Abstract
Key message
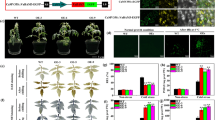
Overexpressing TaUb2 promoted stem growth and resulted in early flowering in transgenic tobacco plants. Ubiquitin are involved in the production, metabolism and proper function of gibberellin.
Abstract
The ubiquitin–26S proteasome system (UPS), in which ubiquitin (Ub) functions as a marker, is a post-translational regulatory system that plays a prominent role in various biological processes. To investigate the impact of different Ub levels on plant growth and development, transgenic tobacco (Nicotiana tabacum L.) plants were engineered to express an Ub gene (TaUb2) from wheat (Triticum aestivum L.) under the control of cauliflower mosaic virus 35S promoter. Transgenic tobacco plants overexpressing TaUb2 demonstrated an accelerated growth rate at early stage and an early flowering phenotype in development. The preceding expression of MADS-box genes also corresponded to the accelerated developmental phenotypes of the transgenic tobacco plants compared to that of wild-type (WT). Total gibberellin (GA) and active GA contents in transgenic tobacco plants were higher than those in WT at the corresponding developmental stages, and some GA metabolism genes were upregulated. Treatment with GA3 conferred a similarly accelerated grown rate in WT plants to that of transgenic tobacco plants, while growth was inhibited when transgenic tobacco plants were treated with a GA biosynthesis inhibitor. Thus, the results suggest that Ub are involved in the production, metabolism and proper function of GA, which is important in the regulation of plant growth and development.








Similar content being viewed by others
Abbreviations
- ELISA:
-
Enzyme-linked immunosorbent assay
- FW:
-
Fresh weight
- GA:
-
Gibberellin
- Ub:
-
Ubiquitin
- UPS:
-
Ubiquitin–26S proteasome system
- WT:
-
Wild type
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. pii:S0003269776699996
Cho SK, Chung HS, Ryu MY, Park MJ, Lee MM, Bahk YY, Kim J, Pai HS, Kim WT (2006) Heterologous expression and molecular and cellular characterization of CaPUB1 encoding a hot pepper U-Box E3 ubiquitin ligase homolog. Plant Physiol 142(4):1664–1682. doi:10.1104/pp.106.087965
Ciechanover A, Elias S, Heller H, Ferber S, Hershko A (1980) Characterization of the heat-stable polypeptide of the ATP-dependent proteolytic system from reticulocytes. J Biol Chem 255(16):7525–7528
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1(4):19–21
Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435(7041):441–445. doi:10.1038/nature03543
Doelling JH, Yan N, Kurepa J, Walker J, Vierstra RD (2001) The ubiquitin-specific protease UBP14 is essential for early embryo development in Arabidopsis thaliana. Plant J 27(5):393–405. doi:tpj1106
Doherty FJ, Dawson S, Mayer RJ (2002) The ubiquitin–proteasome pathway of intracellular proteolysis. Essays Biochem 38:51–63
Fleet CM, Sun TP (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8(1):77–85. doi:10.16/j.pbi.2004.11.015
Fu X, Richards DE, Ait-Ali T, Hynes LW, Ougham H, Peng J, Harberd NP (2002) Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 14(12):3191–3200
Gallego-Giraldo L, Garcia-Martinez JL, Moritz T, Lopez-Diaz I (2007) Flowering in tobacco needs gibberellins but is not promoted by the levels of active GA1 and GA4 in the apical shoot. Plant Cell Physiol 48(4):615–625. doi:10.1093/pcp/pcm034
Gallego-Giraldo L, Ubeda-Tomas S, Gisbert C, Garcia-Martinez JL, Moritz T, Lopez-Diaz I (2008) Gibberellin homeostasis in tobacco is regulated by gibberellin metabolism genes with different gibberellin sensitivity. Plant Cell Physiol 49(5):679–690. doi:10.1093/pcp/pcn042
Gilmour SJ, Zeevaart JA, Schwenen L, Graebe JE (1986) Gibberellin metabolism in cell-free extracts from spinach leaves in relation to photoperiod. Plant Physiol 82(1):190–195
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414(6861):271–276. doi:10.1038/3510450035104500
Guo Q, Zhang J, Gao Q, Xing S, Li F, Wang W (2008) Drought tolerance through overexpression of monoubiquitin in transgenic tobacco. J Plant Physiol 165(16):1745–1755. doi:10.1016/j.jplph.2007.10.002
Harberd NP, King KE, Carol P, Cowling RJ, Peng J, Richards DE (1998) Gibberellin: inhibitor of an inhibitor of…? Bioessays 20 (12):1001–1008. doi:10.1002/(SICI)1521-1878(199812)20:12\1001::AID-BIES6[3.0.CO;2-O
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479. doi:10.1146/annurev.biochem.67.1.425
Hooley R (1994) Gibberellins: perception, transduction and responses. Plant Mol Biol 26(5):1529–1555
Jang S, An K, Lee S, An G (2002) Characterization of tobacco MADS-box genes involved in floral initiation. Plant Cell Physiol 43(2):230–238
Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, Valverde F, Coupland G (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27(8):1277–1288. doi:10.1038/emboj.2008.68
Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435(7041):446–451. doi:10.1038/nature03542
Kusaba S, Kano-Murakami Y, Matsuoka M, Tamaoki M, Sakamoto T, Yamaguchi I, Fukumoto M (1998) Alteration of hormone levels in transgenic tobacco plants overexpressing the rice homeobox gene OSH1. Plant Physiol 116(2):471–476
McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15(5):1120–1130
Melotto M, Mecey C, Niu Y, Chung HS, Katsir L, Yao J, Zeng W, Thines B, Staswick P, Browse J, Howe GA, He SY (2008) A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J 55(6):979–988. doi:10.1111/j.1365-313X.2008.03566.x
Miao Y, Zentgraf U A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J 63(2):179–188. doi:10.1111/j.1365-313X.2010.04233x
Mukhopadhyay D, Riezman H (2007) Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315 (5809):201–205. doi:10.1126/science.1127085
Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev 12(2):198–207
Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, Matsuoka M (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299(5614):1896–1898. doi:10.1126/science.1081077299/5614/1896
Smalle J, Vierstra RD (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 55:555–590. doi:10.1146/annurev.arplant.55.031903.141801
Tanaka-Ueguchi M, Itoh H, Oyama N, Koshioka M, Matsuoka M (1998) Over-expression of a tobacco homeobox gene, NTH15, decreases the expression of a gibberellin biosynthetic gene encoding GA 20-oxidase. Plant J 15(3):391–400
Thomas SG, Rieu I, Steber CM (2005) Gibberellin metabolism and signaling. Vitam Horm 72:289–338. doi:10.16/S0083-6729(05)72009-4
van Nocker S, Vierstra RD (1993) Multiubiquitin chains linked through lysine 48 are abundant in vivo and are competent intermediates in the ubiquitin proteolytic pathway. J Biol Chem 268(33):24766–24773
Vidal AM, Gisbert C, Talon M, Primo-Millo E, Lopez-Diaz I, Garcia-Martinez JL (2001) The ectopic overexpression of a citrus gibberellin 20-oxidase enhances the non-13-hydroxylation pathway of gibberellin biosynthesis and induces an extremely elongated phenotype in tobacco. Physiol Plant 112(2):251–260. doi:ppl1120214
Weiler EW, Jourdan PS, Conrad W (1981) Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta 153:561–571
Weston DE, Elliott RC, Lester DR, Rameau C, Reid JB, Murfet IC, Ross JJ (2008) The Pea DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth. Plant Physiol 147(1):199–205. doi:10.1104/pp108115808
Wilkinson KD (2000) Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol 11(3):141–148. doi:10.1006/scdb.2000.0164S1084-9521(00)90164-2
Wu K, Li L, Gage DA, Zeevaart JA (1996) Molecular cloning and photoperiod-regulated expression of gibberellin 20-oxidase from the long-day plant spinach. Plant Physiol 110(2):547–554
Wu YH, Li Q, Zhang JS, Zheng ZF, Xue S, Li Y (2000) Molecular cloning and characterization of two tobacco MADS-box genes. Sex Plant Reprod 13:163–169
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251. doi:10.1146/annurev.arplant.59.032607.092804
Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001) Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol 127(1):315–323
Yang CW, Gonzalez-Lamothe R, Ewan RA, Rowland O, Yoshioka H, Shenton M (2006) The E3 ubiquitin ligase activity of Arabidopsis plant U-BOX17 and its functional tobacco homolog ACRE276 are required for cell death and defense. Plant Cell 18:1084–1098
Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, Sun TP (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19(10):3037–3057. doi:10.1105/tpc.107.054999
Zhang J, Guo Q, Feng Y, Li F, Gong J, Fan Z, Wang W (2011) Manipulattion of monoubiquitin improves salt tolerance in transgenic tobacco. Plant Biol. doi:10.1111/j.1438-8677.2011.00512.x
Zhu S, Gao F, Cao X, Chen M, Ye G, Wei C, Li Y (2005) The rice dwarf virus P2 protein interacts with ent-kaurene oxidases in vivo, leading to reduced biosynthesis of gibberellins and rice dwarf symptoms. Plant Physiol 139(4):1935–1945. doi:10.1104/pp105072306
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30671259).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Zou.
Rights and permissions
About this article
Cite this article
Wang, GK., Zhang, M., Gong, JF. et al. Increased gibberellin contents contribute to accelerated growth and development of transgenic tobacco overexpressing a wheat ubiquitin gene. Plant Cell Rep 31, 2215–2227 (2012). https://doi.org/10.1007/s00299-012-1331-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1331-z