Abstract
The use of artificial intelligence (AI) in high-resolution computed tomography (HRCT) for diagnosing systemic sclerosis-associated interstitial lung disease (SSc-ILD) is relatively limited. This study aimed to analyse lung HRCT images of patients with systemic sclerosis with interstitial lung disease (SSc-ILD) using artificial intelligence (AI), conduct correlation analysis with clinical manifestations and prognosis, and explore the features and prognosis of SSc-ILD. Overall, 72 lung HRCT images and clinical data of 58 patients with SSC-ILD were collected. ILD lesion type, location, and volume on HRCT images were identified and evaluated using AI. The imaging characteristics of diffuse SSC (dSSc)-ILD and limited SSc-ILD (lSSc-ILD) were statistically analysed. Furthermore, the correlations between lesion type, clinical indicators, and prognosis were investigated. dSSc and lSSc were more prevalent in patients with a disease duration of < 1 and ≥ 5 years, respectively. SSc-ILD mainly comprises non-specific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), and unclassifiable idiopathic interstitial pneumonia. HRCT reveals various lesion types in the early stages of the disease, with an increase in the number of lesion types as the disease progresses. Lesions appearing as grid, ground-glass, and nodular shadows were dispersed throughout both lungs, while those appearing as consolidation shadows and honeycomb were distributed across the lungs. Ground-glass opacity lesion type was absent on HRCT images of patients with SSc-ILD and pulmonary hypertension. This study showed that AI can efficiently analyse imaging characteristics of SSc-ILD, demonstrating its potential to learn from complex images with high generalisation ability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterised by local or extensive sclerosis of the skin, progressive fibrosis of the internal organs, and vascular disease. SSc commonly affects the respiratory system because the alveolar septa are rich in connective tissues and vascular components. Statistically, approximately 80% of patients with SSc develop interstitial lung disease (ILD) [1]. Owing to the insidious onset of ILD, early clinical symptoms are atypical and mainly manifest as cough, expectoration, and activity-induced dyspnoea, making it often difficult to diagnose. ILD is characterised by irreversible pulmonary fibrosis and severe damage to lung function. The mortality rate of patients with SSc within 10 years of ILD onset is reportedly approximately 40%, and ILD is the main cause of death in patients with SSc [2]. Therefore, the early diagnosis and treatment of SSc-associated ILD (SSc-ILD) is crucial.
Currently, ILD is diagnosed mainly by combining clinical, imaging, and pathological examination. However, since pathological examination is invasive and may lead to physical and psychological burdens and potential complications, its clinical use is limited. High-resolution computed tomography (HRCT) is considered the mainstay for diagnosing SSc-ILD as it is used to interpret imaging data by assessing the range and distribution of various ILD textures within scans [3]. According to the updated classification method for idiopathic interstitial pneumonia (IIP) published by the American Thoracic Society/European Respiratory Society (ATS/ERS) in 2013 [4], SSc-ILD can be categorised into primary, rare, and unclassifiable IIP. Primary IIP includes usual interstitial pneumonia (UIP), non-specific interstitial pneumonia (NSIP), respiratory bronchiolitis-associated interstitial lung disease (RB-ILD), desquamative interstitial pneumonia (DIP), cryptogenic organising pneumonia (COP), and acute interstitial pneumonia (AIP). Rare IIP includes idiopathic pleuroparenchymal fibroelastosis (PPEE) and lymphocytic interstitial pneumonia (LIP). Unclassifiable IIP mainly results from incomplete clinical data or a lack of characteristic presentations, overlapping histopathological types, such as NSIP with UIP, and certain IIP types that may not have been recognised yet. HRCT of ILD showed that it manifests in the following five typical patterns: ground-glass opacities, consolidations, reticular opacities, honeycombing, and nodular shadows [5, 6]. Classifying ILD using HRCT facilitates the early detection of lesions, dynamic observation of disease progression, and assessment of therapeutic efficacy.
Presently, few imaging studies on have focused on SSc-ILD, both locally and internationally [7, 8]. Although ILD is different from SSc histologically, their clinical manifestations are mostly similar. Even for experienced physicians, differential diagnosis of ILD is challenging. The inherent nature of ILD, characterised by its complexity, combined with the absence of strict clinical guidelines and the demand for radiologists to analyse extensive radiographic images within tight time frames meticulously, contributes to the disease's low diagnostic accuracy and the substantial inter- and intra-observer variability, which can reach approximately 50% [9], leading to frequent clinical misdiagnoses and underdiagnoses of ILD.
Artificial intelligence (AI) is a scientific technology that simulates and enhances human intelligence. As a leading contemporary technology, AI has been widely used to assist diagnostic radiologists in imaging diagnosis to improve diagnostic accuracy [10,11,12,13,14]. Deep learning, a subset of AI, has attracted considerable attention because of its capacity to extract features and predict outcomes from raw data [15,16,17]. Recent research on AI in pulmonary computed tomography (CT) has predominantly focused on lung segmentation, quantitative analysis of pulmonary diseases, and differential diagnosis [18, 19]. However, studies utilising AI to analyse SSc-ILD remain relatively limited, with no local studies. Therefore, this study, conducted in collaboration with the Mobile Video Research Center at Peking University Shenzhen Graduate School, aimed to use AI algorithms to analyse SSc-ILD imaging features, examine the radiological characteristics of SSc-ILD and integrate clinical data to explore the correlation between SSc-ILD lesion type, clinical indicators, and prognosis and provide valuable insights into lesion identification and prognostic evaluation of SSc-ILD.
Methods
A retrospective analysis was performed on 72 datasets of lung HRCT images of 58 patients with SSc-ILD who received medical care at the Rheumatology and Immunology Department of the First Affiliated Hospital of Jishou University and Xiangya Hospital between November 2015 and December 2022.
Patients
All patients who met the classification criteria for SSc as established by the American College of Rheumatology in 1980 and who underwent at least one lung HRCT examination during hospitalisation or outpatient visits were included. Patients with concurrent non-ILDs, such as pulmonary oedema, infection, pulmonary haemorrhage, pulmonary embolism, or tumours; history of clinically evident primary lung diseases, such as chronic obstructive pulmonary disease, bronchiectasis, pulmonary tuberculosis, or thoracic surgery; severe systemic diseases and/or organ dysfunction; history of malignant tumours; or poor image quality and considerable artefacts that could affect the results were excluded.
Study factors
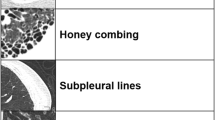
Currently, there is no consensus regarding the diagnostic criteria for SSc-ILD. This study primarily follows the definitions of lung HRCT terminologies established by the Fleischner Society Naming Committee. Pulmonary interstitial lesions are classified as ground-glass opacity, characterised by a hazy appearance, mild increase in lung density on imaging, and visible broncho-vascular bundles; consolidation, characterised by the disappearance of air-containing spaces, absence of vascular markings, and increased lung density, often caused by atelectasis and alveolar fluid accumulation, and is commonly distributed in lung lobes or segments; honeycomb pattern, characterised by alveolar septal destruction and walled cystic spaces with low-density foci on imaging, a characteristic feature of pulmonary interstitial fibrosis; reticular pattern, characterised by changes, such as the thickening of interlobular septa and subpleural lines; and nodular pattern, characterised by clear margins and cystic changes or bronchovascular markings on CT with nodular vascularity appearing indistinct, sometimes showing a ‘halo sign’.
Procedures
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Xiangya Hospital, Central South University (approval number: 201212074; date: 31 December 2012). Informed consent was obtained from all patients participating in the study.
The chest CT scan encompassed the area from the thoracic inlet to the lung bases and was conducted using a Siemens Sensation 16, Somatom Definition AS 64, or Somatom Force dual-source spiral CT scanner. The scan parameters were set at a tube voltage of 100–120 kV and automatically adjusted tube current with a pitch of 1.2–1.5. Reconstruction parameters include a convolution kernel of I70 f, with a lung window level ranging from − 450 to − 600 HU and a window width of 1200–1500 HU. The slice interval and thickness were set to 1 mm. All images used in this study were axial images with lung window settings.
The lung fields were transversely divided by drawing horizontal lines anteriorly at the inferior margins of the second and fourth ribs. The area above the upper line was defined as the upper lung field, extending roughly from the lung apices to the level of the aortic arch. The area between the two lines constituted the middle lung field, extending approximately from the aorta to the inferior vena cava. Below the lower line lies the lower lung field spanning from the level of the inferior vena cava to the lung base. In this classification, the involvement of both the upper and middle lung fields is defined as upper-middle lung field involvement, involvement of both the middle and lower lung fields as middle-lower lung field involvement, and involvement of the upper to lower lung fields as total lung involvement.
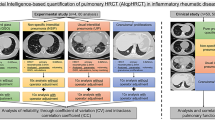
Two experienced thoracic radiologists, each with over a decade of expertise, performed a comprehensive morphological evaluation of lung HRCT images of 58 patients with SSc. These patients fulfilled the criteria for various classifications of IIP, as outlined in the 2013 ATS and ERS updates and 2015 statements on immune-related pulmonary interstitial lesions. The assessment covered nine IIP types, including UIP, NSIP, COP, LIP, AIP, RB-ILD, DIP, PPEE, and unclassified IIP. HRCT images were annotated using ITK SNAP software to identify the five distinct classes of pulmonary manifestations: ground-glass opacities, consolidation, reticular patterns, honeycombing, and nodular shadows. Each manifestation was distinctly marked with specific colours—red, purple, yellow, green, and blue—to facilitate easy recognition of the extent and distribution of the lesions. These annotated images, encompassing a total of 72 cases, were further analysed by the Mobile Video Research Center at Peking University Shenzhen Graduate School. Advanced AI techniques involving a deep-learning U-NET network for lung detection and a watershed algorithm combined with a dilation method for lesion identification were used to enhance the precision of the analysis.
Our approach began with the preprocessing of annotated images, encompassing normalisation, denoising, histogram equalisation, and bilateral filtering to refine image quality for subsequent analysis. Then, we continued with data augmentation, followed by the construction and rigorous training of our models.
The model architecture was bifurcated into two specialised modules:
Lesion Area Detection Module: we selected the DeepLabV3 neural network model, which is initially pre-trained on the PASCAL VOC dataset to obtain foundational weights. This model then underwent mixed training, leveraging both our proprietary training dataset and an open-source dataset, to bolster its semantic feature learning capabilities. Finally, fine-tuning was applied to accurately detect the lesion areas.
Lesion Area Feature Extraction and Classification Module: the Inception-ResNet model was engaged to extract and depict the lesion area's characteristics, including its size, boundary, and texture. A multi-task classification network was trained to refine the feature descriptions derived from the lesion area, using these features in conjunction with the lesion images as input to facilitate precise classification of the affected regions.
To validate our model's efficacy, the hospital's annotated dataset was meticulously partitioned into training, validation, and test sets at a ratio of 75:15:10. The joint model achieved an average accuracy of 88.9% in classifying lesion areas, showcasing its adeptness in the categorization and quantification of HRCT images from our patient sample (Figs. 1, 2; Table 1) [20,21,22,23].
The information collected included general patient information, such as sex, age, and duration of illness were collected; patients’ clinical features, including arthritis, arthralgia, chest tightness, dyspnoea, fingertip ulcers, Raynaud's phenomenon, the extent of skin involvement, and dysphagia; additional information, primarily history of medication use, including steroids and immunosuppressants, and treatment details; results of laboratory tests, including erythrocyte sedimentation rate (ESR), complement components (C3/C4), C-reactive protein (CRP), immunoglobulins (IgA/IgG/IgM), and various autoantibodies, such as antinuclear antibodies (ANA), anti-double stranded DNA antibodies (anti-dsDNA), anti-topoisomerase I/Scl-70 antibodies (anti-Scl-70), anti-U1 ribonucleoprotein antibodies (anti-RNP), anti-SSA antibodies, and anti-SSB antibodies. Additionally, pulmonary function tests, lung HRCT descriptions, and diagnostic outcomes were included in the routine laboratory examinations.
Statistical analysis
Clinical data and HRCT classification data are represented using different methods based on their distribution characteristics. Specifically, continuous variables following a normal distribution are presented as mean ± standard deviation (x ± s), whereas continuous variables without a normal distribution are presented as the median (interquartile range). Non-continuous variables are expressed as composition ratio (%). The volume occupied by each imaging was determined through imaging annotation and modelling. The volume sizes of each group, conforming to a normal distribution, are expressed as mean ± standard deviation (x ± s). Prior to conducting parametric tests, normality of all continuous variables was assessed using the Kolmogorov–Smirnov test or Shapiro–Wilk test, and homogeneity of variances was assessed using the Levene test. Unpaired t-tests were used to compare data between two groups when continuous variables met normal distribution and homogeneity of variance, while analysis of variance was used to compare between multiple groups. The Mann–Whitney U test was employed for nonparametric comparisons of continuous variables that did not meet these conditions. Categorical variables were compared between groups using the chi-square test. Analysis of the correlation between lesion type and time involved the quadratic term distribution test and chi-square test. The relationship between different lesions and clinical indicators was evaluated using the chi-square test and analysis of variance. We used SPSS 26.0 software (IBM Corp.) to perform data analysis.
The performance of the AI model was assessed through indicators such as the area under the receiver operating characteristic curve, accuracy, sensitivity, and specificity. Statistical results were interpreted on the basis of P values and 95% confidence intervals.
In this study, data from three out of 58 participants were incomplete owing to missing anti-SCL-70 antibody levels. However, all other data, including CT findings and clinical information, were available for these patients. To maintain statistical integrity, especially in correlating autoantibodies with clinical parameters, these three cases were excluded from the correlation analysis presented in Table 3, which now includes data from 55 participants with complete antibody profiles. Tables 4, 5, 7, and 8 reflect analyses based on this adjusted dataset.
Results
General characteristics of the patients
Among the 58 patients with SSc-ILD, 36 (62.1%) had diffuse SSc-ILD (dSSc-ILD), 22 (37.9%) had limited SSc-ILD (lSSc-ILD), 32 (58.2%) were positive for anti-Scl-70 antibodies, and 23 (41.8%) were negative for anti-Scl-70 antibodies, with antibody data missing in three cases. The patients were divided into two groups based on the extent of skin involvement and anti-Scl-70 antibody results for the comparison of general characteristics. The patients in the dSSc-ILD and lSSc-ILD groups were predominantly female. ILD was more common in patients with dSSc within the first year of disease onset and in patients with ISSc with illness duration of ≥ 5 years, the difference between both groups being statistically significant (P = 0.009). Compared with that in the ISSc-ILD group, anti-RNP antibodies were more prevalent, and the incidence of oesophageal involvement was higher in the dSSc-ILD group. No significant difference was observed in disease duration between the anti-SCL-70 antibody positive and negative groups. Patients with ILD who tested negative for anti-Scl-70 antibody had increased levels of anti-centromere and anti-RNP antibodies. Oesophageal involvement was more frequent in the anti-Scl-70 positive group. Pulmonary hypertension was more commonly observed in patients with ILD who tested negative for anti-Scl-70 antibodies (Tables 2, 3).
Classification of lung HRCT in patients with SSc-ILD
In patients with SSc-ILD, NSIP, UIP, and unclassifiable ILD are the most common manifestations observed on lung HRCT images. No cases of LIP, COP, AIP, RB-ILD, or DIP were identified in this study. Over 50% of SSc-ILD cases are categorised as unclassifiable ILD. No significant differences were observed in the distribution of SSc-ILD subtypes, lSSc, and dSSc, as well as between the anti-Scl70(+) and anti-Scl70(−) groups (Table 4).
Distribution of lung HRCT manifestation types in patients with SSc-ILD
We observed 36, 20, 18, 14, and four cases of nodular shadow, consolidation shadow, grid-like shadow, honeycomb structure, and ground-glass shadow, respectively. No significant difference was observed in the composition ratio between groups with different disease types, antibodies, and disease durations. As the disease progresses, the number of lesions typically increases, with individuals who have had the disease for > 5 years often having more than two imaging lesions, and this difference was statistically significant (Table 5; Fig. 3).
Distribution characteristics of various lung HRCT manifestation types in patients with SSc-ILD
The various lesions exhibited distinct distribution characteristics. Grid, ground-glass, and nodular shadow patterns were dispersed throughout both lungs. Grid and nodular shadow patterns shared a similar distribution pattern and were scattered across the middle and outer sides of the lungs, whereas ground glass shadow patterns were less prevalent. Consolidation and honeycomb patterns were distributed throughout the entire lung, with consolidation patterns more frequently observed in the central regions. Additionally, honeycomb structures were notably more voluminous in dSSc-ILD, whereas the reticular pattern was predominant in lSSc-ILD. Honeycomb structures were more frequently observed in both the anti-Scl-70 (−) and anti-Scl-70 (+) groups. Moreover, multiple lesion types coexisted, with consolidated shadows, grid shadows, and honeycomb structures being the most common types (Fig. 4; Tables 6, 7).
Relationship between HRCT findings in SSc-ILD, clinical symptoms, and laboratory parameters
Patients with SSc-ILD with Raynaud's phenomenon were more likely to experience honeycomb patterns, whereas those with digital ulcers were inclined towards the ground-glass opacity patterns. Additionally, patients with SSc-ILD with concomitant pulmonary arterial hypertension and arthritis tended to exhibit honeycomb patterns. Notably, HRCT showed the absence of ground-glass opacity patterns in patients with SSc-ILD with pulmonary arterial hypertension (P < 0.001). Elevated levels of inflammatory markers, such as ESR, were observed in consolidation patterns, whereas immunoglobulin A levels increased in ground-glass opacity patterns. Elevated levels of autoantibodies, including anti-RNP and anti-SSA, were present in patients with honeycomb patterns, while increased levels of anti-Scl-70 antibodies were present in those with ground-glass opacity patterns (Table 8).
Changes in SSc-ILD pulmonary HRCT
Among the patients, eight had more than one radiological record, including five with dSSc-ILD and three with lSSc-ILD. Following treatment, lung lesion volume decreased in three patients with dSSc and increased in two patients with dSSc, whereas all three patients with lSSc experienced an increase in lesion volume. The type of lung lesion can change from one to several forms, for example, from consolidation to honeycomb pattern or from consolidation, reticular shadowing, nodules, and ground-glass opacity patterns to other types. It can also change from two types to another distinct type, such as nodule, reticular shadow, and honeycomb patterns.
Discussion
This study examined 58 patients with SSc-ILD based on the specific inclusion and exclusion criteria. Pulmonary complications were more prevalent in patients with dSSc. dSSc tends to progress rapidly in the early stages, with a higher likelihood of skin and lung involvement and progressive lung fibrosis in other internal organs. Conversely, lSSc exhibits slower progression, with clinical symptoms primarily manifesting as mild fibrosis of the skin and internal organs. This study revealed that dSSc was more commonly associated with SSc-ILD in patients with a disease duration of less than 1 year. Additionally, the lSSc-ILD group had a higher proportion of individuals with a disease duration of ≥ 5 years compared with the dSSc-ILD group, and this difference was statistically significant. This observation suggests that patients with lSSc may experience less severe disease involvement and milder clinical symptoms and potentially survive longer than patients with dSSc.
We analysed 72 HRCT images of the lungs from 58 patients with SSc-ILD. Our findings indicate that NSIP, UIP, and unclassifiable IIP are the predominant manifestations of SSc-ILD on lung HRCTs, which is consistent with previous research [2, 5, 20]. The challenge in classifying most imaging data lies in the lack of characteristic manifestations in clinical data or overlapping lesion types. Notably, unclassifiable ILD is more prevalent within the first year of the disease, possibly because of limited imaging studies on SSc-ILD and insufficient experience among doctors and radiologists in recognising HRCT manifestations of ILD. This, which underscores the non-specific nature of early HRCT findings of interstitial pulmonary lesions, contributes to missed diagnoses. The pathogenesis of the various types of lesions remains unclear. Some studies suggest a potential association with salivary glycan antigen-6 and pulmonary surfactant-related protein-D [21, 24]. Using AI, this study demonstrated that HRCT can reveal various types of lesions in the early stages of the disease that increase in number as the condition progresses, often transitioning from a single type to multiple types. Lesions of more than two types are frequently observed in cases over 5 years, indicating the irreversible and progressive nature of pulmonary fibrosis [25]. Grid patterns, ground-glass opacities, and nodular shadows were dispersed within the lungs, with grid patterns and nodular shadows showing similar distributions primarily around the middle and outer sides of the lungs. Ground-glass opacity patterns exhibited the lowest distribution in the lungs. Consolidation and honeycomb patterns were present throughout the lungs, with consolidations more commonly found in the middle parts, suggesting that the different types of lesions had distinct distribution characteristics. The distribution of these lesions can be used to predict the progression of pulmonary fibrosis and for prognostic assessments [26,27,28]. Schniering et al. suggested that ground-glass opacities, reticular patterns, and honeycombing are relatively common, which differs from the results of this study. This difference may be related to three-dimensional recognition by AI and the relatively small sample size [7, 29,30,31].
The most common clinical symptom in patients with SSc-ILD across the five different types of pulmonary HRCT phenotypes is Raynaud's phenomenon, followed by respiratory distress, such as chest tightness, shortness of breath, and coughing. Raynaud's phenomenon, respiratory symptoms, pulmonary hypertension, and arthritis are more frequently associated with honeycomb patterns; however, pulmonary hypertension is not associated with lesions with ground-glass opacities. Pulmonary hypertension is characterised by increased pulmonary artery pressure and pulmonary vascular resistance and is a serious complication of severe pulmonary fibrosis that often occurs in the late stages of pulmonary fibrosis. Young reported that the incidence of pulmonary hypertension in the late stage of pulmonary fibrosis is 85%, severely affecting lung function, with a high mortality rate and poor prognosis, and currently, there are no effective treatments available [32]. Ground-glass opacities represent early inflammatory changes in interstitial lung disease and did not occur concurrently with pulmonary hypertension in the present study, consistent with the clinical pathological staging. This indicates that pulmonary hypertension and honeycombing are pathological changes in the late stage of the disease and are closely related to the severity of clinical symptoms, consistent with previous research findings [33, 34].
Inflammatory responses play a crucial role in the pathogenesis of ILD, leading to an influx of inflammatory cells into the lungs and damaging the alveolar epithelium. This damage activates the lung interstitial fibroblasts, resulting in the development of a fibrotic environment in the affected lung tissue. Studies have indicated that the extent of alveolar epithelial damage is a key factor in the progression of ILD [35,36,37]. Acute-phase reactants, such as ESR and CRP, are commonly used as markers of inflammatory activity in SSc. In this study, the ESR significantly increased in all types of interstitial pulmonary lesions observed on HRCT, with the most noticeable increase observed in consolidation shadows. After eliminating confounding factors, such as pulmonary infection, it was found that consolidation shadows were mainly attributed to alveolar leakage caused by outflow and inflammatory cell infiltration. This suggests a potential relationship between ESR and the activity of interstitial lung lesions, aligning with findings from recent studies on biological markers of systemic sclerosis-associated interstitial lung disease (SSC-ILD) [38, 39]. However, no significant increase in CRP levels was observed, indicating a potential imbalance between pulmonary interstitial fibrosis and the overall disease activity.
Patients with SSc display a diverse range of specific autoantibodies in their blood that are crucial for the diagnosis, treatment response, and prognosis of SSc. Among these, anti-Scl-70 antibodies are particularly important and are frequently detected in patients with SSc and ILD, which is a significant risk factor for ILD development [40, 41]. The prevalence of anti-Scl-70 antibodies in patients with SSc-ILD varied from 20 to 46% [42]. In our study, the prevalence of anti-Scl-70 antibodies in patients with SSc-ILD was 58.5%, with approximately half of the patients presenting with fibrotic lesions. Therefore, the presence of anti-Scl-70 antibodies in ILD should alert clinicians to the possibility of interstitial fibrosis and prompt early initiation of anti-fibrotic therapy to prevent progressive lung damage. Anti-RNP and anti-centromere antibodies are often associated with honeycomb lesions, indicating disease progression to a moderate-to-severe stage where drug efficacy is limited. At this stage, steroid treatment may not be optimal, and timely initiation of anti-fibrotic therapy is recommended [43, 44].
The therapeutic drugs commonly used for SSc-ILD include glucocorticoids, immunosuppressants, and anti-fibrotic agents [45]. This study collected data from 58 patients with SSc-ILD using a total of 72 imaging scans. Patients were categorised into pre- and post-treatment groups based on treatment timing. AI was used to measure the lesion size and compare the results. The findings indicated that some patients responded well to treatment with a reduced lesion volume, whereas others experienced limited effectiveness with increasing lesion volume.Lesion types can change during treatment, transitioning from single to multiple types, such as consolidated, ground-glass opaque, nodular, or honeycomb-structured lesions. Additionally, some patients showed evolving lesion types, such as nodular and consolidation shadows, which transformed into honeycomb structures. Most patients exhibit overlapping imaging findings, transitioning from early to mid-stage exudative lesions, including consolidation, grid shadows, and eventually honeycomb-like results. We observed that pulmonary fibrosis lesions tended to worsen over time, often manifesting as honeycomb shadows in later stages. Salaffi et al. noted that honeycomb or grid-like shadows on pulmonary HRCT scans and other fibrotic patterns typically indicate irreversible disease and a poor prognosis. Overall, this study underscores the uncertain therapeutic outcomes and poor prognosis of SSc-ILD, highlighting the lack of effective treatment options for interstitial pulmonary lesions associated with this condition [46,47,48].
Current research on AI in ILD has primarily centred on lung and lesion-based segmentation, disease classification, and severity assessment [18]. Early studies, such as the work by Anthimopoulos et al. [49], developed and trained one of the initial convolutional neural networks (CNNs), achieving an accuracy of 88.9% in identifying six lesion patterns of ILD and normal lung tissue. Subsequently, Kim et al. [50] enhanced the accuracy to 95.12% by increasing the number of CNN convolutional layers, showcasing the potential of deep learning in recognising lung tissue specificity. Aliboni et al. [51] created a CNN algorithm that not only quantified different lesion patterns of hypersensitivity pneumonitis but also revealed a significant negative correlation between the extent of CT signs of fibrosis and lung function indices.
AI represents a substantial advancement in the staging and quantitative analysis of ILD, offering valuable guidance for developing, implementing, and evaluating clinical strategies [52, 53]. Despite these advancements, there remains a scarcity of research focused on the quantitative analysis of lesions, necessitating further studies to validate previous findings [54, 55]. Current research predominantly centres on the general application of ILD, with limited attention given to CTD-ILD. There is a lack of literature addressing algorithms and prognostic factors specifically for SSc-ILD. It is important to note that the pathogenesis, treatment, and prognosis of CTD-ILD differ significantly from idiopathic pulmonary fibrosis (IPF), which has the highest incidence rate among ILDs. Unlike IPF, which is characterised by malignant progressive fibrosis and a decline in lung function, CTD-ILD has a benign immune-related pathogenesis and treatment approach, warranting further in-depth investigation [56].
Our research employed an innovative approach to develop and train an advanced medical image processing model with the goal of enhancing the detection and classification capabilities of SSc-ILD, and by extension, CTD-ILD. By combining the DeepLabV3 and Inception-ResNet neural network architectures [57, 58], we aim to improve feature extraction and classification tasks for lesion areas through a multi-task learning framework. This study represents the first instance in China in which AI technology has been used to analyse HRCT imaging characteristics of patients with SSc-ILD, quantify lesion sizes, and correlate them with clinical data to investigate the relationship between SSc-ILD lesion types, clinical indicators, and prognosis. Our findings demonstrate the potential for AI systems to effectively learn from complex images with limited data and exhibit strong generalisation capabilities [59, 60]. Our work complements recent studies by Schniering et al. [52] and Chassagnon et al. [8], collectively advancing the field of AI in medical image analysis. With the expansion of the sample size and research cohort and ongoing technical enhancements, we anticipate that our model will make considerale progress in medical image analysis, offering valuable support for clinical diagnosis and treatment of SSc-ILD and CTD-ILD.
Nevertheless, we acknowledges several study limitations. First, as a retrospective cross-sectional study conducted at two centres, this study does not fully encapsulate the characteristics of individuals with SSc and ILD across different regions. Second, the evaluation of treatment efficacy was constrained by the absence of a large enough sample size for robust statistical analyses. Third, the small sample size could have limited the study's statistical power, affecting the robustness of the findings. Lastly, the study's focus was exclusively on patients with SSc-ILD, indicating a need for further research to ascertain the applicability and generalisability of the AI model to broader patient populations.
Conclusions
This study highlights the significant impact of AI in effectively analysing the detailed imaging characteristics of SSc-ILD, demonstrating its robust ability to generalise when processing complex HRCT images. SSc-ILD commonly presents with patterns such as UIP, NSIP, or IIP. HRCT imaging in patients with SSc-ILD reveals distinct distribution patterns of grid shadow, ground-glass shadow, nodular shadow, consolidation shadow, and honeycomb structure in the lungs. Specifically, honeycomb structures are more prevalent in SSc-ILD patients with pulmonary hypertension, whereas ground-glass opacity is less common. Additionally, this study suggests that lesion types in patients with SSc-ILD can change post-treatment.
Data availability
Data are available upon reasonable request from the corresponding author.
References
Volkmann ER, Andréasson K, Smith V (2023) Systemic sclerosis. Lancet 401:304–318. https://doi.org/10.1016/S0140-6736(22)01692-0
Perelas A, Silver RM, Arrossi AV, Highland KB (2020) Systemic sclerosis-associated interstitial lung disease. Lancet Respir Med 8:304–320. https://doi.org/10.1016/S2213-2600(19)30480-1
Morais A, Duarte AC, Fernandes MO, Borba A, Ruano C, Marques ID, Calha J, Branco JC, Pereira JM, Salvador MJ, Bernardes M, Khmelinskii N, Pinto P, Pinto-Basto R, Freitas S, Campainha S, Alfaro T, Cordeiro A (2023) Early detection of interstitial lung disease in rheumatic diseases: a joint statement from the Portuguese Pulmonology Society, the Portuguese Rheumatology Society, and the Portuguese Radiology and Nuclear Medicine Society. Pulmonology. https://doi.org/10.1016/j.pulmoe.2023.11.007
Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D, Travis WD, King TE, Costabel U, Wells AU, Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Ryu JH, Behr J, Brown KK, Collard HR, Cordeiro CR, Cottin V, Drent M, Egan J, Flaherty K, Inoue Y, Kim DS, Martinez FJ, Raghu G, Richeldi L, Valeyre D, Hansell DM, Lynch DA, Johkoh T, Sverzellati N, Nicholson AG, Colby TV, Kitaichi M, Myers J, Selman M, Crestani B, Hogaboam C, Loyd J, Ryerson CJ, Swigris J, Dudden RF, Protzko S (2013) An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188:733–748. https://doi.org/10.1164/rccm.201308-1483ST
Abida H, Meddeb Z, Abdelkefi C, El Ouni A, Toujani S, Hamzeoui S, Larbi T, Bouslama K (2023) AB0893 correlations between CT scan, clinical and respiratory functional data in systemic sclerosis-associated interstitial lung disease. Ann Rheum Dis 82:1661–1661. https://doi.org/10.1136/annrheumdis-2023-eular.5890
Ferrazza AM, Gigante A, Gasperini ML, Ammendola RM, Paone G, Carbone I, Rosato E (2020) Assessment of interstitial lung disease in systemic sclerosis using the quantitative CT algorithm CALIPER. Clin Rheumatol 39:1537–1542. https://doi.org/10.1007/s10067-020-04938-3
Schniering J, Maciukiewicz M, Gabrys HS, Brunner M, Blüthgen C, Meier C, Braga-Lagache S, Uldry AC, Heller M, Guckenberger M, Fretheim H, Nakas CT, Hoffmann-Vold AM, Distler O, Frauenfelder T, Tanadini-Lang S, Maurer B (2022) Computed tomography-based radiomics decodes prognostic and molecular differences in interstitial lung disease related to systemic sclerosis. Eur Respir J 59:2004503. https://doi.org/10.1183/13993003.04503-2020
Chassagnon G, Vakalopoulou M, Régent A, Sahasrabudhe M, Marini R, Hoang-Thi TN, Dinh-Xuan AT, Dunogué B, Mouthon L, Paragios N, Revel MP (2021) Elastic registration-driven deep learning for longitudinal assessment of systemic sclerosis interstitial lung disease at CT. Radiology 298:189–198. https://doi.org/10.1148/radiol.2020200319
Poerio A, Carlicchi E, Zompatori M (2023) Diagnosis of interstitial lung disease (ILD) secondary to systemic sclerosis (SSc) and rheumatoid arthritis (RA) and identification of “progressive pulmonary fibrosis” using chest CT: a narrative review. Clin Exp Med 23:4721–4728. https://doi.org/10.1007/s10238-023-01202-1
Haug CJ, Drazen JM (2023) Artificial intelligence and machine learning in clinical medicine, 2023. N Engl J Med 388:1201–1208. https://doi.org/10.1056/NEJMra2302038
He B, Kwan AC, Cho JH, Yuan N, Pollick C, Shiota T, Ebinger J, Bello NA, Wei J, Josan K, Duffy G, Jujjavarapu M, Siegel R, Cheng S, Zou JY, Ouyang D (2023) Blinded, randomized trial of sonographer versus AI cardiac function assessment. Nature 616:520–524. https://doi.org/10.1038/s41586-023-05947-3
Ray PP (2024) Advancing AI in rheumatology: critical reflections and proposals for future research using large language models. Rheumatol Int 44(3):573–574. https://doi.org/10.1007/s00296-023-05488-y
Gomes B, Ashley EA (2023) Artificial intelligence in molecular medicine. N Engl J Med 388(26):2456–2465. https://doi.org/10.1056/NEJMra2204787
Pranav R, Matthew PL (2023) The current and future state of AI interpretation of medical images. N Engl J Med. https://doi.org/10.1056/nejmra2301725
Vakalopoulou M, Christodoulidis S, Burgos N, Colliot O, Lepetit V (2023) Learning Basics and convolutional neural networks (CNNs). In: Colliot O (ed) Machine learning for brain disorders. Humana Press, New York, pp 77–115
Wang Y-R, Yang K, Wen Y, Wang P, Hu Y, Lai Y, Wang Y, Zhao K, Tang S, Zhang A, Zhan H, Lu M, Chen X, Yang S, Dong Z, Wang Y, Liu H, Zhao L, Huang L, Li Y, Wu L, Chen Z, Luo Y, Liu D, Zhao P, Lin K, Wu JC, Zhao S (2024) Screening and diagnosis of cardiovascular disease using artificial intelligence-enabled cardiac magnetic resonance imaging. Nat Med 30(5):1471–1480. https://doi.org/10.1038/s41591-024-02971-2
Sohn JH, Fields BKK (2024) Radiomics and deep learning to predict pulmonary nodule metastasis at CT. Radiology 311(1):e233356. https://doi.org/10.1148/radiol.233356
Mei X, Liu Z, Singh A, Lange M, Boddu P, Gong JQX, Lee J, DeMarco C, Cao C, Platt S, Sivakumar G, Gross B, Huang M, Masseaux J, Dua S, Bernheim A, Chung M, Deyer T, Jacobi A, Padilla M, Fayad ZA, Yang Y (2023) Interstitial lung disease diagnosis and prognosis using an AI system integrating longitudinal data. Nat Commun 14:2272. https://doi.org/10.1038/s41467-023-37720-5
Oh AS, Lynch DA, Swigris JJ, Baraghoshi D, Dyer DS, Hale VA, Koelsch TL, Marrocchio C, Parker KN, Teague SD, Flaherty KR, Humphries SM (2024) Deep learning-based fibrosis extent on computed tomography predicts outcome of fibrosing interstitial lung disease independent of visually assessed computed tomography pattern. Ann Am Thorac Soc 21(2):218–227. https://doi.org/10.1513/AnnalsATS.202301-084OC
Li L, Qin L, Xu Z, Yin Y, Wang X, Kong B, Bai J, Lu Y, Fang Z, Song Q, Cao K, Liu D, Wang G, Xu Q, Fang X, Zhang S, Xia J, Xia J (2020) Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology 296:E65–E71. https://doi.org/10.1148/radiol.2020200905
Zhu Q, Che P, Li M, Guo W, Ye K, Yin W, Chu D, Wang X, Li S (2024) Artificial intelligence for segmentation and classification of lobar, lobular, and interstitial pneumonia using case-specific CT information. Quant Imaging Med Surg 14:579–591. https://doi.org/10.21037/qims-23-945
Soffer S, Morgenthau AS, Shimon O, Barash Y, Konen E, Glicksberg BS, Klang E (2022) Artificial intelligence for interstitial lung disease analysis on chest computed tomography: a systematic review. Acad Radiol 29(Suppl 2):S226-s235. https://doi.org/10.1016/j.acra.2021.05.014
Le Gall A, Hoang-Thi TN, Porcher R, Dunogué B, Berezné A, Guillevin L, Le Guern V, Cohen P, Chaigne B, London J, Groh M, Paule R, Chassagnon G, Vakalopoulou M, Dinh-Xuan AT, Revel MP, Mouthon L, Régent A (2024) Prognostic value of automated assessment of interstitial lung disease on CT in systemic sclerosis. Rheumatology (Oxford) 63(1):103–110. https://doi.org/10.1093/rheumatology/kead164
Konopka KE, Myers JL (2021) Interstitial lung disease pathology in systemic sclerosis. Ther Adv Musculoskelet Dis 13:1759720X211032437. https://doi.org/10.1177/1759720X211032437
De Santis M, Isailovic N, Ceribelli A, Motta F, Vecellio M, Selmi C (2021) OP0249 serum proteomic biomarkers define patients with systemic sclerosis with interstitial lung disease. Ann Rheum Dis 80(Supplement 1):152.2-15152. https://doi.org/10.1136/annrheumdis-2021-eular.3099
Györfi AH, Filla T, Dickel N, Möller F, Li YN, Bergmann C, Matei AE, Harrer T, Kunz M, Schett G, Distler JHW (2024) Performance of serum biomarkers reflective of different pathogenic processes in systemic sclerosis-associated interstitial lung disease. Rheumatol (Oxford) 63:962–969. https://doi.org/10.1093/rheumatology/kead332
Mouawad JE, Feghali-Bostwick C (2023) The molecular mechanisms of systemic sclerosis-associated lung fibrosis. Int J Mol Sci 24:2963. https://doi.org/10.3390/ijms24032963
Hunninghake GM, Goldin JG, Kadoch MA, Kropski JA, Rosas IO, Wells AU, Yadav R, Lazarus HM, Abtin FG, Corte TJ, de Andrade JA, Johannson KA, Kolb MR, Lynch DA, Oldham JM, Spagnolo P, Strek ME, Tomassetti S, Washko GR, White ES, Abtin F, Antoniou K, Blackwell T, Brown K, Chung J, Corte T, Crestani B, Crossno P, Culver D, de Andrade J, Deveraj A, Flaherty K, Gudmundsson G, Hatabu H, Jacob J, Johansson K, Kanne J, Kazerooni E, Kolb M, Lynch D, Maher T, Martinez F, Morais A, Nathan SD, Noth I, Oldham J, Podolanczuk A, Poletti V, Ravaglia C, Renzoni E, Richeldi L, Rubin G, Ryerson C, Sahoo D, Tomassetti S, Spagnolo P, Strek ME, Suh R, Sverzellati N, Valeyre D, Walsh S, Washko G, White ES (2022) Detection and early referral of patients with interstitial lung abnormalities: an expert survey initiative. Chest 161:470–482. https://doi.org/10.1016/j.chest.2021.06.035
Zheng B, Marinescu DC, Hague C, Muller N, Murphy D, Churg A, Wright J, Al-Arnawoot A, Cox G, Guenther Z, Grant-Orser A, Huynh J, Elliot T, Fladeland D, Ellis J, Karjala G, Goobie G, Johannson K, Lok S, Sedlic T, Khalil N, Marcoux V, Kolb M, Scallan C, Hambly N Macisaac S, Leipsic J, Tan V, Durand C, Manganas H, Haider E, Fisher J, Mcinnis M, Shapera S, Bilawich AM, Mayo J, Bourgouin P, Morisset J, Sun K, Wong A, Ryerson C (2023) POS1242 computed tomography findings in connective tissue disease-related interstitial lung disease. https://doi.org/10.1136/annrheumdis-2023-eular.2153
Kwong JCC, Khondker A, Lajkosz K, McDermott MBA, Frigola XB, McCradden MD, Mamdani M, Kulkarni GS, Johnson AEW (2023) APPRAISE-AI tool for quantitative evaluation of ai studies for clinical decision support. JAMA Netw Open 6(9):e2335377. https://doi.org/10.1001/jamanetworkopen.2023.35377
Majdik ZP, Graham SS, Shiva Edward JC, Rodriguez SN, Karnes MS, Jensen JT, Barbour JB, Rousseau JF (2024) Sample size considerations for fine-tuning large language models for named entity recognition tasks: methodological study. Jmir ai 3:e52095. https://doi.org/10.2196/52095
Williamson L (2021) New reference atlas for pulmonary fibrosis severity score in systemic sclerosis. Lancet Respir Med 9:130–131. https://doi.org/10.1016/S2213-2600(20)30565-8
Rusu I, Muntean L, Tamas MM, Felea I, Damian L, Filipescu I, Simon SP, Pamfil C, Marinescu CM, Man MA, Csutak C, Rednic S (2020) AB0605 clinical profile and chest high-resolution computed tomography (HRCT) findings in patients with connective tissue diseases and interstitial lung disease: experience of a single reference rheumatology centre. Ann Rheum Dis 79(Supplement 1):1598.2-151599. https://doi.org/10.1136/annrheumdis-2020-eular.3758
Young A, Vummidi D, Visovatti S, Homer K, Wilhalme H, White ES, Flaherty K, McLaughlin V, Khanna D (2019) Prevalence, treatment, and outcomes of coexistent pulmonary hypertension and interstitial lung disease in systemic sclerosis. Arthritis Rheumatol 71:1339–1349. https://doi.org/10.1002/art.40862
Fayed H, Schreiber BE, Denton CP, Coghlan JG (2021) Impact of routine screening on detection, severity and outcome of pulmonary arterial hypertension in systemic sclerosis. Eur Heart J 42(Supplement):1. https://doi.org/10.1093/eurheartj/ehab724.1976
Hassan HJ, Naranjo M, Ayoub N, Housten T, Hsu S, Balasubramanian A, Simpson CE, Damico RL, Mathai SC, Kolb TM, Hassoun PM (2023) Improved survival for patients with systemic sclerosis-associated pulmonary arterial hypertension: the Johns Hopkins Registry. Am J Respir Crit Care Med 207:312–322. https://doi.org/10.1164/rccm.202204-0731OC
Khanna D, Tashkin DP, Denton CP, Renzoni EA, Desai SR, Varga J (2020) Etiology, risk factors, and biomarkers in systemic sclerosis with interstitial lung disease. Am J Respir Crit Care Med 201:650–660. https://doi.org/10.1164/rccm.201903-0563CI
Amanda G, Sutoyo DK (2021) Interstitial lung disease in systemic sclerosis: from immunopathogenesis to treatment. Pneumol (Bucharest Romania) 70(4):2–9. https://doi.org/10.2478/pneum-2023-0001
Wittwer MF, Kim SY, Leichtle A, Berezowska S, Guler SA, Geiser T, Heverhagen J, Maurer B, Poellinger A (2023) Signs of alveolar collapse in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis, and systemic sclerosis revealed by inspiration and expiration computed tomography. Biomed 3(4):471–483. https://doi.org/10.3390/biomed3040038
Yomono K, Kuwana M (2023) AB0810 performance of circulating biomarkers for predicting progression of interstitial lung disease in patients with systemic sclerosis. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2023-eular.1060
Fajardo Hermosillo LD, María Karina LR (2023) OP0237 use of neutrophil/lymphocyte and PLATELET/lymphocyte ratios to detect systemic sclerosis-associated interstitial lung disease. Use of neutrophil/lymphocyte and platelet/lymphocyte ratios to detect systemic sclerosis-associated interstitial lung disease. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2023-eular.1582
Shah S, Denton CP (2022) Scleroderma autoantibodies in guiding monitoring and treatment decisions. Curr Opin Rheumatol 34:302–310. https://doi.org/10.1097/BOR.0000000000000904
Mendez Diaz LM, Gil Velez RJ, Madroñal García I, Ricardo Juan Gil V, Isabel Madroñal G (2020) AB0591 analysis of a cohort of patients with systemic sclerosis and interstitial lung disease. Ann Rheum Dis 79(Supplement 1):1592.1-151592. https://doi.org/10.1136/annrheumdis-2020-eular.2673
Jandali B, Salazar GA, Hudson M, Fritzler MJ, Lyons MA, Estrada-Y-Martin RM, Charles J, Terracina KA, Mayes MD, Assassi S (2022) The effect of anti-Scl-70 antibody determination method on its predictive significance for interstitial lung disease progression in systemic sclerosis. ACR Open Rheumatol 4:345–351. https://doi.org/10.1002/acr2.11398
De Lorenzis E, Natalello G, Di Donato S, Verardi L, Cerasuolo PG, Kakkar V, D’agostino Ma, Del Galdo F, Bosello SL (2023) AB0841 concordance and prognostic relevance of different definitions of systemic sclerosis interstitial lung disease progression. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2023-eular.5786
Stochmal A, Czuwara J, Trojanowska M, Rudnicka L (2020) Antinuclear antibodies in systemic sclerosis: an update. Clin Rev Allergy Immunol 58:40–51. https://doi.org/10.1007/s12016-018-8718-8
Raghu G, Montesi SB, Silver RM, Hossain T, Macrea M, Herman D, Barnes H, Adegunsoye A, Azuma A, Chung L, Gardner GC, Highland KB, Hudson M, Kaner RJ, Kolb M, Scholand MB, Steen V, Thomson CC, Volkmann ER, Wigley FM, Burlile D, Kemper KA, Knight SL, Ghazipura M (2024) Treatment of systemic sclerosis-associated interstitial lung disease: evidence-based recommendations. An Official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 209:137–152. https://doi.org/10.1164/rccm.202306-1113ST
Liakouli V, Ciancio A, Del Galdo F, Giacomelli R, Ciccia F (2024) Systemic sclerosis interstitial lung disease: unmet needs and potential solutions. Nat Rev Rheumatol 20:21–32. https://doi.org/10.1038/s41584-023-01044-x
Anthimopoulos M, Christodoulidis S, Ebner L, Christe A, Mougiakakou S (2016) Lung pattern classification for interstitial lung diseases using a deep convolutional neural network. IEEE Trans Med Imaging 35:1207–1216. https://doi.org/10.1109/TMI.2016.2535865
Kim GB, Jung KH, Lee Y, Kim HJ, Kim N, Jun S, Seo JB, Lynch DA (2018) Comparison of shallow and deep learning methods on classifying the regional pattern of diffuse lung disease. J Digit Imaging 31:415–424. https://doi.org/10.1007/s10278-017-0028-9
Aliboni L, Dias OM, Pennati F, Baldi BG, Sawamura MVY, Chate RC, Carvalho CRR, de Albuquerque ALP, Aliverti A (2022) Quantitative CT analysis in chronic hypersensitivity pneumonitis: a convolutional neural network approach. Acad Radiol 29(Suppl 2):S31–S40. https://doi.org/10.1016/j.acra.2020.10.009
Schniering J, Maciukiewicz M, Gabrys HS, Brunner M, Blüthgen C, Meier C, Braga-Lagache S, Uldry AC, Heller M, Guckenberger M, Fretheim H, Nakas CT, Hoffmann-Vold AM, Distler O, Frauenfelder T, Tanadini-Lang S, Maurer B (2022) Computed tomography-based radiomics decodes prognostic and molecular differences in interstitial lung disease related to systemic sclerosis. Eur Respir J. https://doi.org/10.1183/13993003.04503-2020
Dwivedi K, Sharkey M, Delaney L, Alabed S, Rajaram S, Hill C, Johns C, Rothman A, Mamalakis M, Thompson AAR, Wild J, Condliffe R, Kiely DG, Swift AJ (2024) Improving prognostication in pulmonary hypertension using AI-quantified fibrosis and radiologic severity scoring at baseline CT. Radiology 310(2):e231718. https://doi.org/10.1148/radiol.231718
Ahn Y, Kim HC, Lee JK, Noh HN, Choe J, Seo JB, Lee SM (2024) Usefulness of CT quantification-based assessment in defining progressive pulmonary fibrosis. Acad Radiol. https://doi.org/10.1016/j.acra.2024.05.005
Koh SY, Lee JH, Park H, Goo JM (2024) Value of CT quantification in progressive fibrosing interstitial lung disease: a deep learning approach. Eur Radiol 34(7):4195–4205. https://doi.org/10.1007/s00330-023-10483-9
Maher TM (2024) Interstitial lung disease: a review. JAMA 331(19):1655–1665. https://doi.org/10.1001/jama.2024.3669
Costanzo E, Polito MS, Bandello F, Querques G (2023) Artificial intelligence’s role in differentiating the origin for subretinal bleeding in pathologic myopia. Retina (Philadelphia, Pa) 43(11):1881–1889. https://doi.org/10.1097/iae.0000000000003884
Wang H, Xu S, Fang KB, Dai ZS, Wei GZ, Chen LF (2023) Contrast-enhanced magnetic resonance image segmentation based on improved U-Net and Inception-ResNet in the diagnosis of spinal metastases. J Bone Oncol 42:100498. https://doi.org/10.1016/j.jbo.2023.100498
Han R, Acosta JN, Shakeri Z, Ioannidis JPA, Topol EJ, Rajpurkar P (2024) Randomised controlled trials evaluating artificial intelligence in clinical practice: a scoping review. Lancet Digit Health 6(5):e367–e373. https://doi.org/10.1016/s2589-7500(24)00047-5
Wang H, Fu T, Du Y, Gao W, Huang K, Liu Z, Chandak P, Liu S, Van Katwyk P, Deac A, Anandkumar A, Bergen K, Gomes CP, Ho S, Kohli P, Lasenby J, Leskovec J, Liu TY, Manrai A, Marks D, Ramsundar B, Song L, Sun J, Tang J, Veličković P, Welling M, Zhang L, Coley CW, Bengio Y, Zitnik M (2023) Scientific discovery in the age of artificial intelligence. Nature 620(7972):47–60. https://doi.org/10.1038/s41586-023-06221-2
Acknowledgements
We thank the reviewers for their insightful comments and suggestions. We also acknowledge that no external editing support was used in the preparation of this manuscript. All authors have contributed to the writing, editing, and finalisation of the content.
Funding
This research was funded by Xiangxi Tujia and Miao Autonomous Prefecture People's Hospital Young Physician Backbone Cultivation Program.
Author information
Authors and Affiliations
Contributions
L.R. contributed to the study design. J.Z., Y.L., S.L., X.L., Y.Z., and J.H. contributed to the collection of cases. L.H. contributed to the image annotation. J.Z. and Y.L. wrote the manuscript. L.R. reviewed and edited the manuscript. All authors have given final approval of the version to be published and take full responsibility for the integrity and accuracy of all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
This study was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University.
Note on AI and editing software usage
The authors confirm that no artificial intelligence tools or editing software were used in the writing or editing process of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, J., Long, Y., Li, S. et al. Use of artificial intelligence algorithms to analyse systemic sclerosis-interstitial lung disease imaging features. Rheumatol Int 44, 2027–2041 (2024). https://doi.org/10.1007/s00296-024-05681-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-024-05681-7