101:6309–6314
Ning J, Li X, Hicks LM, Xiong LZ (2010) A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 152(2):876–890
Park S, Lee CM, Doherty CJ, Gilmour SJ, Kim Y, Thomashow MF (2015) Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J 82:193–207
Peterman TK, Ohol YM, McReynolds LJ, Luna EJ (2004) Patellin1, a novel Sec14-like protein, localizes to the cell plate and binds phosphoinositides. Plant Physiol 136:3080–3094
Phillips SE, Vincent P, Rizzieri KE, Schaaf G, Bankaitis VA, Gaucher EA (2006) The diverse biological functions of phosphatidylinositol transfer proteins in eukaryotes. Crit Rev Biochem Mol 41:21–49
Pical C, Westergren T, Dove SK, Larsson C, Sommarin M (1999) Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4, 5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J Biol Chem 274:38232–38240
Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6:1301–1310
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Ritter A, Dittami SM, Goulitquer S, Correa JA, Boyen C, Potin P, Tonon T (2014) Transcriptomic and metabolomic analysis of copper stress acclimation in Ectocarpus siliculosus highlights signaling and tolerance mechanisms in brown algae. BMC Plant Biol 14:116
Saito K, Tautz T, Mustelin T (2007) The lipid-binding SEC14 domain. BBA Mol Cell Biol L 1771:719–726
Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-shinozaki K (2004) Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought-, cold-, and high-salinity-stress conditions. Plant Physiol 136:2734–2746
Schaaf G, Ortlund EA, Tyeryar KR, Mousley CJ, Ile KE, Garrett TA, Ren JH, Woolls MJ, Raetz CRH, Redinbo MR, Bankaitis VA (2008) Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol Cell 29:191–206
Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Zheng G, Zheng CC (2007) Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol 176(1):70–81
Shan XH, Wang XY, Yang G, Wu Y, Su SZ, Li SP, Liu HK, Yuan YP (2013) Analysis of the DNA methylation of maize (Zea mays L.) in response to cold stress based on methylation-sensitive amplified polymorphisms. J Plant Biol 56:32–38
Singh A, Bhatnagar N, Pandey A, Pandey GK (2015) Plant phospholipase C family: regulation and functional role in lipid signaling. Cell Calcium 58:139–146
Smolenska-Sym G, Kacperska A (1994) Phosphatidylinositol metabolism in low temperature-affected winter oilseed rape leaves. Physiol Plant 91:1–8
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94:1035–1040
Suprunova T, Krugman T, Distelfeld A, Fahima T, Nevo E, Korol A (2007) Identification of a novel gene (Hsdr4) involved in water-stress tolerance in wild barley. Plant Mol Biol 64:17–34
Takahashi S, Katagiri T, Hirayama T, Yamaguchi-Shinozaki K, Shinozaki K (2001) Hyperosmotic stress induced a rapid and transient increase in inositol 1,4,5-trisphosphate independent of abscisic acid in Arabidopsis cell culture. Plant Cell Physiol 42:214–222
Tasma IM, Brendel V, Whitham SA, Bhattacharyya MK (2008) Expression and evolution of the phosphoinositide-specific phospholipase C gene family in Arabidopsis thaliana. Plant Physiol Bioch 46:627–637
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Trovato M, Mattioli R, Costantino P (2008) Multiple roles of proline in plant stress tolerance and development. Rend Lincei 19:325–346
Ueno S, Yasutake K, Tohyama D, Fujimori T, Dai Ayusama, Fujii M (2012) Systematic screen for genes involved in the regulation of oxidative stress in the nematode Caenorhabditis elegans. Biochem Bioph Res Co 420:552–557
Umezawa T, Mizumo K, Fujimura T (2002) Discrimination of genes expressed in response to the ionic or osmotic effect of salt stress in soybean with cDNA-AFLP. Plant Cell Environ 25:1617–1625
Venkateswarlu B, Shanker AK, Shanker C, Maheswari M (2012) Crop stress and its management: perspectives and strategies. In: Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M (eds) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor, 1st edn. Springer, Netherlands, pp 261–315
Vincent P, Chua M, Nogue F, Fairbrother A, Mekeel H, Xu Y, Allen N, Bibikova TN, Gilroy S, Bankaittis VA (2005) A Sec14p nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J Cell Biol 168:801–812
Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005) Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 41:195–211
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M (1995) AFLP: a new technique for DNA fingerprinting. Nuc Acids Res 23:4407–4414
Wirtz KWA (1991) Phospholipid transfer proteins. Annu Rev Biochem 60:73–99
Xiong LM, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:S165–S183
Xu WR, Jiao YT, Li RM, Zhang NB, Xiao DM, Ding XL, Wang ZP (2014) Chinese wild-growing Vitis amurensis ICE1 and ICE2 encode MYC-type bHLH transcription activators that regulate cold tolerance in Arabidopsis. PloS one 9:e102303
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yang YN, Li RG, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–551
Yang G, Zou HD, Wu Y, Liu HK, Yuan YP (2011) Identification and characterization of candidate genes involved in chilling responses in maize (Zea mays L.). Plant Cell Tissue Organ Cult 106:127–141
Zhao CZ, Lang ZB, Zhu JK (2015) Cold responsive gene transcription becomes more complex. Trends Plant Sci 20:466–468
Zong XJ, Li DP, Gu LK, Li DQ, Liu LX, Hu XL (2009) Abscisic acid and hydrogen peroxide induce a novel maize group CMAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 229(3):485–495
Acknowledgments
This work was supported by the National Transgenic Crops of New Varieties Breeding Major Project-New Germplasm Combination Breeding of Cold Tolerance Transgenic Maize (20142X0800305B).
Conflict of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C-H Dong.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, X., Shan, X., Xue, C. et al. Isolation and functional characterization of a cold responsive phosphatidylinositol transfer-associated protein, ZmSEC14p, from maize (Zea may L.). Plant Cell Rep 35, 1671–1686 (2016). https://doi.org/10.1007/s00299-016-1980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1980-4