Abstract
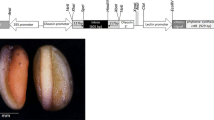
The ability of genetic manipulation to yield greatly increased concentrations of free amino acids (FAAs) in seeds of soybean was evaluated by introduction of a feedback-insensitive mutant enzyme of tryptophan (Trp) biosynthesis into two transformation-competent breeding lines deficient in major seed storage proteins. The storage protein-deficient lines exhibited increased accumulation of certain other seed proteins as well as of FAAs including arginine (Arg) and asparagine in mature seeds. Introduction of the gene for a feedback-insensitive mutant of an α subunit of rice anthranilate synthase (OASA1D) into the two high-FAA breeding lines by particle bombardment resulted in a >10-fold increase in the level of free Trp in mature seeds compared with that in nontransgenic seeds. The amount of free Trp in these transgenic seeds was similar to that in OASA1D transgenic seeds of the wild-type cultivar Jack. The composition of total amino acids in seeds of the high-FAA breeding lines remained largely unaffected by the expression of OASA1D with the exception of an increase in the total Trp content. Our results therefore indicate that the extra nitrogen resource originating from storage protein deficiency was used exclusively for the synthesis of inherent alternative nitrogen reservoirs such as free Arg and not for deregulated Trp biosynthesis conferred by OASA1D. The intrinsic null mutations responsible for storage protein deficiency and the OASA1D transgene affecting Trp content were thus successfully combined and showed additive effects on the amino acid composition of soybean seeds.



Similar content being viewed by others
References
Delhaye S, Landry J (1986) High-performance liquid chromatography and ultraviolet spectrophotometry for quantification of tryptophan in barytic hydrolysates. Anal Biochem 159:175–178
El-Shemy HA, Teraishi M, Khalafalla MM, Katsube-Tanaka T, Utsumi S, Ishimoto M (2004) Isolation of soybean plants with stable transgene expression by visual selection based on green fluorescent protein. Mol Breed 14:227–238
Falco SC, Guida T, Locke M, Mauvais J, Sanders C, Ward RT, Webber P (1995) Transgenic canola and soybean seeds with increased lysine. Biotechnology 13:577–582
Finer JJ, Nagasawa A (1988) Development of an embryogenic suspension culture of soybean [Glycine max (L.) Merrill]. Plant Cell Tissue Organ Cult 15:125–136
Goldraij A, Polacco JC (1999) Arginase is inoperative in developing soybean embryos. Plant Physiol 119:297–303
Hajika M, Takahashi M, Sakai S, Igita M (1996) A new genotype of 7S globulin (β-conglycinin) detected in wild soybean (Glycine soja Sieb. et Zucc.). Breed Sci 46:385–386
Hajika M, Takahashi M, Sakai S, Matsunaga R (1998) Dominant inheritance of a trait lacking β-conglycinin detected in a wild soybean line. Breed Sci 48:383–386
Hanafy MS, Rahman MS, Khalafalla MM, El-Shemy HA, Nakamoto Y, Ishimoto M, Wakasa K (2006) Accumulation of free tryptophan in azuki bean (Vigna angularis) induced by expression of a gene (OASA1D) for a modified α-subunit of rice anthranilate synthase. Plant Sci 171:670–676
Harada K, Toyokawa Y, Kitamura K (1983) Genetic analysis of the most acidic 11S globulin subunit and related characters in soybean seeds. Jpn J Breed 33:23–30
Herrmann KM (1995) The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell 7:907–919
Herrmann KM, Weaver LM (1999) The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol 50:473–503
Huang S, Frizzi A, Florida CA, Kruger DE, Luethy MH (2006) High lysine and high tryptophan transgenic maize resulting from the reduction of both 19- and 22-kD α-zeins. Plant Mol Biol 61:525–535
Inaba Y, Brotherton JE, Ulanov A, Widholm JM (2007) Expression of a feedback insensitive anthranilate synthase gene from tobacco increases free tryptophan in soybean plants. Plant Cell Rep 26:1763–1771
Ishimoto M, Rahman SM, Hanafy MS, Khalafalla MM, El-Shemy HA, Nakamoto Y, Kita A, Takahashi K, Matusda F, Murano Y, Funabashi T, Miyagawa H, Wakasa K (2009) Evaluation of amino acid content and nutritional quality of transgenic soybean seeds with high-level tryptophan accumulation. Mol Breed (in press). doi:10.1007/s11032-009-9334-3
Iwabuchi S, Yamauchi F (1987) Determination of glycinin and β-conglycinin in soybean proteins by immunological method. J Agric Food Chem 35:200–205
Kaizuma N, Miura S (1974) Variations of seed protein percentage and sulfur-containing amino acid content among various leguminous species. Jpn J Breed 24:125–132
Khalafalla MM, Rahman SM, El-Shemy HA, Nakamoto Y, Wakasa K, Ishimoto M (2005) Optimization of particle bombardment conditions by monitoring of transient sGFP(S65T) expression in transformed soybean. Breed Sci 55:257–263
Kinney AJ, Jung R, Herman EM (2001) Cosuppression of the α subunits of β-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum-derived protein bodies. Plant Cell 13:1165–1178
Kita Y, Nishizawa K, Takahashi M, Kitayama M, Ishimoto M (2007) Genetic improvement of the somatic embryogenesis and regeneration in soybean and transformation of the improved breeding lines. Plant Cell Rep 26:755–764
Kitamura K, Kaizuma N (1981) Mutant strains with low level of subunits of 7S globulin in soybean (Glycine max Merr.) seeds. Jpn J Breed 31:353–359
Komatsu A, Ohtake M, Hasegawa H, Terakawa T, Wakasa K (2006) Transgenic rice for animal feed with high tryptophan content generated by a selectable marker- and vector backbone-free technology. Plant Biotechnol 23:39–46
Micallef BJ, Shelp BJ (1989) Arginine metabolism in developing soybean cotyledons. II. Biosynthesis. Plant Physiol 90:631–634
Misra PS, Mertz ET, Glover DV (1975) Studies on corn proteins: VIII. Free amino-acid content of opaque 2 double mutants. Cereal Chem 52:844–848
Mori T, Utsumi S, Inaba H, Kitamura K, Harada K (1981) Differences in subunit composition of glycinin among soybean cultivars. J Agric Food Chem 29:20–23
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nielsen NC (1985) Structure and complexity of the 11S polypeptides in soybean. J Am Oil Chem Soc 62:1680–1686
Nielsen NC, Dickinson CD, Cho T-J, Thanh VH, Scallon BJ, Fischer RL, Sims TL, Drews GN, Goldberg RB (1989) Characterization of the glycinin gene family from soybean. Plant Cell 1:313–328
Odanaka H, Kaizuma N (1989) Mutants on soybean storage proteins induced with γ-ray irradiation. Jpn J Breed 39(Suppl 1):430–431
Schubert D, Lechtenberg B, Forsbach A, Gils M, Bahadur S, Schmidt R (2004) Silencing in Arabidopsis T-DNA transformants: the predominant role of a gene-specific RNA sensing mechanism versus position effects. Plant Cell 16:2561–2572
Simmonds DH, Donaldson PA (2000) Genotype screening for proliferative embryogenesis and biolistic transformation of short-season soybean genotypes. Plant Cell Rep 19:485–490
Slocum RD (2005) Genes, enzymes and regulation of arginine biosynthesis in plants. Plant Physiol Biochem 43:729–745
Staswick PE, Hermodson MA, Nielsen NC (1981) Identification of the acidic and basic subunit complexes of glycinin. J Biol Chem 256:8752–8755
Takahashi K, Banba H, Kikuchi A, Ito M, Nakamura S (1994) An induced mutant line lacking the α-subunit of β-conglycinin in soybean (Glycine max (L.) Merrill). Breed Sci 46:65–66
Takahashi K, Mizuno Y, Yumoto S, Kitamura K, Nakamura S (1996) Inheritance of the α-subunit deficiency of β-conglycinin in soybean (Glycine max (L.) Merrill). Breed Sci 46:251–255
Takahashi M, Uematsu Y, Kashiwaba K, Yagasaki K, Hajika M, Matsunaga R, Komatsu K, Ishimoto M (2003) Accumulation of high levels of free amino acids in soybean seeds through integration of mutations conferring seed protein deficiency. Planta 217:577–586
Thanh VH, Shibazaki K (1978) Major proteins of soybean seeds. Subunit structure of β-conglycinin. J Agric Food Chem 26:692–695
Tomlin ES, Branch SR, Chamberlain D, Gabe H, Wright MS, Stewart JCN (2002) Screening of soybean, Glycine max (L.) Merrill, lines for somatic embryo induction and maturation capability from immature cotyledons. In Vitro Cell Dev Biol 38:543–548
Tumer NE, Richiter JD, Nielsen NC (1982) Structural characterization of glycinin precursors. J Biol Chem 257:4016–4018
Ufaz S, Galili G (2008) Improving the content of essential amino acids in crop plants: goals and opportunities. Plant Physiol 147:954–961
Wang X, Larkins BA (2001) Genetic analysis of amino acid accumulation in opaque-2 maize endosperm. Plant Physiol 125:1766–1777
Yagasaki K, Kaizuma N, Kitamura K (1996) Inheritance of glycinin subunits and characterization of glycinin molecules lacking the subunits in soybean (Glycine max (L.) Merr.). Breed Sci 46:11–15
Yagasaki K, Takagi T, Sakai M, Kitamura K (1997) Biochemical characterization of soybean protein consisting of different subunits of glycinin. J Agric Food Chem 45:656–660
Yagasaki K, Kousaka F, Kitamura K (2000) Potential improvement of soymilk gelation properties by using soybeans with modified protein subunit compositions. Breed Sci 50:101–107
Acknowledgments
This work was supported by CREST of the Japan Science and Technology Agency (K.W. and M.I.). We thank Yasuo Niwa (School of Food and Nutritional Science, University of Shizuoka, Japan) for providing the sGFP(S65T) gene.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Ebinuma.
Rights and permissions
About this article
Cite this article
Kita, Y., Nakamoto, Y., Takahashi, M. et al. Manipulation of amino acid composition in soybean seeds by the combination of deregulated tryptophan biosynthesis and storage protein deficiency. Plant Cell Rep 29, 87–95 (2010). https://doi.org/10.1007/s00299-009-0800-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0800-5