Abstract
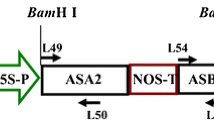
A novel acetyltransferase (Mpr1) found in Saccharomyces cerevisiae (strain Σ1278b) has been shown to specifically detoxify a proline analog, l-azetidine-2-carboxylic acid (A2C) in yeast cells [M. Shichiri et al. (2001) J Biol Chem 276: 41998–42002]. We investigated whether the yeast MPR1 gene would function similarly in a plant system and if its expression could confer resistance to proline analogs. The MPR1 gene coding sequence driven by two different constitutive promoters, with or without the 5′- and 3′-noncoding sequence from the MPR1 gene adjacent to the conventional NOS terminator, was transformed into tobacco (Nicotiana tabacum L. cv. Xanthi) plants via Agrobacterium tumefaciens infection. The presence of the yeast 5′- and 3′-noncoding sequences appeared to increase the likelihood of MPR1 gene expression in the transgenic plants. The kanamycin-selected transgenic plants with a high level of Mpr1 activity grew normally, and their progeny expressed acetyltransferase activity that could utilize A2C, azetidine-3-carboxylic acid and 4-hydroxy-l-proline as substrates. Resistance to A2C, but not to the other two analogs, was exhibited during leaf tissue culture and seed germination. The A2C toxicity to the wild-type plants was reversed by the addition of proline, suggesting that A2C acts as a proline analog. Our studies confirm that MPR1 can function in a similar fashion in tobacco as in yeast to detoxify the toxic proline analog A2C, so it could potentially be used as a new selectable marker for plant transformation. However, our attempts to utilize MPR1 as an efficient selectable marker gene for the A. tumefaciens-mediated transformation of tobacco were unsuccessful.






Similar content being viewed by others
Abbreviations
- A2C::
-
l-Azetidine-2-carboxylic acid
- A3C::
-
Azetidine-3-carboxylic acid
- Hyp::
-
4-Hydroxy-l-proline
- hpt::
-
Hygromycin phosphotransferase II
- NPTII::
-
Neomycin phosphotransferase II
References
Berlin J, Widholm JM (1977) Correlation between phenylalanine ammonia lyase activity and phenolic biosynthesis in p-fluorophenylalanine-sensitive and -resistant tobacco and carrot tissue cultures. Plant Physiol 59:550–553
Cella R, Parisi B, Nielsen E (1982) Characterization of a carrot cell line resistant to azetidine-2-carboxylic acid. Plant Sci Lett 24:125–135
Cleland R, Olson AC (1968) Direct incorporation of hydroxyproline into Avena coleoptile proteins. Biochemistry 7:1745–1751
Donn G, Tischer E, Smith JA, Goodman HM (1984) Herbicide-resistant alfalfa cells: an example of gene amplification in plants. J Mol Appl Genet 2:621–635
Fowden L (1963) Amino-acid analogues and the growth of seedlings. J Exp Bot 14:387–398
Goddijn OJM, Schouten PMD, Schilperoort RA, Hoge JHC (1993) A chimaeric tryptophan decarboxylase gene as a novel selectable marker in plant cells. Plant Mol Biol 22:907–912
Hasagawa H, Mori S (1986) Non-proline accumulating rice mutants resistant to hydroxy-l-proline. Theor Appl Genet 72:226–230
Hauptmann RM, Widholm JM (1982) Cryostorage of cloned amino acid analog-resistant carrot and tobacco suspension cultures. Plant Physiol 70:30–34
Hewitt EJ, Notton BA (1967) Inhibition by l-azetidine-2-carboxylic acid of induction of nitrate reductase in plants and its reversal by l-proline. Phytochemistry 6:1329–1335
Hyun DY, Lee IS, Kim DS, Lee SJ, Seo YW, Lee YI (2003) Selection of azetidine-2-carboxylic acid resistant cell lines by in vitro mutagenesis in rice (Oryza sativa L.). J Plant Biotechnol 5:43–49
Kueh JHS, Bright SWJ (1982) Biochemical and genetical analysis of three proline accumulating barley mutants. Plant Sci Lett 27:233–241
Leete E (1964) The biosynthesis of azetidine-2-carboxylic acid. J Am Chem Soc 86:3162
Maliga P, Klessig DF, Cashmore AR, Gruissem W, Varner JE (1995) Methods in plant molecular biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 66–69
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco cultures. Physiol Plant 15:473–479
Narasimhulu SB, Deng X-b, Sarria R, Gelvin SB (1996) Early transcription of Agrobacterium T-DNA genes in tobacco and maize. Plant Cell 8:873–886
Ni M, Cui D, Einstein J, Narasimhulu S, Vergara CE, Gelvin SB (1995) Strength and tissue specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J 7:661–676
Peterson PJ, Fowden L (1963) Different specificities of proline-activating enzymes from some plant species. Nature 200:148–151
Sasse F, Buchholz M, Berlin J (1983a) Selection of cell lines of Catharanthus roseus with increased tryptophan decarboxylase activity. Z Naturforsch 38c:916–922
Sasse F, Buchholz M, Berlin J (1983b) Site of action of growth inhibitory tryptophan analogues in Catharanthus roseus cell suspension cultures. Z Naturforsch 38c:910–915
Shichiri M, Hoshikawa C, Nakamori S, Takagi H (2001) A novel acetyltransferase found in Saccharomyces cerevisiae Σ1278b that detoxifies a proline analogue, azetidine-2-carboxylic acid. J Biol Chem 276:41998–42002
Singh TN, Paleg LG, Aspinall D (1973) Stress metabolism I. Nitrogen metabolism and growth in the barley plant during water stress. Aust J Biol Sci 26:45–56
Takagi H, Shichiri M, Takemura M, Mohri M, Nakamori S (2000) Saccharomyces cerevisiae strain Σ1278b has novel genes of the N-acetyltransferase gene superfamily required for l-proline analogue resistance. J Bacteriol 182:4249–4256
Verbruggen N, Borstlap AC, Jacobs M, Van Montagu M, Messens E (1996) The raz1 mutant of Arabidopsis thaliana lacks the activity of a high-affinity amino acid transporter. Planta 200:247–253
Widholm JM (1972) Cultured Nicotiana tabacum cells with an altered anthranilate synthetase which is less sensitive to feedback inhibition. Biochim Biophys Acta 261:52–58
Widholm JM (1976) Selection and characterization of cultured carrot and tobacco cells resistant to lysine, methionine, and proline analogs. Can J Bot 54:1523–1529
Zhang X-H, Widholm JM, Portis AR Jr (2001) Photosynthetic properties of two different soybean suspension cultures. J Plant Physiol 158:357–365
Acknowledgements
We thank R. Bressan for the E1494 vector and Xiangxia Luo for help with the tissue culture work. This work was supported in part by the United Soybean Board and the Illinois Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Wang
Rights and permissions
About this article
Cite this article
Zhang, XH., Takagi, H. & Widholm, J.M. Expression of a novel yeast gene that detoxifies the proline analog azetidine-2-carboxylate confers resistance during tobacco seed germination, callus and shoot formation. Plant Cell Rep 22, 615–622 (2004). https://doi.org/10.1007/s00299-003-0741-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0741-3