Abstract
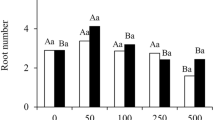
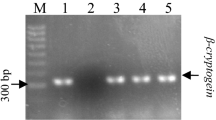
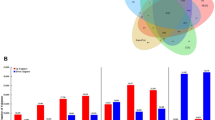
Alternative oxidase (AOX) has been proposed as a functional marker candidate in a number of events involving cell differentiation, including rooting efficiency in semi-hardwood shoot cuttings of olive (Olea europaea L.). To ascertain the general importance of AOX in olive rooting, the auxin-induced rooting process was studied in an in vitro system for microshoot propagation. Inhibition of AOX by salicylhydroxamic acid (SHAM) significantly reduced rooting efficiency. However, the inhibitor failed to exhibit any effect on the preceding calli stage. This makes the system appropriate for distinguishing dedifferentiation and de novo differentiation during root induction. Metabolite analyses of microshoots showed that total phenolics, total flavonoids and lignin contents were significantly reduced upon SHAM treatment. It was concluded that the influence of alternative respiration on root formation was associated to adaptive phenylpropanoid and lignin metabolism. Transcript profiles of two olive AOX genes (OeAOX1a and OeAOX2) were examined during the process of auxin-induced root induction. Both genes displayed stable transcript accumulation in semi-quantitative RT-PCR analysis during all experimental stages. In contrary, when the reverse primer for OeAOX2 was designed from the 3′-UTR instead of the ORF, differential transcript accumulation was observed suggesting posttranscriptional regulation of OeAOX2 during metabolic acclimation. This result confirms former observations in olive semi-hardwood shoot cuttings on differential OeAOX2 expression during root induction. It further points to the importance of future studies on the functional role of sequence and length polymorphisms in the 3′-UTR of this gene.
Key message The manuscript reports the general importance of AOX in olive adventitious rooting and the association of alternative respiration to adaptive phenylpropanoid and lignin metabolism.






Similar content being viewed by others
Abbreviations
- AOX:
-
Alternative oxidase
- Cyt:
-
Cytochrome
- DMSO:
-
Dimethyl sulfoxide
- IBA:
-
Indole-3-butyric acid
- ROS:
-
Reactive oxygen species
- SHAM:
-
Salicylhydroxamic acid
- SQ-RT-PCR:
-
Semi-quantitative RT-PCR
- TGA:
-
Thioglycolic acid
- UTR:
-
Untranslated region
References
Ali MB, Singh N, Shohael AM, Hahn EJ, Paek KY (2006) Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci 171:147–154
Amirsadeghi S, Robson CA, McDonald AE, Vanlerberghe GC (2006) Changes in plant mitochondrial electron transport alter cellular levels of reactive oxygen species and susceptibility to cell death signaling molecules. Plant Cell Physiol 47:1509–1519
Arnholdt-Schmitt B, Costa JH, Fernandes de Melo D (2006a) AOX—a functional marker for efficient ell reprogramming under stress? Trends Plant Sci 6:281–287
Arnholdt-Schmitt B, Santos Macedo E, Peixe A, Cardoso HCG, Cordeiro AM (2006b) AOX—A potential functional marker for efficient rooting in olive shoot cuttings. In: Proceedings Second International Seminar Olivebioteq, Marsala Mazara del Vallo, Italy, vol 1. November 5th–10th, pp 249–254
Balakrishnamurthy G, Rao VN (1988) Changes in phenols during rhizogenesis in rose (Rosa bourboniana Desp). Curr Sci 17:960–962
Booker FL, Miller JE (1998) Phenylpropanoid metabolism and phenolic composition of soybean [Glycine max (L.) Merr.] leaves following exposure to ozone. J Exp Bot 49:1191–1202
Boudet AM, LaPierre C, Grima-Pettenati J (1995) Biochemistry and molecular biology of lignification. New Phytol 129:203–236
Bracci T, Busconi M, Fogher C, Sebastiani L (2011) Molecular studies in olive (Olea europaea L.): overview on DNA markers applications and recent advances in genome analysis. Plant Cell Rep 30:449–462
Campos MD, Cardoso HG, Linke B, Costa JH, Fernandes de Melo D, Justo L, Frederico AM, Arnholdt-Schmitt B (2009) Differential expression and co-regulation of carrot AOX genes (Daucus carota L.). Physiol Plant 137:578–591
Chakraborty D, Sircar D, Mitra A (2008) Phenylalanine ammonia-lyase-mediated biosynthesis of 2-hydroxy-4-methoxybenzaldehyde in roots of Hemidesmus indicus. J Plant Physiol 165:1033–1040
Curir P, Van Sumere CF, Termini A, Barthe P, Marchesini A, Dolci M (1992) Flavonoid accumulation is correlated with adventitious root formation in Eucalyptus gunnii Hook micropropagated through axillary bud stimulation. Plant Physiol 92:1148–1153
Cvikrová M, Malá J, Eder J, Hrubcová M, Vágner M (1998) Abscisic acid, polyamines and phenolic acids in sessile oak somatic embryos in relation to their conversion potential. Plant Physiol Biochem 36:247–255
Cvikrová M, Malá J, Hrubcová M, Eder J, Zoń J, Machác Ková I (2003) Effect of inhibition of biosynthesis of phenylpropanoids on sessile oak somatic embryogenesis. Plant Physiol Biochem 41:251–259
Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
Dutilleul C, Garmier M, Noctor G, Mathieu C, Chetrit P, Foyer CH, de Paepe R (2003) Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell 15:1212–1226
Fiorani F, Umbach AL, Siedow JN (2005) The Alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1a transgenic plants. Plant Physiol 139:1795–1805
Frederico AM, Campos MD, Cardoso HCG, Imani J, Arnholdt-Schmitt B (2009) Alternative Oxidase involvement in Daucus carota L. somatic embrogenesis. Physiol Plant 137:498–508
Fu Z, Xu P, He S, Jaime A (2011) Dynamic changes in enzyme activities and phenolic content during in vitro rooting of tree peony (Paeonia suffruticosa Andr.) plantlets. Maejo Int J Sci Technol 5(02): 252–265
Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Harvey Millar A, Whelan J (2008) The absence of alternative oxidase 1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol 147:595–610
Gray G, Maxwell D, Villarimo A, McIntosh L (2004) Mitochondria/nuclear signaling of alternative oxidase gene expression occurs through distinct pathways involving organic acids and reactive oxygen species. Plant Cell Rep 23:497–503
Grieb B, Schäfer F, Imani J, NezamabadiMashayekhi K, Arnholdt-Schmitt B, Neumann KH (1997) Changes in soluble proteins and phytohormone concentrations of cultured carrot petiole explants during induction of somatic embryogenesis (Daucus carota L.). J Appl Bot 71:94–103
Hartman HT, Kester DE, Davies FT, Geneve RL (1996) Plant propagation: principles and practices, 6th edn. Prentice hall of India, New Delhi, pp 280–284
Hase A (1987) Changes in respiratory metabolism during callus growth and adventitious root formation in Jerusalem artichoke tuber tissues. Plant Cell Physiol 28:833–841
Hilal M, Zenoff AM, Ponessa G, Moreno H, Massa ED (1998) Saline stress alters the temporal patterns of xylem differentiation and alternative oxidase expression in developing soybean roots. Plant Physiol 117:695–701
Lehmann M, Schwarzlander M, Obata T, Sirikantaramas S, Burow M, Olsen CE, Tohge T, Fricker MD, Moller L, Fernie AR, Sweetlove LJ, Laxa M (2009) The metabolic response of Arabidopsis roots to oxidative stress is distinct from that of heterotrophic cells in culture and highlights a complex relationship between the levels of transcripts, metabolites, and flux. Molecular Plant 2:390–410
Martins VP, Dinamarco TM, Soriani FM, Tudella VG, Oliveira SC, Goldman GH, Curti C, Uyemura SA (2011) Involvement of an alternative oxidase in oxidative stress and mycelium-to-yeast differentiation in Paracoccidioides brasiliensis. Eukaryot Cell .doi:10.1128/EC.00194-10
Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96:8271–8276
Moore AL, Albury MS, Crichton PG, Affourtit C (2002) Function of the alternative oxidase: is it still a scavenger? Trends Plant Sci 7:478–481
Ozyigit II, Kahraman MV, Ercan O (2007) Relation between explant age, total phenols and regeneration response in tissue cultured cotton (Gossypium hirsutum L.) African. J Biotechnol 6:3–8
Palama TL, Menard P, Fock I, Choi YH, Bourdon E, Govinden-Soulange J, Bahut M, Payet B, Verpoorte R, Kodja H (2010) Shoot differentiation from protocorm callus cultures of Vanilla planifolia (Orchidaceae): proteomic and metabolic responses at early stage. BMC Plant Biol. doi:10.1186/1471-2229-10-82
Peixe A, Raposo A, Lourenço RS, Cardoso H, Santos Macedo E (2007) Coconut water and BAP successfully replaced zeatin in olive (Olea europaea L.) micro-propagation. Sci Hortic 113:1–7
Peixe, A. Santos Macedo E, Vieira CM, Arnholdt-Schmitt B (2010). A histological evaluation of adventitious root formation in olive (Olea europaea L. cv. ‘Galega vulgar’) microshoots cultured in vitro. In: 28th International Horticultural Congress, Lisbon-Portugal, August 22–27, pp S08–S214 (Abstract Book, p 379)
Polidoros AN, Mylona PV, Arnholdt-Schmitt B (2009) AOX gene structure, transcript variation and expression in plants. Physiol Plant 137:342–353
Popov VN, Simonian RA, Skulachev VP, Starkov AA (1997) Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Lett 415:87–90
Rasmusson AG, Fernie AR, van Dongen JT (2009) Alternative oxidase: a defence against metabolic fluctuations? Physiol Plant 137:371–382
Santos Macedo E, Cardoso HCG, Hernandez A, Peixe AA, Polidoros A, Ferreira A, Cordeiro A, Arnholdt-Schmitt B (2009) Physiological responses and gene diversity indicate olive alternative oxidase as a potential source for markers involved in efficient adventitious root induction. Physiol Plant 137:532–552
Sieger SM, Kristensen BK, Robson CA, Amirsadeghi S, Eng EWY, Abdel-Mesih A, Moller IM, Vanlerberghe GC (2005) The role of alternative oxidase in modulating carbon use efficiency and growth during macronutrient stress in tobacco cells. J Exp Bot 56:1466–1515
Sircar D, Cardoso HG, Mukherjee C, Mitra A, Arnholdt-Schmitt B (2012) Alternative oxidase (AOX) and phenolic metabolism in methyl-jasmonate-treated hairy root cultures of Daucus carota L. J Plant Physiol 169:657–663
Tamagnone L, Merida A, Stacey N, Plaskitt K, Parr A, Chang CF, Lynn D, Dow JM, Roberts K, Martin C (1998) Inhibition of phenolic acid metabolism results in precocious cell death and altered cell morphology in leaves of transgenic tobacco plants. Plant Cell 10:1801–1816
Umbach AL, Fiorani F, Siedow JN (2005) Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol 139:1806–1820
Vanlerberghe GC, McIntosh L (1996) Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol 111:589–595
Vanlerberghe GC, Cyetkovska M, Wang J (2009) Is the maintenance of homeostatic mitochondria signaling during stress a physiological role for alternative oxidase? Physiol Plant 137:392–406
Wang H, Gao X, Zhou GC, Cai L, Yao WB (2008) In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaries fruit. Food Chem 106:888–895
Zavattieri MA, Frederico AM, Lima M, Arnholdt-Schmitt B (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Elec J Biotechnol 13(1). http://www.ejbiotechnology.cl/content/vol13/issue1/full/4/index.html (ISSN 0717-3458)
Acknowledgments
Research of this paper was financially supported by FEDER Funds through the Operational Programme for Competitiveness Factors (COMPETE) and National Funds through Foundation for Science and Technology (FCT) under the project PTDC/AGR-AAM/103377/2008 and the scholarships to Elisete Santos Macedo (SFRH/BD/22061/2005) and to Debabrata Sircar (SFRH/BPD/65788/2009). The authors gratefully acknowledge Dr. Jan T. Svensson (University of Copenhagen) for comments on the manuscript and language revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Petersen.
E. Santos Macedo and D. Sircar contributed equally.
Rights and permissions
About this article
Cite this article
Santos Macedo, E., Sircar, D., Cardoso, H.G. et al. Involvement of alternative oxidase (AOX) in adventitious rooting of Olea europaea L. microshoots is linked to adaptive phenylpropanoid and lignin metabolism. Plant Cell Rep 31, 1581–1590 (2012). https://doi.org/10.1007/s00299-012-1272-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1272-6