Abstract
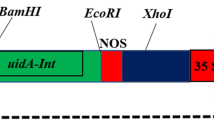
The feedback-insensitive anthranilate synthase (ASA2) cDNA—isolated from a 5-methyltryptophan (5MT)-resistant tobacco cell line—driven by the CaMV 35S promoter or 606 bp of the native ASA2 promoter, was introduced into the forage legume plant Astragalus sinicus or soybean (Glycine max), using Agrobacterium rhizogenes strains DC-AR2 or K599, respectively. Hairy roots of A. sinicus transformed with 35S-ASA2 but not 606-ASA2 could be directly selected using 20–75 µM 5MT. ASA2 mRNA was expressed in all A. sinicus lines selected with 5MT, but nptII mRNA was expressed only in some lines even though the gene was present. Free tryptophan was increased 8- to 26-fold in A. sinicus and 3- to 6-fold in soybean (selected with kanamycin). An HPLC method was used to measure anthranilate synthase (AS) activity since there was a fluorescent compound or compounds present in the soybean hairy root extracts. The transformed soybean hairy roots contained more feedback-resistant AS activity, showing that there is interaction of the tobacco ASA2 α-subunit with the soybean β-subunit to form an active enzyme. Soybean hairy roots that express ASA2 also exhibit 5MT resistance. These results demonstrate that the tobacco feedback-insensitive ASA2 gene can be used as a selectable marker for transformation of the legume A. sinicus.










Similar content being viewed by others
Abbreviations
- AS :
-
Anthranilate synthase
- Kan :
-
Kanamycin
- 5MT :
-
5-Methyltryptophan
References
Anderson PC, Chomet PS, Griffor MC, Kriz AL (1997) Anthranilate synthase gene and its use thereof. World Intellectual Property Organization, July 24 1997, 97/26366
Berardino MB, Roingeard FC, Fukagawa NK (1990) Plasma tryptophan and tyrosine concentrations: determination using high performance liquid chromatography and fluorometric detection. J Nutr Biochem 1:220–222
Cho H-J, Widholm JM, Tanaka N, Nakanishi Y, Murooka Y (1998) Agrobacterium rhizogenes-mediated transformation and regeneration of the legume Astragalus sinicus (Chinese milk vetch). Plant Sci 138:53–65
Cho H-J, Brotherton JE, Song H-S, Widholm JM (2000a) Increasing tryptophan synthesis in a forage legume Astragalus sinicus by expressing the tobacco feedback-insensitive anthranilate synthase (ASA2) gene. Plant Physiol 123:1069–1076
Cho H-J, Farrand SK, Noel GR, Widholm JM (2000b) High efficiency induction of soybean hairy roots and propagation of the soybean cyst nematode. Planta 210:195–204
Daniell H, Muthukumar B, Lee SB (2001) Marker free transgenic plants: engineering the chloroplast genome without the use of antibiotic selection. Curr Genet 39:109–116
Dellaporta S (1994) Plant DNA miniprep and microprep. In: Freeling M, Walbot V (eds) The maize handbook. Springer, New York Berlin Heidelberg, pp 522–525
Grady H, Palmer RG, Imsande J (1995) Isoflavonoids in root and hypocotyls of soybean seedlings (Glycine max, Fabaceae). Am J Bot 82:964–968
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Joersbo M, Donaldson I, Kreiberg J, Peterson S, Brunstedt J, Okkels F (1998) Analysis of mannose selection used for transformation of sugar beet. Mol Breed 4:111–117
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning; a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Siehl DL, Subramanian MV, Walters EW, Blanding JH, Niderman TH, Weinmann C (1997) Evaluating anthranilate synthase as a herbicide target. Weed Sci 45:628–633
Singh M, Widholm JM (1974) Measurement of the five enzymes which convert chorismate to tryptophan in wheat plants (Triticum aestivum L.). Physiol Plant 32:362–366
Song H-S, Brotherton JE, Gonzales RA, Widholm JM (1998) Tissue culture specific expression of a feedback-insensitive Nicotiana tabacum anthranilate synthase. Plant Physiol 117:533–543
Tozawa Y, Hasegawa H, Terakawa T, Wakasa K (2001) Characterization of rice anthranilate synthase α-subunit genes OASA1 and OASA2. Tryptophan accumulation in transgenic rice expressing a feedback-insensitive mutant of OASA1. Plant Physiol 126:1493–1506
Wakasa K, Widholm JM (1987) A 5-methyltryptophan resistant rice mutant MTR1, selected in tissue culture. Theor Appl Genet 74:49–54
Wang CS, Todd JJ, Vodkin LO (1994) Chalcone synthase mRNA and activity are reduced in yellow soybean seed coats with dominant I alleles. Plant Physiol 105:739–748
Weeks JT, Koshiyama KY, Maier-Greiner U, Schaeffner T, Anderson OD (2000) Wheat transformation using cyanamide as a new selective agent. Crop Sci 40:1749–1754
Widholm JM (1972a) Anthranilate synthase from 5-methyltryptophan-susceptible and -resistant cultured Daucus carota cells. Biochim Biophys Acta 279:48–57
Widholm JM (1972b) Cultured Nicotiana tabacum cells with an altered anthranilate synthetase which is less sensitive to feedback inhibition. Biochim Biophys Acta 261:52–58
Widholm JM (1974) Control of aromatic amino acid biosynthesis in cultured plant tissues: effect of intermediates and aromatic amino acids on free levels. Physiol Plant 30:13–18
Wilmink A, Dons JJM (1993) Selective agents and marker genes for use in transformation of monocotyledonous plant. Plant Mol Biol Rep 11:165–185
Zhang X-H, Takagi H, Widholm JM (2004) Expression of a novel yeast gene that detoxifies the proline analog azetidine-2-carboxylate confers resistance during tobacco seed germination, callus and shoot formation. Plant Cell Rep 22:615–622
Acknowledgements
This work was carried out with the technical assistance of Xiangxia Luo and Juli Chikaraishi, and was supported by funds from the Illinois Council on Food and Agricultural Research, the Illinois Soybean Program Operating Board, the United Soybean Board, the Consortium for Plant Biotechnological Research and the Illinois Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Gleddie
Rights and permissions
About this article
Cite this article
Cho, HJ., Brotherton, J.E. & Widholm, J.M. Use of the tobacco feedback-insensitive anthranilate synthase gene (ASA2) as a selectable marker for legume hairy root transformation. Plant Cell Rep 23, 104–113 (2004). https://doi.org/10.1007/s00299-004-0789-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0789-8