Abstract
Introduction
Axial spondyloarthritis (AxSpA) is a chronic inflammatory condition primarily affecting the axial skeleton. Peripheral features such as peripheral arthritis (PA) and dactylitis are common in AxSpA disease. This study aimed to investigate the independent impact of these manifestations on patient presentation and disease outcomes within an Irish AxSpA cohort.
Methods
912 Irish AxSpA patients were analyzed in this study. Disease outcomes in patients with and without peripheral arthritis or dactylitis were compared using univariate and multivariate methods. The prevalence of extra-spinal manifestations was further assessed in relation to AxSpA disease duration.
Results
30.2% of patients reported PA, while 6.6% had dactylitis. PA and dactylitis were strongly linked, with 70% of patients presenting with dactylitis also showing features of PA. Psoriasis was more common in both patients with PA (OR 2.2, P < 0.001) and dactylitis (OR 3.38, P < 0.001). Dactylitis, but not PA was strongly linked to uveitis (OR 2.91, P < 0.001) and inflammatory bowel disease (OR 3.15, P < 0.001), while PA was associated with worse patient functioning and reduced quality of life. PA, but not dactylitis was linked with increased AxSpA disease duration.
Discussion
Despite high concurrence of PA and dactylitis in AxSpA patients, each manifestation is independently associated with worse outcomes. While some of these overlapped, several outcomes are specific to either PA or dactylitis. Due to its strong association with uveitis and inflammatory bowel disease, an early presentation of dactylitis may represent a unique subset of patients and serve as a valuable predictive marker for the later onset of these conditions.
Key points
Peripheral arthritis and dactylitis are closely correlated but independent manifestations in AxSpA, each uniquely contributing to increased disease severity.
Dactylitis is uniquely associated with increased risk of uveitis and inflammatory bowel disease, while peripheral arthritis is uniquely linked with depression.
An early presentation of dactylitis in AxSpA patients may be predictive of later uveitis episodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Axial Spondylarthritis (AxSpA) is a chronic rheumatic inflammatory disease characterised by inflammation of the lumbar spine and sacroiliac joints. In addition to spinal involvement, AxSpA may present with peripheral arthritic symptoms, including peripheral arthritis (PA), enthesitis and dactylitis, together with a range of extra-musculoskeletal manifestations (EMMs). The presence of peripheral involvement is a significant clinical finding which may impact on both the severity of AxSpA disease and the decision making process for selecting appropriate therapies [1].
Peripheral arthritis and dactylitis are peripheral features of AxSpA which are closely related to each other. PA is a common manifestation reported in AxSpA patients, and although wide variations in prevalence, ranging 18–58% are reported by individual studies, its overall prevalence in radiographic of AxSpA is approximately 23% [2]. Dactylitis is a form of peripheral enthesitis which is characterized by complete or proximal diffuse digital swelling (sausage-shaped digits). It is observed in a much smaller proportion (around 6%) of AxSpA patients than peripheral arthritis [2, 3]. However, a strong overlap exists between these manifestations, with the prevalence of dactylitis being up to 9 times higher in AxSpA patients with PA than in those without [4]. Despite this strong correlation, there remains proportions of patients who present with either PA or dactylitis in isolation. The potential for patients initially presenting with one manifestation to eventually develop the other remains unclear, as no follow-up studies have specifically examined the concurrence of dactylitis and peripheral arthritis. However, the prevalence of PA in AxSpA has been shown to increase over a 20 year follow-up period [5], suggesting that at least a proportion of patients with earlier presentation with dactylitis will eventually develop concomitant arthritis. Conversely, a 5 year follow-up from the DESIR cohort showed that the prevalence of dactylitis remained steady over this period, implying that dactylitis is less likely to occur later in AxSpA disease [2, 4].
Of these two manifestations, PA remains the best described in the context of AxSpA. Studies of AxSpA and SpA as a whole indicate that PA is more often observed in older patients and has a female predilection [4, 6]. It is also more frequent in HLA-B27 negative disease and in never smokers [4, 6]. PA is robustly linked with worse patient outcomes in AxSpA, including increased risk of psoriasis, raised C-reactive protein and worse outcomes as measured by patient reported outcomes (PROs) for disease activity and functioning [4, 7]. However, there is some evidence to support a reduced risk of uveitis in AxSpA patients with PA [4].
Limited research has been conducted to explore the clinical relevance of dactylitis in terms of AxSpA disease presentation and severity. A notable study identified a connection between dactylitis and other peripheral manifestations, including enthesitis and psoriasis, as well as increased inflammation [8]. However it is not clear whether these symptoms are caused by the frequently co-occurring PA or are independently associated with (and potentially caused by) dactylitis specific disease processes.
In the present study we aimed to evaluate the significance of PA and dactylitis in terms of AxSpA disease severity and the occurrence of additional peripheral features within a large Irish AxSpA cohort. Furthermore, we aimed to disentangle the disease associations of dactylitis and PA, which are often concurrent manifestations, independently contribute to patient outcomes.
Methods
Study design
Data for this study were obtained from the Ankylosing Spondylitis Registry of Ireland (ASRI), a multi-center, cross-sectional cohort study focusing on Axial Spondylarthritis (AxSpA). The registry was established in 2013 with the primary objective to identify predictive factors of disease outcome. ASRI collects comprehensive demographic and phenotypic information from enrolled individuals.
Patients eligible for recruitment are over 18 years old and have received a diagnosis of AxSpA based on the modified New York (mNY) criteria or the Assessment of SpondyloArthritis International Society (ASAS) criteria for AxSpA from a practising rheumatologist. Additionally, participants must have attended secondary or tertiary care within the preceding 3 years. Patients with any impairment which may prevent informed consent are excluded. Patients diagnosed with AxSpA who received diagnosis with other spondyloarthropathy were excluded. Each participating centre has a designated individual responsible for local oversight of the study. Overall responsibility for oversight is managed by the principal investigator (author FOS).
Data collection
Written informed consent was obtained from all participants. Data from the study participants were collected using a structured interview format conducted by trained professionals. Information was obtained through clinical assessments performed during registration or self-reporting if clinical records were unavailable.
The collected data encompassed various aspects, including demographic information such as age, sex, ethnicity, and family history of AxSpA. Clinical characteristics recorded included self-reported age of disease onset, age of diagnosis, presence of extra-axial manifestations, comorbidities, HLA-B27 status, conformity with the modified New York (mNY) criteria, and compliance with the Assessment of SpondyloArthritis International Society (ASAS) criteria for axial spondyloarthritis. Additionally, information regarding current therapies was documented.
Physical examination included modified Schober test, tragus to wall distance, lumbar side flexion, intermalleolar distance, cervical rotation and chest expansion. All of these examinations were performed in accordance with standardised technique [9].
Validated outcome measures used to assess patients included:
Bath Ankylosing Spondylitis Disease Activity Index (BASDAI): measured in the range 0–10. Higher scores indicate more severe disease [10].
Bath Ankylosing Spondylitis Functional Index (BASFI): measured in the range 0–10. Higher scores indicate worse functioning [11].
Bath Ankylosing Spondylitis Metrology Index (BASMI): measured in the range 0–10. Higher scores reflect worse spinal mobility [9].
Ankylosing Spondylitis Quality of Life (ASQoL): measured in the range 0–18. Higher scores indicate worse quality of life [12].
Health Assessment Questionnaire modified for the Spondyloarthropathies (HAQ-S): assessed on a scale of 1–3. Higher scores indicate greater disability [13].
Disease activity was assessed using the BASDAI. Disease severity and functionality were evaluated using the BASMI and BASFI, respectively. Quality of life and disability were measured using the ASQoL and the HAQ-s questionnaires, respectively.
HLA-B27 types were obtained from genotyping data, where available. Among the 912 patients included in the study, 641 had available genotyping data for HLA-B27 status. For the remaining patients, HLA types were derived from the ASRI database records, obtained from medical records, or self-reported, if available.
Not all patients had all measurements recorded; patients with missing data were excluded from respective analyses.
Statistical analysis
The collected data were formatted for analysis using Microsoft Excel. Descriptive statistics are presented as median with interquartile range or frequencies with percentage, as appropriate.
Statistical analyses were performed using Minitab V21.1 software. For comparison of groups, the Mann Whitney U test or Kruskal-Wallis test was employed for continuous data, depending on the number of groups being compared. Proportions were analyzed using Fisher’s exact test.
Regression analyses, including logistic regression, binary logistic regression, and multivariate regression, were conducted using Minitab V21.1. Covariates included in multivariate models included age, gender, HLA-B27 status, Axial Spondylarthritis (AxSpA) disease duration, smoking status (categorized as current, past, or never), and BMI.
Results from all analyses were considered statistically significant where P < 0.05. All graphical representations of the data were created using Minitab V21.1 software.
Results
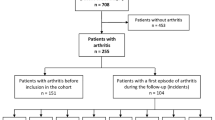
At the time of analysis, data from 912 patients were included in the study. Table 1 provides a summary of the characteristics of these patients.
Within the ASRI cohort, 685 patients (75.1%) were male. The median age of the participants was 44 years, and the median age at the onset of the first Axial Spondylarthritis (AxSpA) symptom was 25 years. The average disease duration among the participants was 16 years.
A majority of the patients, 694 (76.1%), met the modified New York (mNY) criteria for radiographic AxSpA (rAxSpA), while 733 patients (84.9%) were found to be HLA-B27 positive. Frequently reported EMMs included uveitis (34.4%), psoriasis (16.8%), inflammatory bowel disease (10.7%), and depression (10.4%).
In terms of treatment, the most frequently administered therapies were tumor necrosis factor (TNF) inhibitors (58.2%) and non-steroidal anti-inflammatory drugs (NSAIDs), which were used by 51.1% of participants. 30.2% of participants were receiving multiple treatment options, while 13.9% were not receiving any treatment.
Features associated with PA and dactylitis
Out of the 912 patients, 276 participants (30.9%) reported peripheral arthritis (PA), while 60 patients (6.6%) had dactylitis. Table 2 provides a summary of the characteristics associated with these peripheral manifestations.
There was a strong association between peripheral arthritis and dactylitis within the cohort. Among the 60 patients with dactylitis, 42 (70%) also presented with peripheral arthritis, indicating a significant overlap between the two conditions (OR 6.31).
In univariate analysis, both peripheral arthritis (OR 0.68, P = 0.019) and dactylitis (OR 0.38, P = 0.003) were inversely associated with male sex. Peripheral arthritis was associated with older patient age (48 vs. 44 years, P < 0.001) and longer AxSpA duration (19 vs. 15 years, P < 0.001). However, no relationship was observed between dactylitis and either age or AxSpA disease duration.
Patients with PA or dactylitis had worse BASDAI scores compared to those without these peripheral features (PA: 4.4 vs. 3.6, P < 0.001; dactylitis: 5.6 vs. 3.5, P < 0.001). These associations remained robust in multivariate regression analysis. Peripheral arthritis, but not dactylitis, showed residual association with worse scores in BASFI (3.7 vs. 2.9, P = 0.005), AsQoL (7 vs. 4, P = 0.002), and HAQ-s (0.5 vs. 0.4, P < 0.001), after accounting for covariates in multivariate analysis.
Among the EMMs, psoriasis was more frequent in patients with both PA (OR 2.2, P < 0.001) and dactylitis (OR 3.38, P < 0.001). However, while dactylitis showed a strong association with uveitis (OR 2.91, P < 0.001) and inflammatory bowel disease (IBD) (OR 3.15, P < 0.001), PA did not show a significant association with either of these manifestations after accounting for covariates. PA was associated with accompanying depression (OR 1.79, P = 0.012), which remained significant after accounting for covariates.
Therapeutic options chosen by clinicians differed based on the presence of PA and dactylitis in patients. Methotrexate was more commonly administered to patients with PA (OR 4.93, P < 0.001) and/or dactylitis (OR 4.32, P = 0.001). Peripheral arthritis was also associated with the administration of sulfasalazine (OR 2.31, P = 0.03) and multiple drug treatments (OR 1.95, P < 0.001). While sulfasalazine and multiple therapies were also, to a lesser degree, more commonly administered to patients with dactylitis (OR 1.47 and 1.29, respectively), these findings were not statistically significant.
Peripheral arthritis but not dactylitis is associated with AxSpA disease duration
Due to the lack of specific dates when peripheral manifestations developed in patients, we employed logistic regression to model the presence or absence of these manifestations against AxSpA disease duration. The results of this analysis are presented in Fig. 1 (a-e).
All of uveitis, peripheral arthritis, and inflammatory bowel disease (IBD) showed a positive association with AxSpA disease duration. Particularly, uveitis exhibited a notable trend, with its prevalence reaching 50% after 40–50 years post-AxSpA onset (Fig. 1f). In contrast, both psoriasis and dactylitis demonstrated no significant association with AxSpA disease duration, suggesting that these features tend to either precede or develop shortly after AxSpA onset.
Discussion
In this study we have demonstrated that PA and dactylitis are very closely related manifestations with a strong tendency to coincide. As much as 70% of patients who present with dactylitis may have concomitant PA. Despite this close relationship, we have shown that each of these discrete manifestations is independently associated with worse disease outcomes in AxSpA patients.
Consistent with previous findings from the ASAS COSMOSPA and a 5-year follow-up of the DESIR cohort, our study found a female predominance in peripheral arthritis [4, 6]. This trend was also observed in the presentation of dactylitis within our cohort, contrasting with the ESPeranza cohort’s results [8]. This discrepancy could be attributed to the ESPeranza cohort’s inclusion of all SpA subtypes.
Moreover, both manifestations were correlated with increased disease activity. Specifically, PA was associated with worse functioning, as measured by BASFI and HAQ-s, and worse quality of life in patients. Depression is an additional risk identified in patients presenting with PA, which is, perhaps, not surprising given its association with worse functioning in patients. It appears likely that dactylitis is also linked to worse functioning in patients, although this was not statistically significant after the presence of PA was accounted for. Both uveitis and IBD were present with approximately three times higher odds in patients with dactylitis. This correlation withstood multivariate regression analysis which accounted for concurrent peripheral arthritis in patients. These findings differ from those of the ESPeranza cohort, which reported no significant association [8]. This discrepancy could be due to their focus on early SpA disease, as evidenced by the low incidence (4.6%) of uveitis—a characteristic typically associated with longer established SpA—reported in their study. A positive correlation between dactylitis and uveitis was also recorded in a recently published report from the Chinese Spondyloarthritis Registry [14]. No residual association of either uveitis or IBD with PA was observed in our models after concurrent dactylitis was accounted for, this being consistent with previous studies [4, 6]. This indicates a unique association between dactylitis and both uveitis and IBD which can not be accounted for by concomitant PA.
Contrary to previously published findings which showed a marked increase in HLA-B27 negative disease amongst patients presenting with peripheral arthritis [4] and dactylitis [15], we did not find a statistically significant difference in HLA-B27 positivity in patients with either PA or dactylitis, although we noted that HLA-B27 carriage rates were lower amongst patents with these manifestations. Dactylitis, but not peripheral arthritis was associated with non-radiographic AxSpA. Our findings are consistent with previously published results by Londono [16], the German Spondyloarthitis inception cohort [17] and SPACE cohort [18]. Notably, no association between dactylitis and non-radiographic AxSpA was reported in either the ASAS-COSMOSPA [19] or DESIR [20] cohorts.
Both PA and dactylitis are important clinical findings which have impacts on the choices of therapy chosen by physicians, with methotrexate being a key drug of choice in the treatment of both of these manifestations. This increased the likelihood of such patients receiving multiple therapeutic treatments. Previously published findings from the DESIR cohort reported increased use of TNF inhibitors in patients with peripheral arthritis [4]. In our study, we observed a slightly higher proportion of patients with peripheral arthritis receiving anti-TNF therapy, although this was not statistically significant. Additionally, we found no evidence of increased anti-TNF therapy use in patients with concomitant dactylitis.
Although we lacked the dates of onset of peripheral features within our cohort, we demonstrated that the prevalence of uveitis, PA and IBD all increase with AxSpA disease duration using regression models. These findings are in line with the previous reports [5, 21, 22].
Contrary to the findings of Zeboulon et al. (2008), we did not observe a plateau of uveitis prevalence at 20 years post-AxSpA onset within the ASRI cohort. Instead, the prevalence of uveitis continued to rise at a relatively steady rate with disease duration with the primary limitation being survivorship. In contrast, dactylitis showed no relationship with AxSpA disease duration, instead maintaining a stable prevalence of approximately 6%. This suggests that the onset of dactylitis is more likely to occur either early following, or possibly preceding AxSpA onset. This perspective aligns with previous findings from the DESIR cohort, which demonstrated no increase in the prevalence of dactylitis over a five-year period [2, 4].
Given its strong association with uveitis, PA and IBD, a presentation of dactylitis may offer predictive value in the subsequent onset of these EMMs in AxSpA patients. Interestingly, dactylitis was, at the same time, inversely correlated with radiographic AxSpA and male sex, both of which are classically positive predictors of uveitis [2]. This suggests that AxSpA patients presenting with dactylitis may represent a unique sub-cohort with increased peripheral symptoms and reduced axial involvement.
Study limitations
This study was limited by cross sectional design. Models describing the likelihood of various manifestations against AxSpA duration lacked dates of onset of these additional manifestations. This limits our ability to assess cause and effect within the cohort.
Due to the limited number of dactylitis patients within our cohort and strong correlation with PA, we were unable to accurately model the relationship between dactylitis and either uveitis or IBD with respect to AxSpA disease duration. Therefore, it would be interesting to observe how the course of AxSpA disease progression differs between patients with and without dactylitis in the context of a longitudinal study.
References
Redeker I, Siegmund B, Ghoreschi K, Pleyer U, Callhoff J et al (2020) The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study. Therapeutic Adv Musculoskelet Disease 12. https://doi.org/10.1177/1759720X20972610
de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL (2016) Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Therapy 18(1):196. https://doi.org/10.1186/s13075-016-1093-z
Rothschild BM, Pingitore C, Eaton M (1998) Dactylitis: implications for clinical practice. Semin Arthritis Rheum 28(1):41–47. https://doi.org/10.1016/S0049-0172(98)80027-9
López-Medina C, Dougados M, Ruyssen-Witrand A, Moltó A (2019) Evaluation of concomitant peripheral arthritis in patients with recent onset axial spondyloarthritis: 5-year results from the DESIR cohort. Arthritis Res Therapy 21(1):139. https://doi.org/10.1186/s13075-019-1927-6
Ginsburg WW, Cohen MD (1983) Peripheral arthritis in ankylosing spondylitis. A review of 209 patients followed up for more than 20 years. Mayo Clin Proc 58(9):593–596
López-Medina C, Moltó A, Dougados M (2020) Peripheral manifestations in Spondyloarthritis and their effect: an ancillary analysis of the ASAS-COMOSPA study. J Rhuematol 47(2):211. https://doi.org/10.3899/jrheum.181331
Capelusnik D, Ramiro S, Schneeberger EE, Citera G (2021) Peripheral arthritis and higher disease activity lead to more functional impairment in axial spondyloarthritis: longitudinal analysis from ESPAXIA. Semin Arthritis Rheum 51(3):553–558. https://doi.org/10.1016/j.semarthrit.2021.04.007
Tévar-Sánchez MI, Navarro-Compán V, Aznar JJ, Linares LF, Castro MC, De Miguel E (2018) Prevalence and characteristics associated with dactylitis in patients with early spondyloarthritis: results from the ESPeranza cohort. Clin Exp Rheumatol 36(5):879–883
Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A (1994) Defining spinal mobility in ankylosing spondylitis (AS). The bath AS Metrology Index. J Rhuematol 21(9):1694–1698
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (1994) A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing Spondylitis Disease Activity Index. J Rhuematol 21(12):2286–2291
Calin A, Garrett S, Whitelock H, Kennedy LG, O’hea J, Mallorie P, Jenkinson T (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing Spondylitis Functional Index. J Rheumatol 21(12):2281–2285
Doward LC, Spoorenberg A, Cook SA, Whalley D, Helliwell PS et al (2003) Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 62(1):20. https://doi.org/10.1136/ard.62.1.20
Daltroy L, Larson M, Roberts N, Liang M (1990) A modification of the Health Assessment Questionnaire for the spondyloarthropathies. J Rhuematol 17(7):946–950
Liu S, Zhang S, Duan X, Wang Y, Xu J et al (2024) Comparison of clinical characteristics between patients with axial spondyloarthritis with and without acute anterior uveitis: a multicentre study of the Chinese Spondyloarthritis Registry. Clin Exp Rheumatol 42(7):1467–1473. https://doi.org/10.55563/clinexprheumatol/icoqy3
Arévalo M, Gratacós Masmitjà J, Moreno M, Calvet J, Orellana C et al (2018) Influence of HLA-B27 on the Ankylosing Spondylitis phenotype: results from the REGISPONSER database. Arthritis Res Therapy 20(1):1–6. https://doi.org/10.1186/s13075-018-1724-7
Londono J, Pacheco-Tena C, Santos AM, Cardiel MH, Rodríguez-Salas G et al (2024) Differences between radiographic and non-radiographic axial spondyloarthritis patients in a Mexican cohort. Sci Rep 14(1):10342. https://doi.org/10.1038/s41598-024-61001-w
Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker-Hermann E, Zeidler H, Braun J, Sieper J (2009) The early disease stage in axial spondylarthritis: results from the German spondyloarthritis inception cohort. Arthr Rhuem 60(3):717–727. https://doi.org/10.1002/art.24483
van den Berg R, de Hooge M, van Gaalen F, Reijnierse M, Huizinga T, van der Heijde D (2013) Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology 52(8):1492–1499. https://doi.org/10.1093/rheumatology/ket164
Kishimoto M, Ono K, Fukui S, Kawaai S, Deshpande GA et al (2021) Clinical characteristics of non-radiographic versus radiographic axial spondyloarthritis in Asia and non-radiographic axial spondyloarthritis in other regions: results of the cross-sectional ASAS-COMOSPA study. RMD Open 7(3):e001752. https://doi.org/10.1136/rmdopen-2021-001752
López-Medina C, Molto A, Claudepierre P, Dougados M (2020) Clinical manifestations, disease activity and disease burden of radiographic versus non-radiographic axial spondyloarthritis over 5 years of follow-up in the DESIR cohort. Ann Rheum Dis 79(2):209. https://doi.org/10.1136/annrheumdis-2019-216218
Gómez-García I, Ladehesa-Pineda ML, Puche-Larrubia MÁ, Ortega-Castro R, Font-Ugalde P et al (2021) Uveitis as the first symptom in spondyloarthritis and its association with the evolution of the disease. Results from the REGISPONSER registry. Joint Bone Spine 88(3):105136. https://doi.org/10.1016/j.jbspin.2021.105136
Rudwaleit M, Baeten D (2006) Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol 20(3):451–471. https://doi.org/10.1016/j.berh.2006.03.010
Funding
This research was supported by contributions from Abbvie and Pfizer corporations. Funding bodies were not involved in study design, data collection or interpretation of results.
Open Access funding provided by the IReL Consortium
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no connections or financial backings which have influenced or biased the content of this work.
Compliance with ethical standards
The study adhered to good clinical practice guidelines at all participating centers. Ethical approval for ASRI (project ID 3610) was obtained from the St. James’ Hospital and Trinity College Dublin (SJH/TCD) Joint Research Ethics Committee, reference REC 2019-07, first approved 08/06/2012 with latest update 19/03/2024.
Congress abstract publication
This work has not been showcased in any congress at the time of publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kenyon, M., Gallagher, P., Dinneen, B. et al. Distinct clinical outcomes linked to peripheral arthritis and dactylitis in axial spondyloarthritis: findings from a retrospective Irish cohort. Rheumatol Int (2024). https://doi.org/10.1007/s00296-024-05707-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00296-024-05707-0