Abstract
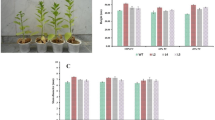
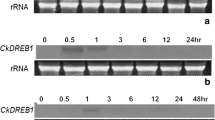
In an attempt to improve chilling stress tolerance, an Arabidopsis C-repeat binding factor 1 (At-CBF1) gene driven by the inducible promoter RD29A was co-transferred into tomato var. Shalimar. Marker (NPTII)-free transgenic were obtained in T1 generation because of unlinked integration of CBF1 and NPTII genes. Reverse transcription-polymerase chain reaction confirmed the expression of CBF1 in T1 transgenic lines. Study of expression pattern in T1 transgenic line showed a gradual increase with increasing chilling stress period and also confirmed the reversibility of expression on removal of stress. The transgenic plants exhibited no morphological and agronomical differences as compared to non-transformed plants. When young transgenic plants were exposed to chilling stress (4°C) for 3 days, increased survival (50%) was observed in transgenic lines than non-transformed plants (10%). Transgenic plants subjected to the chilling stress showed a significant decrease in membrane injury index and lipid peroxidation and also increased significantly free proline content in the leaf tissues as compared to non-transformed plants. Thus, these findings indicate that marker-free transgenic tomato plants expressing Arabidopsis CBF1 gene provided protection and conferred cold tolerance to transgenic tomato without any phenotypic variation.







Similar content being viewed by others
Abbreviations
- BAP:
-
6-Benzylaminopurine
- CBF1 :
-
C-repeat binding factor-1
- CTAB:
-
Cetyl tri-methyl ammonium bromide
- IAA:
-
Indole acetic acid
- MII:
-
Membrane injury index
- MS:
-
Murashige and Skoog
- NPTII :
-
Neomycin phosphotransferase-II
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
References
Afolabi AS, Worland B, Snape J, Vain P (2005) Multiple T-DNA co-cultivation as a method of producing marker-free (clean gene) transgenic rice (Oryza sativa L.) plant. Afr J Biotech 4:531–540
Baker SS, Wilhelm KS, Thomoshow MF (1994) The 5′-region of Arabidopsis thaliana COR15a has cis-acting elements that confer cold-, drought- and ABA regulated gene expression. Plant Mol Biol 24:701–713
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust J Biol Sci 15:413–428
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39(1):205–207
Beck EH, Fetitig S, Knake C, Hartig K, Bhattarai T (2007) Specific and unspecific responses of plants to cold and drought stress. J Biosci 32(3):501–510
Benveniste R, Davies J (1973) Mechanism of antibiotic resistance in bacteria. Ann Rev Biochem 42:471–506
Brasileiro ACM (1998) Neomicina Fosfotransferase II (NPT II). In: Brasileiro ACM, Carneiro VTC (eds) Manual de Transformação Genética de Plantas. Embrapa-SPI/Embrapa-Cenargen, Brasília, Brazil, pp 143–154
Chen THH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5:250–257
Dibax R, Deschamps C, Bespalhok-Filho JC, Vieira LGE, Molinari HBC, De Campos MKF, Quoirin M (2010) Organogenesis and Agrobacterium tumifaciens-mediated transformation of Eucalyptus saligna with P5CS gene. Biol Plant 54(1):6–12
Djilianov D, Georgieva T, Moyankova D, Atanassov A, Shinozaki K, Smeeken SCM, Verma DPS, Murata N (2005) Improved abiotic stress tolerance in plants by accumulation of osmoprotectants-gene transfer approach. Biotech Biotech Eq 19(3):65–71
Doyle JJ, Doyle JI (1987) Isolation of plant DNA from fresh tissue. Phytochem Bull Bot Soci Am 19:11–15
Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold induced COR geen expression. Plant J 16:433–442
Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124:1854–1865
Goyary D, Gupta N, Khare N, Anandhan S, Rathore M, Ahmed Z (2010) In vitro propagation of long melon var. Karnal selection (Cucumis melo L.) from shoot tip. Int J Appl Agric Res 5(1):55–62
Graham D, Patterson GR (1982) Responses of plants to low nonfreezing temperatures: proteins, metabolism and acclimation. Annu Rev Plant Physiol 33:347–372
Hoekstra FA, Golovina EA, Buitink J (2001) Mechanism of plant desiccation tolerance. Trends Plant Sci 6:1360–1385
Hsieh TH, Lee JT, Charng YY, Chan MT (2002) Tomato plants ectopically expressing Arabidopsis CBF1 show enhanced resistance to water deficit stress. Plant Physiol 130:618–626
Jaglo KR, Kleff S, Amundsen KL, Zhang X, Haake V, Zhang JZ, Deits T, Thomashow MF (2001) Components of the Arabidopsis C-repeat/dehydration responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol 127:910–917
Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280:104–106
Janero DR (1990) Malondialdehyde and thiobarbituric activity as diagnostic indices of lipid peroxidation and peroxidative lipid injury. Free Radical Biol Med 9:515–540
Jia GX, Zhu ZQ, Chang FQ, Li YX (2002) Transformation of tomato with the BADH gene from Atriplex improves salt tolerance. Plant Cell Rep 21:141–146
Kavi KPB, Hong J, Miao GH, Hu CAA, Verma DPS (1995) Overexpression of ∆1-pyrroline-5-corboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1382–1394
Lee JT, Prasad V, Yang PT, Wu JF, David Ho TH, Charng YY, Chan MT (2003) Expression of Arabidopsis CBF1 regulated by an ABA/stress inducible promoter in transgenic tomato confers stress tolerance without affecting yield. Plant Cell Environ 26(7):1181–1190
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R (1986) Leaf disc transformation of cultivated tomato (Lycopersicon esculentum) using Agrobacterium tumefaciens. Plant Cell Rep 5:81–84
McKersie BD, Leshem YY (1994) Chilling stress. In: Stress and stress coping in cultivated plants. Kluwer, Dordrecht, pp 79–100
Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RV, Aparicio-Trejo P (1994) Drought induces oxidative stress in pea plant. Planta 194:346–352
Park EJ, Jeknic Z, Sakamoto A, Denoma J, Yuwansiri R, Murata N, Chen TH (2004) Genetic engineering of glycine-betaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 40:474–487
Patterson BD, Mutton L, Paull RE, Nguyen VQ (1997) Tomato pollen development: stages sensitive to chilling and a natural environment for the selection of resistant genotypes. Plant Cell Environ 10:363–368
Paull RE (1990) Chilling injury of tropical and subtropical origin. In: Wang CY (ed) Chilling injury of horticultural crops. CRC Press Inc., Boca Raton, pp 17–36
Pearce RS (2001) Plant freezing and damage. Ann Bot 87:417–424
Prasad TK (1996) Mechanism of chilling induced oxidative stress injury and tolerance in developing maize seedlings; changes in antioxidant system, oxidation of proteins and lipids and protease activity. Plant J 10:1017–1026
Raj SK, Singh A, Pandey SK, Singh BP (2005) Agrobacterium mediated tomato transformation and regeneration of transgenic lines expressing tomato leaf curl virus coat protein gene for resistance against TLCV infection. Curr Sci 88(10):1674–1679
Roberto A, Goxiola Jisheng L, Undurrage S, Dang LM, Allen GJ, Alper LS, Fink GR (2001) Drought- and salt-tolerant plants resulted from overexpression of the AVP1 H1-pump. Proc Natl Acad Sci 98:11444–11449
Roy R, Purty RS, Agrawal V, Gupta SC (2006) Transformation of tomato cultivar ‘‘Pusa Ruby’’ with bspA gene from Populus tremula for drought tolerance. Plant Cell Tissue Organ Cult 84:55–67
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York
Sarad N, Rathore M, Singh NK, Kumar N (2004) Genetically engineering tomatoes: new vista for sustainable agriculture in high altitude regions. In: 4th international crop science congress, Brisbane, Australia
Scutt CP, Zubko E, Meyer P (2002) Techniques for the removal of marker genes from transgenic plants. Biochimie 84:1119–1126
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcription activator that bind to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci 94:1035–1040
Strand A, Foyer CH, Gustafsson P, Gardestrom P, Hurry V (2003) Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant Cell Environ 26:523–535
Thomashow MF, Gilmour SJ, Stockinger EJ, Jaglo-Ottosen KR, Zarka DG (2001) Role of Arabidopsis CBF transcriptional activators in cold acclimation. Physiol Plant 112:171–175
Uemura M, Joseph RA, Steponkus PL (1995) Cold acclimation in Arabidopsis thaliana: effect on plasma membrane lipid composition and freeze-induced lesion. Plant Physiol 109:15–30
Verlues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2005) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539
Wang Y, Qiu L, Dai C, Wang J, Luo J, Zhang F, Ma J (2008) Expression of insect (Microderma puntipennis dzungarica) antifreeze protein MpAFP149 confers the cold tolerance to transgenic tobacco. Plant Cell Rep 27(8):1349–1358
Yuwansiri R, Park EJ, Jeknic Z, Chen THH (2002) Enhancing cold tolerance in plants by genetic engineering of glycine betaine synthesis. In: Li PH, Palva T (eds) Plant cold hardiness: gene regulation and genetic engineering. Kluwer, Dordrecht, pp 259–275
Acknowledgments
Authors are grateful to DRDO HQ, Ministry of Defence, Govt. of India for the financial help. The generous gift of the co-transformation constructs by Dr. A. K. Sharma (Centre for Plant Molecular Biology, University of Delhi, South Campus, India) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Feher.
Rights and permissions
About this article
Cite this article
Singh, S., Rathore, M., Goyary, D. et al. Induced ectopic expression of At-CBF1 in marker-free transgenic tomatoes confers enhanced chilling tolerance. Plant Cell Rep 30, 1019–1028 (2011). https://doi.org/10.1007/s00299-011-1007-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1007-0