Abstract
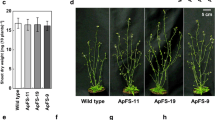
The effects of the cytosolic expression of Escherichia coli pyrophosphatase (ppa) were investigated in the rosette leaves of transgenic Arabidopsis plants. During the daytime, glucose and fructose were found to accumulate at levels that were approximately two- to threefold higher in these plants than in the wild type. Interestingly, however, neither sucrose nor starch levels showed any distinctive build up in transgenic plants except under continuous white light growth conditions, during which they accumulated at high levels. Additionally, the leaves of transgenic Arabidopsis plants contain two- to threefold higher levels of inorganic phosphate (Pi) and two- to sixfold higher levels of uridine diphosphate-glucose than wild type plants during the diurnal cycle. In contrast, triose phosphate contents in the leaves of E. coli ppa transformants were either similar or slightly decreased when compared with wild type leaves. Furthermore, the photosynthetic activity of these transgenic plants was found to be reduced by 20–40% compared to normal levels. These results indicate that induction of ppa activity in the cytosol affects carbon partitioning between source and sink organs and also that the concomitant increase in Pi caused the accumulation of carbon metabolites and reduced photosynthetic activity.








Similar content being viewed by others
Abbreviations
- PFP::
-
Pyrophosphate-dependent fructose-6-phosphate-1 phosphotransferase
- Pi::
-
Inorganic phosphate
- ppa::
-
Pyrophosphatase
- PPi::
-
Inorganic pyrophosphate
- UDP-Glc::
-
UDP-glucose
- UGPase::
-
UDP glucose pyrophosphorylase
References
Borisjuk L, Walenta S, Weber H, Mueller-Klieser W, Wobus U (1998) High-resolution histographical mapping of glucose concentrations in developing cotyledons of Vicia fava in relation to mitotic activity and storage process: glucose as a possible developmental trigger. Plant J 15:583–591
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Dancer JE, ap Rees T (1989) Phosphoribosyl pyrophosphate and the measurement of inorganic pyrophosphate in plant tissues. Planta 177:261–266
du Jardin P, Rojas-Beltran J, Gebhardt C, Brasseur R (1995) Molecular cloning and characterization of a soluble inorganic pyrophosphatase in potato. Plant Physiol 109:853–860
Farre EM, Bachmann A, Willmitzer L, Trethewey RN (2001) Acceleration of potato tuber sprouting by the expression of a bacterial pyrophosphatase. Nat Biotechnol 19:268–272
Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M (1998) Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205:428–437
Geigenberger P (2003) Regulation of sucrose to starch conversion in growing potato tubers. J Exp Bot 54:457–465
Gross P, ap Rees T (1986) Alkaline inorganic pyrophosphatase and starch synthesis in amyloplasts. Planta 167:140–145
Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25:989–994
Hajirezaei M, Sonnewald U, Viola R, Carlisle S, Dennis D, Stitt M (1994) Transgenic potato plants with strongly decreased expression of pyrophosphate:fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta 192:16–30
Hajirezaei M, Bornke F, Peisker M, Takahata Y, Lerchl J, Kirakosyan A, Sonnewald U (2003) Decreased sucrose content triggers starch breakdown and respiration in stored potato tubers (Solanum tuberosum). J Exp Bot 4:477–488
Häusler RE, Schlieben NH, Schulz B, Flügge UI (1998) Compensation of decreased triose phosphate/phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta 204:366–376
Heinonen JK, Lahti RJ (1981) A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem 113:313–317
Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M (1992) Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188:238–244
Karthikeyan AS, Varadarajan DK, Mukatira UT, D’Urzo MP, Damsz B, Raghothama KG (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130:221–233
Knappe S, Flugge UI, Fischer K (2003) Analysis of the plastidic phosphate translocator gene family in Arabidopsis and identification of new phosphate translocator-homologous transporters, classified by their putative substrate-binding site. Plant Physiol 131:1178–1190
Lerchl J, Geigenberger P, Stitt M, Sonnewald U (1995) Impaired photoassimilate partitioning caused by phloem-specific removal of pyrophosphate can be complemented by a phloem-specific cytosolic yeast-derived invertase in transgenic plants. Plant Cell 7:259–270
Mersereau M, Pazour GJ, Das A (1990) Efficient transformation of Agrobacterium tumefaciens by electroporation. Gene 90:149–151
Nielsen TH, Stitt M (2001) Tobacco transformants with strongly decreased expression of pyrophosphate:fructose-6-phosphate expression in the base of their young growing leaves contain much higher levels of fructose-2,6-bisphosphate but no major changes in fluxes. Planta 214:106–116
Paul M, Sonnewald U, Hajirezaei M, Dennis D, Stitt M (1995) Transgenic tobacco plants with strongly decreased expression of pyrophosphate:Fructose-6-phosphate 1-phosphotransferase do not differ significantly from wild type in photosynthate partitioning, plant growth or their ability to cope with limiting phosphate, limiting nitrogen and suboptimal temperatures. Planta 196:277–283
Rea P, Sanders DA (1987) Tonoplast energisation: two H+ pumps, one membrane. Physiol Plant 71:131–141
Sokolov LN, Déjardin A, Kleczkowski LA (1998) Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress). Biochem J 336:681–687
Sonnewald U (1992) Expression of E. coli inorganic pyrophosphatse in transgenic plants alters photoassimilate partitioning. Plant J 2:571–581
Stitt M, Lilley RM, Gerhardt R, Heldt HW (1989) Determination of metabolite levels in specific cells and subcellular compartments of plant leaves. Method Enzymol 174:518–522
Takeshige K, Tazawa M (1989) Determination of the inorganic pyrophosphate level and its subcellular localization in Characorallina. J Biol Chem 264:3262–3266
Visser K, Heimovaara-Dijkstra S, Kijne JW, Wang M (1998) Molecular cloning and characterization of an inorganic pyrophosphatase from barley. Plant Mol Biol 37:131–140
Von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Walters RG, Ibrahim DG, Horton P, Kruger NJ (2004) A mutant of Arabidopsis lacking the triose-phosphate translocator reveals metabolic regulation of starch breakdown in the light. Plant Physiol 135:1–16
Weber A, Schneidereit J, Voll LM (2004) Using mutants to probe the in vivo function of plastid envelope membrane metabolite transporters. J Exp Bot 55:1231–1244
Weber A (2004) Solute transporters as connecting elements between cytosol and plastid stroma. Curr Opin Plant Biol 7:247–253
Weiner H, Stitt M, Heldt HW (1987) Subcellular compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim Biophys Acta 893:13–21
Zrenner R, Willmitzer L, Sonnewald U (1993) Analysis of the expression of potato uridinediphosphoglucose pyrophosphorylase and its inhibition by antisense RNA. Planta 190:247–252
Acknowledgement
This research was supported by a grant from the Korean Science and Engineering Foundation through the Plant Metabolism Research Centre, Kyung Hee University, Suwon, Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.S. Chung
Rights and permissions
About this article
Cite this article
Lee, JW., Lee, DS., Bhoo, S.H. et al. Transgenic Arabidopsis plants expressing Escherichia coli pyrophosphatase display both altered carbon partitioning in their source leaves and reduced photosynthetic activity. Plant Cell Rep 24, 374–382 (2005). https://doi.org/10.1007/s00299-005-0951-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0951-y