Abstract
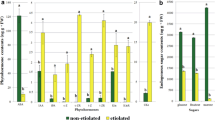
Auxin induces in vitro somatic embryogenesis in coconut plumular explants through callus formation. Embryogenic calli and non-embryogenic calli can be formed from the initial calli. Analysis of endogenous cytokinins showed the occurrence of cytokinins with aromatic and aliphatic side chains. Fourteen aliphatic cytokinins and four aromatic cytokinins were analysed in the three types of calli and all the cytokinins were found in each type, although some in larger proportions than others. The most abundant cytokinins in each type of callus were isopentenyladenine-9-glucoside, zeatin-9-glucoside, zeatin riboside, isopentenyladenine riboside, dihydrozeatin and dihydrozeatin riboside in decreasing order. Total cytokinin content was compared between the three types of calli, and it was found to be lower in embryogenic calli compared to non-embryogenic calli or initial calli. The same pattern was observed for individual cytokinins. When explants were cultured in media containing exogenously added cytokinins, the formation of embryogenic calli in the explants was reduced. When 8-azaadenine (an anticytokinin) was added the formation of embryogenic calli and somatic embryos was increased. These results suggest that the difference in somatic embryo formation capacity observed between embryogenic calli and non-embryogenic calli is related to their endogenous cytokinin contents.





Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxiacetic acid
- BAP:
-
6-Benzylaminopurine
- BAP9G:
-
6-Benzylaminopurine-9-glucoside
- BAPR:
-
6-Benzylaminopurine riboside
- BAPR5′P:
-
6-Benzylaminopurine ribotide
- DHZ:
-
Dihydrozeatin
- DHZR:
-
Dihydrozeatin riboside
- DHZ9G:
-
Dihydrozeatin-9-glucoside
- DHZR5′P:
-
Dihydrozeatin ribotide
- iP:
-
Isopentenyladenine
- iPR:
-
Isopentenyladenine riboside
- iP9G:
-
Isopentenyladenine-9-glucoside
- iPR5′P:
-
Isopentenyladenine ribotide
- mT:
-
Meta-Topolin 6-(3-hydroxybenzylamino)purine
- mT9G:
-
Meta-Topolin-9-glucoside
- mTR:
-
Meta-Topolin-riboside
- oT:
-
Ortho-Topolin 6-(2-hydroxybenzylamino)purine
- oT9G:
-
Ortho-Topolin 9-glucoside
- oTR:
-
Ortho-Topolin riboside
- Z:
-
Zeatin
- ZR:
-
Zeatin riboside
- Z9G:
-
Zeatin-9-glucoside
- ZR5′P:
-
Zeatin ribotide
- ZOG:
-
Zeatin-O-glucoside
- ZROG:
-
Zeatin riboside-O-glucoside
- FW:
-
Fresh weight
- SE:
-
Somatic embryogenesis
References
Ahmadi M, Baker DA (2000) Identification and quantification of the major endogenous cytokinins in pistachio seedlings. Plant Growth Regul 32:351–357
Centeno ML, Rodríguez R, Berros B, Rodríguez A (1997) Endogenous hormonal content and somatic embryogenic capacity of Corylus avellana L. cotyledons. Plant Cell Rep 17:139–144
Chan JL, Sáenz L, Talavera C, Hornung R, Robert M, Oropeza C (1998) Regeneration of coconut (Cocos nucifera L.) from plumule explants through somatic embryogenesis. Plant Cell Rep 17:515–521
Danin M, Upfold SJ, Levin N, Nadel BL, Altman A, van Staden J (1993) Polyamines and cytokinins in celery embryogenic cell cultures. Plant Growth Regul 12:245–254
Davies PJ (1995) The plant hormones: their nature, occurrence and functions. In: Davies PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Kluwer, Dordrecht, pp 1–38
Dieleman JA, Verstappen FWA, Perik PRJ, Kuiper D (1997) Quantification of the export cytokinins from root to shoots of Rosa hybrida and their degradation rate in the shoot. Physiol Plant 101:342–352
Eeuwens CJ (1976) Mineral requirements for growth and callus initiation of tissue explants excised from mature coconut palms (Cocos nucifera) and cultured in vitro. Physiol Plant 36:23–28
Ernst D, Schaffer W, Oesterhelt D (1983) Isolation and identification of a new, naturally occurring cytokinin (6-benzylaminopurine riboside) from anise cell culture (Pimpinella anisum L.). Planta 159:222–225
Faiss M, Zalubilová J, Strnad M, Schmulling T (1997) Conditional expression of the ipt gene indicates a function for cytokinins in paracrine signalling in whole tobacco plants. Plant J 12:401–415
Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 43:27–47
Gaudinová A, Süssenbeková H, Vojtechová M, Kamínek M, Eder J, Kohout L (1995) Different effects of two brassinosteroids on growth, auxin and cytokinin content in tobacco callus tissue. Plant Growth Regul 17:121–126
George EF (1993) Plant propagation by tissue culture, Part 1, 2nd edn. Exegetics, England, p 444
Holub J, Hanuš J, Hanke DE, Strnad M (1998) Biological activity of cytokinins derived from ortho- and meta-hydroxybenzyladenine. Plant Growth Regul 26:109–115
Ivanova A, Velcheva M, Denchev P, Atanassov A, Onckelen A (1994) Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant 92:85–89
Jones LH (1990) Endogenous cytokinins in oil palm (Elaeis guineensis L.) callus, embryoids and regenerant plants measured by radioimmunoassay. Plant Cell Tiss Org Cult 20:201–209
Jones LH, Hughes WA (1989) Oil palm (Elaeis guineensis Jacq). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry 5. Trees II. Springer, Berlin, pp 176–202
Jones LH, Hanke DE, Eeuwens CJ (1995) An evaluation of the role of cytokinins in the development of abnormal inflorescences in oil palm cultures (Elaeis guineensis Jacq.) regenerated from tissue culture. J Plant Growth Regul 14:135–142
Jones LH, Martínková H, Strnad M, Hanke DE (1996) Occurrence of aromatic cytokinins in oil palm (Elaeis guineensis Jacq.). J Plant Growth Regul 15:39–49
Kraigher H, Grayling A, Wang T, Hanke DE (1991) Cytokinin production by two ectomycorrhizal fungi in liquid culture. Phytochemistry 30:2249–2254
Krikorian D (1995) Hormones in tissue culture and micropropagation. In: Davies PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Kluwer, London, pp 774–796
MacDonald EMS, Akioshi DE, Morris RO (1981) Combined high performance liquid chromatography–radioimmunoassay for cytokinins. J Chromatogr 214:101–109
Nandi SK, Palni LM, Parker CW (1990) Dynamics of endogenous cytokinins during the growth cycle of hormone-autotrophic genetic tumor line of tobacco. Plant Physiol 94:1084–1089
Rajasekaran K, Hein MB, Davis GC, Carnes MG, Vasil IK (1987) Endogenous growth regulators in leaves and tissue cultures of Pennisetum purpureum Schum. J Plant Physiol 130:13–25
Sáenz L, Jones LH, Oropeza C, Vlácil D, Strnad M (2003) Cytokinins in Cocos nucifera L. Plant Growth Regul 39:205–215
Somleva MM, Kapchina V, Alexieva V, Golovinsky E (1995) Anticytokinin effects on in vitro response of embryogenic and non embryogenic genotypes of Dactylis glomerata L. Plant Growth Regul 16:109–112
Strnad M (1996) Enzyme immunoassays of N6-benzyladenine and N6-(meta-hydroxybenzyl)adenine cytokinins. J Plant Growth Regul 15:179–188
Verdeil J-L, Hocher V (1997) Coconut: development of methods for the clonal propagation of elite, disease resistant palms by somatic embryogenesis. Third Annual Report. EC STD3
Wagner BM, Beck E (1993) Cytokinins in the perennial herb Urtica dioica L. as influenced by its nitrogen status. Planta 190:511–518
Wenck AR, Conger BV, Trigiano RN, Sams CE (1988) Inhibition of somatic embryogenesis in Orchardgrass by endogenous cytokinins. Plant Physiol 88:990–992
Acknowledgments
We are grateful to H. Martínková for excellent technical assistance. L. Sáenz would like to acknowledge the continuing support from the Centro de Investigación Científica de Yucatán and CONACyT (88207). A. Azpeitia would like to acknowledge continuing support from Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias and CONACyT (119335). Research in the Czech Republic was supported by Ministry of Education Grant No. MSM 6198959216 and Academy of Science Grant No. IBS5038351.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Jordan.
Rights and permissions
About this article
Cite this article
Sáenz, L., Azpeitia, A., Oropeza, C. et al. Endogenous cytokinins in Cocos nucifera L. in vitro cultures obtained from plumular explants. Plant Cell Rep 29, 1227–1234 (2010). https://doi.org/10.1007/s00299-010-0906-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0906-9