Abstract
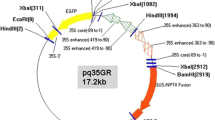
A genetic transformation protocol for green ash (Fraxinus pennsylvanica) hypocotyl explants was developed. Green ash hypocotyls were transformed using Agrobacterium tumefaciens strain EHA105 harboring binary vector pq35GR containing the neomycin phosphotransferase (nptII) and β-glucuronidase (GUS) fusion gene, and an enhanced green fluorescent protein gene. Pre-cultured hypocotyl explants were transformed in the presence of 100 μM acetosyringone using 90 s sonication plus 10 min vacuum-infiltration. Kanamycin at 20 mg l−1 was used for selecting transformed cells. Adventitious shoots regenerated on Murashige and Skoog medium supplemented with 13.3 μM 6-benzylaminopurine, 4.5 μM thidiazuron, 50 mg l−1 adenine sulfate, and 10% coconut water. GUS- and polymerase chain reaction (PCR)-positive shoots from the cut ends of hypocotyls were produced via an intermediate callus stage. Presence of the GUS and nptII genes in GUS-positive shoots were confirmed by PCR and copy number of the nptII gene in PCR-positive shoots was determined by Southern blotting. Three transgenic plantlets were acclimatized to the greenhouse. This transformation and regeneration system using hypocotyls provides a foundation for Agrobacterium-mediated transformation of green ash. Studies are underway using a construct containing the Cry8Da protein of Bacillus thuringiensis for genetic transformation of green ash.




Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzylaminopurine
- EGFP:
-
Enhanced green fluorescent protein
- GUS:
-
β-Glucuronidase
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog
- nptII:
-
Neomycin phosphotransferase
- PCR:
-
Polymerase chain reaction
- TDZ:
-
Thidiazuron
References
Bates SA (1997) Developing protocols to genetically transform white ash (Fraxinus americana L.). PhD Dissertation. Southern Illinois University at Carbondale
Cappaert D, McCullough DG, Poland TM, Siegert NW (2005) Emerald ash borer in North America: a research and regulatory challenge. Am Entomol 51:51–53
Chakrabarty R, Viswakarma N, Bhat SR, Kirti PB, Singh BD, Chopra VL (2002) Agrobacterium-mediated transformation of cauliflower: optimization of protocol and development of Bt-transgenic cauliflower. J Biosci 27(5):495–502
Dobesberger EJ (2002) Emerald ash borer, Agrilus planipennis: pest risk assessment. Canadian Food Inspection Agency, Plant Health Risk Assessment Unit, Nepean
Dong JZ, McHughen A (1993) An improved procedure for production of transgenic flax plants using Agrobacterium tumefaciens. Plant Sci 88:61–71
Du N, Pijut PM (2008) Regeneration of plants from Fraxinus pennsylvanica hypocotyls and cotyledons. Sci Hortic 118(1):74–79
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gonzalez-Padilla IM, Webb K, Scorza R (2003) Early antibiotic selection and efficient rooting and acclimatization improve the production of transgenic plum plants (Prunus domestica L.). Plant Cell Rep 22(1):38–45
Haack RA, Jendek E, Liu H, Marchant KR (2002) The emerald ash borer: a new exotic pest in North America. Newsl Mich Entomol Soc 47(3&4):1–5
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kennedy HE (1990) Green ash (Fraxinus pennsylvanica Marsh.). In: Burns RM, Honkala BH (tech cords) Silvics of North America. Agric. handbook 654. USDA, Forest Service, Washington, pp 348–354
Kim KH, Lee YH, Kim D, Park YH, Lee JY, Hwang YS, Kim YH (2004) Agrobacterium-mediated genetic transformation of Perilla frutescens. Plant Cell Rep 23:386–390
Koroch A, Kapteyn J, Juliani HR, Simon JE (2002) In vitro regeneration and Agrobacterium transformation of Echinacea purpurea leaf explants. In: Janick J, Whipkey A (eds) Trends in new crops and new uses. ASHS Press, Alexandria, pp 522–526
Lee YH, Lee SB, Suh SC, Byun MO, Kim HO (2000) Herbicide resistant cabbage (Brassica oleracea ssp. capitata) plants by Agrobacterium-mediated transformation. J Plant Biotechnol 2:35–41
Lefort F, Douglas GC (1999) An efficient micro-method of DNA isolation from mature leaves of four hardwood tree species Acer, Fraxinus, Prunus and Quercus. Ann Des Sci Forestières 56:259–263
Li ZJ, Jayasankar S, Gray DJ (2004) Bi-directional duplex promoters with duplicated enhancers significantly increase transgene expression in grape and tobacco. Transgenic Res 13:143–154
Liu ZC, Park BJ, Kanno A, Kameya T (2005) The novel use of a combination of sonication and vacuum infiltration in Agrobacterium-mediated transformation of kidney bean (Phaseolus vulgaris L.) with lea gene. Mol Breed 16:189–197
Lloyd G, McCown B (1981) Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc Int Plant Prop Soc 30:421–427
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray BE, Handyside RJ, Keller WA (1977) In vitro regeneration of shoots on stem explants of haploid and diploid flax (Linum usitatissimum). Can J Genet Cytol 19:177–186
Park BJ, Liu ZC, Kanno A, Kameya T (2005) Genetic improvement of Chinese cabbage for salt and drought tolerance by constitutive expression of a B. napus LEA gene. Plant Sci 169(3):553–558
Pathak MR, Hamzah RY (2008) An effective method of sonication-assisted Agrobacterium-mediated transformation of chickpeas. Plant Cell Tissue Organ Cult 93:65–71
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J For 104(3):118–124
Rohini VK, Manjunath NH, Rao KS (2005) Studies to develop systems to mobilize foreign genes into parasitic flowering plants. Physiol Mol Biol Plants 11(1):111–120
Roome WJ (1992) Agrobacterium-mediated transformation of two forest tree species Prunus serotina and Fraxinus pennsylvanica. MS Thesis, State University of New York, College of Environmental Science and Forestry
SAS Institute (1999) The SAS system for Windows, ver. 8. SAS Inst., Cary
Shin D, Park HS (2006) Enhancement of genetic transformation for seedlings of Brassica juncea L. Czern var. Laciniata Makino by chemical wounding. Korean J Breed 38(5):366–369
Stachel SE, Messens E, Van Montagu M, Zambryski P (1985) Identification of signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629
Steward FC, Caplin SM, Shantz EM (1955) Investigations on the growth and metabolism of plant cells. Ann Bot 19:29–47
Supartana P, Shimizu T, Nogawa M, Shioiri H, Nakajima T, Haramoto N, Nozue M, Kojima M (2006) Development of simple and efficient in planta transformation method for wheat (Triticum aestivum L.) using Agrobacterium tumefaciens. J Biosci Bioeng 102(3):162–170
Trick HN, Finer JJ (1997) Sonication-assisted Agrobacterium-mediated transformation. Transgenic Res 6(5):329–336
USDA FAS (2004) Foreign agricultural service export commodity aggregations. Department of Commerce, US Census Bureau, Foreign Trade Statistics
Wang X, Zhou Z, Li Q, Gu XH, Ma DF (2006) Optimization of genetic transformation using SAAT in sweetpotato. Jiangsu J Agric Sci 22(1):14–18
Wei X, Guo X, Yuan T, Russell SD (2006) A highly efficient in vitro plant regeneration system and Agrobacterium-mediated transformation in Plumbago zeylanica. Plant Cell Rep 25(6):513–521
Yookongkaew N, Srivatanakul M, Narangajavana J (2007) Development of genotype-independent regeneration system for transformation of rice (Oryza sativa ssp. indica). J Plant Res 120(2):237–245
Yu C (1992) Agrilus arcopoli Obenberger. In: Xiao GR (ed) Forest insects of China, 2nd edn. China Forestry Publishing House, Beijing, pp 400–401
Acknowledgments
This work was supported financially by a Fred M. van Eck scholarship for Purdue University to Ningxia Du. The authors gratefully acknowledge Dr. Dennis J. Gray, University of Florida, for the transformation vector pq35GR, and Drs. Shujun Chang and Zhijan Li for their constructive review and suggestions for the improvement of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Harwood.
Rights and permissions
About this article
Cite this article
Du, N., Pijut, P.M. Agrobacterium-mediated transformation of Fraxinus pennsylvanica hypocotyls and plant regeneration. Plant Cell Rep 28, 915–923 (2009). https://doi.org/10.1007/s00299-009-0697-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0697-z