Abstract
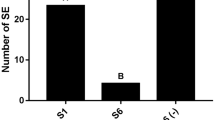
In white spruce (Picea glauca), an improvement of somatic embryo yield and quality can be achieved by applications of dl-buthionine-[S,R]-sulfoximine (BSO), which inhibits the biosynthesis of reduced glutathione (GSH), thereby switching the total glutathione pool towards its oxidized form (GSSG). Applications of BSO almost tripled the embryogenic output of two cell lines by increasing the number of embryos produced by 100 mg−1 tissue from 65 to 154 in the (E)WS1 line and from 59 to 130 in the (E)WS2 line. This increase in embryo number was ascribed to a higher production of morphologically normal embryos with four or more cotyledons (group A embryos), at the expense of group B embryos, characterized by fewer cotyledons. The quality of the embryos produced, estimated by their post-embryonic performance, was also different between treatments. In both cell lines applications of BSO in the maturation medium increased the conversion frequency, i.e. root and shoot emergence, of group A embryos while it enhanced root emergence in group B embryos. Compared to their control counterparts, BSO-treated embryos had normal shoot apical meristems as in their zygotic counterparts. Such meristems were characterized by large apical cells and vacuolated sub-apical cells. They also lacked intercellular spaces, which were present in the apical poles of control embryos where they contributed to cell–cell separation and meristem degradation. Furthermore, storage product accumulation was also improved in the presence of BSO, with protein bodies prevailing over starch. These data show that an oxidized glutathione environment is beneficial for spruce embryo production in vitro.





Similar content being viewed by others
References
Belmonte M, Stasolla C, Loukanina N, Yeung EC, Thorpe TA (2003) Effects of reduced and oxidized glutathione on purine nucleotide metabolism of white spruce embryogenic tissue. Plant Sci 165:1377–1385
Belmonte M, Stasolla C, Loukanina N, Yeung EC, Thorpe TA (2004) Effects of reduced and oxidized glutathione on purine nucleotide metabolism of white spruce embryogenic tissue. Plant Sci 165:1377–1385
Belmonte MF, Yeung ECY (2004) The effects of reduced and oxidized glutathione on white spruce somatic embryogenesis. In Vitro Cell Dev Biol Plant 40:61–66
Belmonte MF, Donald G, Reid DM, Yeung EC, Stasolla C (2005) Alterations of the glutathione redox state improve apical meristem structure and somatic embryo quality in white spruce (Picea glauca). J Exp Bot 56:2355–2364
Belmonte MF, Ambrose SJ, Ross AR S, Abrams SR, Stasolla C (2006) Improved development of microspore derived embryo cultures of Brassica Napus cv Topaz following changes in glutathione metabolism. Physiol Plant 127:690–700
De Gara L, de Pinto MC, Moliterni VM C, D’Egidio MG (2003) Redox regulation and storage processes during maturation in kernels of Triticum durum. J Exp Bot 54:249–258
de Pinto MC, Francis D, De Gara L (1999) The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209:90–97
Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (s-n-butyl homocysteine sulfoximine). J Biochem Chem 254:7558–7560
Grossnickle SC (2000) Ecophysiology of northern spruce species: the performance of planted seedlings. NRC Research Press, Ottawa
Jain SM, Newton RJ, Sopltes EJ (1988) Enhancement of somatic embryogenesis in Norway spruce (Picea abies L.). Theor Appl Genet 76:501–506
Jiang K, Meng YL, Feldman LJ (2002) Quiescent center formation in maize roots is associated with an auxin-regulated oxidizing environment. Development 130:1429–1438
Litvay JD, Verma DC, Johnson MA (1985) Influence of a loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep 4:325–328
Lu C-Y, Thorpe TA (1987) Somatic embryogenesis and plantlet regeneration in cultured immature embryos of Picea glauca. J Plant Physiol 128:297–302
Shi Z-Z, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Lieberman MW (2000) Glutathione synthesis is essential for mouse development but not cell growth in culture. Proc Natl Acad Sci USA 97:5101–5106
Stasolla C, Yeung EC (2003) Advances on embryogenesis in culture of coniferous species: improving somatic embryo quality. Plant Cell Tiss Org Cult 74:15–35
Stasolla C, Belmonte MF, van Zyl L, Craig D, Liu W, Yeung EC, Sederoff RR (2004) The effect of reduced glutathione on morphology and gene expression of white spruce (Picea glauca) somatic embryos. J Exp Bot 55:695–709
Yeung EC (1984) Histological and histochemical staining procedures. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol 1. Academic Press, Orlando, pp 689–697
Yeung EC (1999) The use of histology in the study of plant tissue culture systems—some practical comments. In Vitro Cell Dev Biol Plant 35:137–143
Yeung EC, Stasolla C (2000) Somatic embryogenesis—apical meristems and embryo conversion. Korean J Plant Tiss Cult 27:299–307
Yeung EC, Stasolla C, Kong L (1998) Apical meristem formation during zygotic embryo development of white spruce. Can J Bot 76:751–761
Yeung EC, Belmonte MF, Tu LTT, Stasolla S (2005) Glutathione modulation of in vitro development. In Vitro Cell Dev Biol Plant 41:584–590
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Englewood Cliff, pp 208–228
Zhang J, Kirkham MB (1996) Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol 132:361–373
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council of Canada Research Grants to CS and an NSERC PGS-D to MFB. The assistance of Mr. Bert Luit is also greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar
Rights and permissions
About this article
Cite this article
Belmonte, M.F., Stasolla, C. Applications of dl-buthionine-[S,R]-sulfoximine deplete cellular glutathione and improve white spruce (Picea glauca) somatic embryo development. Plant Cell Rep 26, 517–523 (2007). https://doi.org/10.1007/s00299-006-0267-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0267-6