Abstract
Sporadic inclusion body myositis (sIBM) is a subgroup of idiopathic inflammatory myopathies characterised by progressive muscle weakness and skeletal muscle inflammation. Quantitative data on the myofibre morphology in sIBM remains scarce. Further, no previous study has examined fibre type association of satellite cells (SC), myonuclei number, macrophages, capillaries, and myonuclear domain (MD) in sIBM patients. Muscle biopsies from sIBM patients (n = 18) obtained previously (NCT02317094) were included in the analysis for fibre type-specific myofibre cross-sectional area (mCSA), SCs, myonuclei and macrophages, myonuclear domain, and capillarisation. mCSA (p < 0.001), peripheral myonuclei (p < 0.001) and MD (p = 0.005) were higher in association with type 1 (slow-twitch) than type 2 (fast-twitch) fibres. Conversely, quiescent SCs (p < 0.001), centrally placed myonuclei (p = 0.03), M1 macrophages (p < 0.002), M2 macrophages (p = 0.013) and capillaries (p < 0.001) were higher at type 2 fibres compared to type 1 fibres. In contrast, proliferating (Pax7+/Ki67+) SCs (p = 0.68) were similarly associated with each fibre type. Type 2 myofibres of late-phase sIBM patients showed marked signs of muscle atrophy (i.e. reduced mCSA) accompanied by higher numbers of associated quiescent SCs, centrally placed myonuclei, macrophages and capillaries compared to type 1 fibres. In contrast, type 1 fibres were suffering from pathological enlargement with larger MDs as well as fewer nuclei and capillaries per area when compared with type 2 fibres. More research is needed to examine to which extent different therapeutic interventions including targeted exercise might alleviate these fibre type-specific characteristics and countermeasure their consequences in impaired functional performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sporadic inclusion body myositis (sIBM) is an acquired slowly progressing disorder within the group of idiopathic inflammatory myopathies [1, 2]. Severe progressive muscle weakness accompanied by impairments in physical function are typical clinical traits of the disease [3, 4]. Histologically, sIBM is characterised by muscle fibre damage, inflammatory infiltrates, and over time, marked myofibre atrophy [2, 5, 6].
Studies investigating pathophysiological changes in sIBM muscles have mainly focused on the immune response, including lymphocytes [7, 8], macrophages [9, 10] and major histocompatibility complex [11], while studies describing other histological features such as myofibre size (cross-sectional area: mCSA), myofibre type distribution and the content of myogenic precursor cells are scarce [12,13,14]. Specifically, no previous studies have examined myofibre type-specific (type 1, slow-twitch and type 2, fast-twitch) association of satellite cells (SC), myonuclei, myofibre area per myonuclei (i.e., myonuclear domain: MD) and capillary supply in sIBM skeletal muscle.
Therefore, by analysing fibre-specific myogenic regenerative capacity (SCs and myonuclei), markers of inflammation (M1- and M2 macrophages), and vascularization the present study aimed to expand our understanding of the pathophysiological changes in myocellular morphology related to sIBM.
Methods
Patient characteristics
General characteristics of the included sIBM patients (n = 18) are presented in Table 1. The average age was 68.8 ± 5.9 years and the age at onset of symptoms was 60.1 ± 6.9 years. The patient group included 14 males (78%) and 4 females (22%). Seven patients (39%) were receiving immunosuppressive medication.
Study outline
The muscle biopsy material from a previous randomised controlled trial (RCT) conducted in our lab (NCT02317094) was analysed [15]. Only baseline biopsies were included in the present study, comprising muscle biopsies from 21 biopsy-validated sIBM patients. Three biopsies were excluded, as the quantitative analysis was impossible due to excessive tissue damage (Figure A, Supplementary Material) and thus the data from 18 sIBMs muscle biopsies were included in the final analysis. Recruitment procedures have been described in detail previously [15].
Muscle biopsy sampling
Muscle biopsies from m. vastus lateralis (VL) or in cases of severe VL atrophy, m. tibialis anterior (TA) were obtained using the Bergström needle (4.0 mm) technique. Immediately after extraction, the muscle samples were embedded in Tissue Tek (4583, Sakura Finetek, Alphen aan den Rijn, The Netherlands) and frozen in nitrogen-cooled 2-methyl butane. The muscle biopsies were stored in a freezer at − 80 °C until further analysis.
Immunohistochemistry and data processing
Immunohistochemistry procedures were performed as described in detail elsewhere [10]; see Supplementary Table A for a detailed listing of the antibodies used.
The data processing with Visiopharm has been described previously [10]. In brief, the image analysis software (Visiopharm A/S, Denmark) was used following a three-step protocol: (1) Identifying muscle fibres. (2) Identifying and quantification of structures of interest (i.e., satellite cells). The linear distance from any fibre determined the allocation of structures of interest, in terms of fibre type (i.e., closest fibre). (3) Normalising the number of structures of interest to the analysed area (counts/mm2). For type 1 and type 2 fibres, the average analysed area was 1.94 ± 0.19 and 0.74 ± 0.01 mm2 per biopsy sample, respectively. To allow comparisons to the existing literature both density (cells/mm2) and structure of interest/fibre profile” for SCs, macrophages and capillaries are presented in the results section (Tables 3, 4, 5).
Analysis of mCSA, sublaminal myonuclei and centrally-placed myonuclei was conducted by manual analysis (AxioVision SE64 Rel. 4.9.1 software: Carl Zeiss, Germany). First, all cross-sectional fibres were identified, hereafter the cross-sectional area and the relative number of type 1 and 2 fibres, as well as myonuclei and central myonuclei, were recorded. A median of 120 fibres (range [21–837]) were analysed per biopsy (type 1; 71 [10–295] fibres, type 2; 62 [11–542]).
Statistical analysis
All data reported in the present study were analysed for normality and homogenous distribution of variance, which could not be verified uniformly for all outcome parameters. The Wilcoxon’s signed-rank test was used to evaluate differences in outcome parameters between type 1 and 2 myofibres. All descriptive data are presented as group mean ± SD unless otherwise stated. The statistical significance level was set at p ≤ 0.05 (two-tailed testing). Statistical analysis was performed using RStudio (Version 2022.7.1.554) [16].
Results
Myofibre cross-sectional area, myonuclei and myonuclear domain
Mean type 1 fibre mCSA (6040 ± 3054 µm2) was ~ 140% larger than type 2 fibre mCSA (2518 ± 1815 µm2) (p = 0.0002). Histograms depicting fibre type-specific mCSA distribution are shown in Fig. 1.
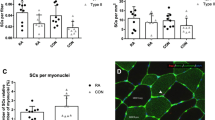
Type 1 fibres demonstrated ~ 61% higher myonuclei (Six1+/DAPI) content per fibre profile as compared to type 2 fibres (p < 0.0001). Despite that myonuclear domain was ~ 65% larger in type 1 fibres as compared to type 2 fibres (p = 0.005) (Table 2). The number of centrally placed myonuclei (Six1+/DAPI) was 67% higher in type 2 than in type 1 fibres (p = 0.03) (Table 2); see Fig. 2 for examples of fibre type differences in size (A) and centrally placed myonuclei (B).
Illustrations of muscle biopsy findings from patients with sporadic inclusion body myositis (sIBM) A Type 1 fibres (black) were observed to be larger in size than type 2 fibres (grey). B The number of centrally placed myonuclei (red arrows) was higher in type 2 than in type 1 fibres. C The number of satellite cells (red arrows) was higher in association to type 2 fibres than to type 1 fibres. D M1 (red arrows) and M2 (green arrows) macrophages were observed to group into clusters in the vicinity of small type 2 fibres. A Green = laminin, Grey = MHC fast. B Green = laminin, Grey = MHC fast, blue = DAPI. C Green = laminin, Grey = MHC fast, blue = DAPI, red = pax7. Grey = MHC fast, blue = DAPI, yellow = CD206, red = CD68
Satellite cells
Quiescent SC number associated with type 2 fibre area was higher as compared to that of type 1 fibre area (p < 0.0001), and this difference that persisted in the fibre profile analysis (i.e., more SCs/myofibre profile) (Table 3); see Fig. 2C for an example of fibre type differences in quiescent SCs.
Proliferating SCs was equally associated with type 1 fibre and type 2 fibre in both the area analysis and the fibre profile analysis (p = 0.08) also when compared with area (p = 0.68; p = 0.08, respectively) (Table 3).
Macrophages
M1 macrophage density in type 2 fibre area was higher as compared to that of type 1 fibre area (p = 0.002). Likewise, M2 macrophages were more densely packed around type 2 fibres (p = 0.01) (Table 4).
In contrast, in the individual fibre profile analysis, both M1 & M2 macrophage number associated with type 1 were observed to be similar compared to type 2 fibres (p = 0.15; p = 0.34, respectively) (Table 4); see Fig. 2D for an example of fibre type differences in M1 and M2 macrophages.
Capillarisation
Capillary density was of a higher abundance in type 2 fibre area compared to type 1 fibre area (p = 0.0002) (Table 5). However, if related to the fibre profile, type 1 fibres had more capillaries associated with the individual fibre as compared to type 2 fibres (p = 0.008) (Table 5).
Discussion
As evaluated for the first time in myositis patients, we observed that mCSA, peripheral myonuclei, myonuclear domain was higher in association to type 1 (slow-twitch) as compared to type 2 (fast-twitch) myofibres. Conversely, quiescent SCs and central myonuclei, were higher in association to type 2 fibres compared to type 1 fibres. For both M1 and M2, the density was observed to be higher in type 2 fibres, however no differences were observed on the “per fibre profile” analyses. No fibre type-specific differences were observed for proliferating SCs. These observations suggest distinct and exclusive pathological changes related to the respective fibre types.
Type 1 hypertrophy and type 2 atrophy
In the present study, mCSA was ~ 140% larger in type 1 fibres as compared to type 2 fibres, which aligns with previous biopsy data (70–100% larger) obtained in sIBM patients [12, 13]. In comparison, healthy age-matched adults (~ 65 years of age) typically demonstrate type 1 fibres that are between 10% smaller and up to ~ 15% larger than type 2 fibres [17,18,19,20]. Our mCSA histogram analysis identified a high number of pathologically large myofibres, sometimes termed megafibres [21], that contributed to the increased mean mCSA, especially for type 1 fibres (cf. Figure 1). Karlsen et al. reported healthy age-matched muscle (VL) to contain less than 10% of type 1 fibres with an mCSA above 7000 µm2 [22], while in the present cohort of sIBM patients ~ 40% of type 1 fibres demonstrated mCSA > 7000 µm2 (cf. Fig. 1).
In healthy lower limb (VL) muscles of adults between 50 and 70 years of age, mCSA of type 2 fibres typically amounts to 4000–6000 µm2, depending on habitual physical activity levels [23]. Specifically, in age-matched muscle biopsies obtained at ~ 65 years of age only a few (< 10%) type 2 fibres demonstrate mCSA ≤ 2000 µm2, irrespective of training history [22]. In contrast, the present group of sIBM patients showed highly marked myofibre atrophy, as 62% of type 2 fibres demonstrated mCSA ≤ 2000 µm2 (cf. Fig. 1 and Suppl. Figure B). These findings underline that the sIBM disease process not only severely affects lower limb muscle fibre morphology in accordance with the previous reports [12, 13], but also supports the findings in a recently published study by Nelke et al., that to our knowledge, is the first to reveal that the atrophic effect of the disease appears to be highly fibre type-specific, preferentially affecting fast-contracting type 2 myofibres [24].
Type 2 fibre atrophy and higher association of quiescent satellite cells
Quiescent SC (Pax7+/Six1+) density associated with type 2 fibres was 52% higher compared to type 1 (cf. Table 3), even though type 1 fibres were larger. In contrast, the number of proliferating SCs (Pax7+/Ki67+) was similarly between type 1 and 2 fibres.
Overall myonuclei number (Six1+/DAPI) was ~ 61% higher in type 1 than type 2 fibres (cf. Table 3), which likely was driven by the larger mCSA of type 1 fibres, given that myonuclei number is known to be positively correlated with fibre size [22, 25, 26]. In contrast, the number of centrally placed myonuclei (Six1+) was ~ 67% higher in type 2 fibres (cf. Table 3), an observation that may be linked to the apparent more substantial type 2 myofiber atrophy.
The present cohort of sIBM patients demonstrated an enlarged (+ 67%) myonuclear domain in type 1 fibres compared to type 2 fibres. This could indicate that the proliferative capacity of satellite cells and their ability to fuse with the type 1 myofibers to donate their myonuclei may be dysregulated, as the mCSA to myonuclei ratio (i.e., myonuclei domain) have been demonstrated to be highly positively associated [27, 28].
Higher degree of inflammation associated with type 2 fibres
Both M1 (CD68 + /CD206-) and M2 (CD68 + /CD206 +) macrophages were found to be threefold more abundant related to type 2 fibres as compared to type 1 fibres. These observations indicate higher levels of proinflammatory activity (M1), and elevated tissue remodelling (M2) associated to type 2 vs. type 1 fibres in sIBM patients. The present study did not obtain fibre type specific data on T-cell content. However, we previously observed spatial overlap between macrophage infiltration and T-cell accumulation in the present sIBM patients, where macrophage infiltration was dominantly concentrated around groups of highly atrophied type 2 fibres [10]. Collectively, the previous and present observations suggest that a fibre type dependent pattern of “disease activity” may exist with sIBM, characterised by vastly atrophied type 2 myofibres, which could be driven by the defective muscle regeneration seen in sIBM and myositis [29, 30].
Metabolically suffering type 1 fibres
The number of capillaries (CD31+) density was greater (~ 65%) in relation to type 2 fibre area than type 1 fibre area. In contrast, observations in healthy age-matched adults typically demonstrate higher capillarisation in type 1 fibres compared to type 2 fibres [20, 31]. This disparity suggests that the sIBM disease might be affecting capillary content, however, based on the present observations it is difficult to conclude whether the disease induces increased type 2 fibre capillarisation, or decreased type 1 fibre capillarisation or both. The lower abundance of capillaries of type 1 fibre area, taken together with the higher myonuclear domain of type 1 fibres (lower abundance of myonuclei per fibre profile), could suggest that type 1 fibres in sIBM are metabolically “suffering”, due to negligence in repair and blood supply. This suggests that the sIBM disease might be affecting angiogenesis, especially within type 1 fibres.
Study limitations
The present data were obtained in a somewhat small and heterogeneous group of sIBM patients, which were characterized by large inter-individual differences in disease duration, plasma creatine kinase levels and immunosuppressive treatment. As the sample size did not allow for more detailed subgroup analysis, cautions should be made against generalisations based on the current findings.” The majority of the muscle biopsies were acquired from m. tibialis anterior, rather than m. vastus lateralis, which could have affected the data, however previous data show no significant difference in inflammation or pathologic features between these muscles [32, 33]. Owing to a high degree of physical inactivity and very pronounced muscle atrophy in some study participants, a number of muscle samples did not allow for quantification of mCSA, SC number, myonuclei content etc. and therefore could not be included in the overall statistical analysis (cf. suppl. Figure A), thus reducing the sample size.
Conclusions
As reported for the first time, sIBM appears to exert differential effects on the morphology as well as density and content of quiescent satellite cells, myonuclei, M1 and M2 macrophages and capillarisation of type 1 and type 2 myofibres. In contrast, no fibre type differences in number of associated proliferating satellite cell could be observed. Somewhat paradoxically, type 2 muscle fibres were characterized by focally elevated levels of quiescent satellite cells and central myonuclei, yet these fibres remained markedly atrophied. Likewise, type 1 fibre morphology was pathologically comprised with the presence of megafibres accompanied by large myonuclear domains. All suggest an impact of the sIBM disease on fibre morphology, presumably due to a preferential imbalance between myofibrillar regeneration and degeneration.
Availability of data and materials
Data are generally not available due to current GDPR regulations.
References
Greenberg SA (2019) Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 15(5):257–272. https://doi.org/10.1038/s41584-019-0186-x
Naddaf E, Barohn RJ, Dimachkie MM (2018) Inclusion body myositis: update on pathogenesis and treatment. Neurotherapeutics 15(4):995–1005. https://doi.org/10.1007/s13311-018-0658-8
Yunis EJ, Samaha FJ (1971) Inclusion body myositis. Lab Invest 25(3):240–248
Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, Rowland LP (1995) Inclusion body myositis and myopathies. Ann Neurol 38(5):705–713. https://doi.org/10.1002/ana.410380504
Karpati G, O’Ferrall EK (2009) Sporadic inclusion body myositis: pathogenic considerations. Ann Neurol 65(1):7–11. https://doi.org/10.1002/ana.21622
Askanas V, Engel WK (2008) Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer’s and Parkinson’s disease brains. Acta Neuropathol 116(6):583–595. https://doi.org/10.1007/s00401-008-0449-0
Dalakas MC, Rakocevic G, Schmidt J, Salajegheh M, McElroy B, Harris-Love MO, Shrader JA, Levy EW, Dambrosia J, Kampen RL, Bruno DA, Kirk AD (2009) Effect of Alemtuzumab (CAMPATH 1-H) in patients with inclusion-body myositis. Brain 132(Pt 6):1536–1544. https://doi.org/10.1093/brain/awp104
Hou Y, Luo YB, Dai T, Shao K, Li W, Zhao Y, Lu JQ, Yan C (2018) Revisiting pathological classification criteria for adult idiopathic inflammatory myopathies: in-depth analysis of muscle biopsies and correlation between pathological diagnosis and clinical manifestations. J Neuropathol Exp Neurol 77(5):395–404. https://doi.org/10.1093/jnen/nly017
de Camargo LV, de Carvalho MS, Shinjo SK, de Oliveira ASB, Zanoteli E (2018) Clinical, histological, and immunohistochemical findings in inclusion body myositis. Biomed Res Int 2018:5069042. https://doi.org/10.1155/2018/5069042
Jensen KY, Jacobsen M, Schrøder HD, Aagaard P, Nielsen JL, Jørgensen AN, Boyle E, Bech RD, Rosmark S, Diederichsen LP, Frandsen U (2019) The immune system in sporadic inclusion body myositis patients is not compromised by blood-flow restricted exercise training. Arthritis Res Ther 21(1):293. https://doi.org/10.1186/s13075-019-2036-2
Muth IE, Barthel K, Bähr M, Dalakas MC, Schmidt J (2009) Proinflammatory cell stress in sporadic inclusion body myositis muscle: overexpression of alphaB-crystallin is associated with amyloid precursor protein and accumulation of beta-amyloid. J Neurol Neurosurg Psychiatry 80(12):1344–1349. https://doi.org/10.1136/jnnp.2009.174276
Arnardottir S, Alexanderson H, Lundberg IE, Borg K (2003) Sporadic inclusion body myositis: pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reaction. J Rehabil Med 35(1):31–35. https://doi.org/10.1080/16501970306110
Arnardottir S, Borg K, Ansved T (2004) Sporadic inclusion body myositis: morphology, regeneration, and cytoskeletal structure of muscle fibres. J Neurol Neurosurg Psychiatry 75(6):917–920. https://doi.org/10.1136/jnnp.2003.018994
Tajsharghi H, Thornell LE, Darin N, Martinsson T, Kyllerman M, Wahlström J, Oldfors A (2002) Myosin heavy chain IIa gene mutation E706K is pathogenic and its expression increases with age. Neurology 58(5):780–786. https://doi.org/10.1212/wnl.58.5.780
Jørgensen AN, Aagaard P, Frandsen U, Boyle E, Diederichsen LP (2018) Blood-flow restricted resistance training in patients with sporadic inclusion body myositis: a randomized controlled trial. Scand J Rheumatol 47(5):400–409. https://doi.org/10.1080/03009742.2017.1423109
Team R (2022) RStudio: Integrated Development Environment for R. In., Boston, MA
Larsson L, Sjödin B, Karlsson J (1978) Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103(1):31–39. https://doi.org/10.1111/j.1748-1716.1978.tb06187.x
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R (2000) Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (Bethesda, Md: 1985) 88(4):1321–1326. https://doi.org/10.1152/jappl.2000.88.4.1321
Mattiello-Sverzut AC, Chimelli L, Moura MS, Teixeira S, de Oliveira JA (2003) The effects of aging on biceps brachii muscle fibers: a morphometrical study from biopsies and autopsies. Arq Neuropsiquiatr 61(3a):555–560. https://doi.org/10.1590/s0004-282x2003000400006
Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, Parise G (2016) Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 7(5):547–554. https://doi.org/10.1002/jcsm.12105
Andersen LL, Suetta C, Andersen JL, Kjær M, Sjøgaard G (2008) Increased proportion of megafibers in chronically painful muscles. Pain 139(3):588–593. https://doi.org/10.1016/j.pain.2008.06.013
Karlsen A, Couppé C, Andersen JL, Mikkelsen UR, Nielsen RH, Magnusson SP, Kjaer M, Mackey AL (2015) Matters of fiber size and myonuclear domain: does size matter more than age? Muscle Nerve 52(6):1040–1046. https://doi.org/10.1002/mus.24669
Tøien T, Nielsen JL, Berg OK, Brobakken MF, Nyberg SK, Espedal L, Malmo T, Frandsen U, Aagaard P, Wang E (2023) The impact of life-long strength versus endurance training on muscle fiber morphology and phenotype composition in older men. J Appl Physiol (Bethesda, Md:1985) 135(6):1360–1371. https://doi.org/10.1152/japplphysiol.00208.2023
Nelke C, Schroeter CB, Theissen L, Preusse C, Pawlitzki M, Räuber S, Dobelmann V, Cengiz D, Kleefeld F, Roos A, Schoser B, Brunn A, Neuen-Jacob E, Zschüntzsch J, Meuth SG, Stenzel W, Ruck T (2023) Senescent fibro-adipogenic progenitors are potential drivers of pathology in inclusion body myositis. Acta Neuropathol 146(5):725–745. https://doi.org/10.1007/s00401-023-02637-2
Horwath O, Envall H, Röja J, Emanuelsson EB, Sanz G, Ekblom B, Apró W, Moberg M (2021) Variability in vastus lateralis fiber type distribution, fiber size, and myonuclear content along and between the legs. J Appl Physiol (Bethesda, Md:1985) 131(1):158–173. https://doi.org/10.1152/japplphysiol.00053.2021
Mackey AL, Andersen LL, Frandsen U, Suetta C, Sjøgaard G (2010) Distribution of myogenic progenitor cells and myonuclei is altered in women with vs those without chronically painful trapezius muscle. J Appl Physiol (Bethesda, Md:1985) 109(6):1920–1929. https://doi.org/10.1152/japplphysiol.00789.2010
Kwon B, Kumar P, Lee HK, Zeng L, Walsh K, Fu Q, Barakat A, Querfurth HW (2014) Aberrant cell cycle reentry in human and experimental inclusion body myositis and polymyositis. Hum Mol Genet 23(14):3681–3694. https://doi.org/10.1093/hmg/ddu077
Morosetti R, Broccolini A, Sancricca C, Gliubizzi C, Gidaro T, Tonali PA, Ricci E, Mirabella M (2010) Increased aging in primary muscle cultures of sporadic inclusion-body myositis. Neurobiol Aging 31(7):1205–1214. https://doi.org/10.1016/j.neurobiolaging.2008.08.011
Gandolfi S, Pileyre B, Drouot L, Dubus I, Auquit-Auckbur I, Martinet J (2023) Stromal vascular fraction in the treatment of myositis. Cell Death Discov 9(1):346. https://doi.org/10.1038/s41420-023-01605-9
Khuu S, Fernandez JW, Handsfield GG (2023) Delayed skeletal muscle repair following inflammatory damage in simulated agent-based models of muscle regeneration. PLoS Comput Biol 19(4):e1011042. https://doi.org/10.1371/journal.pcbi.1011042
Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, van Loon LJ (2014) Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (Bethesda, Md:1985) 116(8):998–1005. https://doi.org/10.1152/japplphysiol.00919.2013
Dahlbom K, Geijer M, Oldfors A, Lindberg C (2019) Association between muscle strength, histopathology, and magnetic resonance imaging in sporadic inclusion body myositis. Acta Neurol Scand 139(2):177–182. https://doi.org/10.1111/ane.13040
Michelle EH, Pinal-Fernandez I, Casal-Dominguez M, Albayda J, Paik JJ, Tiniakou E, Adler B, Mecoli CA, Danoff SK, Christopher-Stine L, Mammen AL, Lloyd TE (2023) Clinical subgroups and factors associated with progression in patients with inclusion body myositis. Neurology 100(13):e1406–e1417. https://doi.org/10.1212/wnl.0000000000206777
Acknowledgements
The authors would like to thank Dorte Mengers Flindt for handling the immunohistochemistry and laboratory procedures and Professor Ingrid Lundberg from Karolinska Institute in Stockholm for valuable input on the study design.
Funding
Open access funding provided by National Hospital. This work was funded by The Region of Southern Denmark [2012 j.nr. 12/7763], The Danish Rheumatism Association [R185-A6606], The A.P. Moller Foundation [20-L-0031], and Helsefonden [21-B-0125].
Author information
Authors and Affiliations
Contributions
KYJ were responsible for designing the framework for the biopsy analyses, acquisition of data, analysis of the data, interpretation of the data, and writing the draft of the article. HDS made contributions to designing the framework for the biopsy analyses, interpreting the data and writing the manuscript. PAA made contributions to interpreting the data and writing the article draft. JN contributed to the interpretation of the analysed data and laboratory work. ANJ handled the development of trial design, planning and managing of the randomised controlled trial, as well as essential insight and feedback on the manuscript. RDB handled the biopsy sampling and gave essential feedback on the manuscript. LPD were the driving force in conceptualising the randomised controlled trial. Further, LPD contributed to the interpretation of the analysed data and was a main reviser of the article. All authors assisted with the revisions of the article and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no conflicts of interest.
Ethical approval
This study was approved by the Ethics Committee of The Region of Southern Denmark (S-20120166) and adhered to the Helsinki declaration II. Furthermore, the study was pre-registered at www.clinicaltrials.gov (NCT02317094) and The Danish Data Protection Agency (14/32001). Before participation in the study informed consent (verbally and written) was collected from all patients according to Danish law.
Consent for publication
All patients gave informed consent (verbally and written) for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Congress abstracts: Jensen KY, Aagaard P, Nielsen JL, Jørgensen AN, Bech RD, Frandsen U, Diederichsen LP, Schrøder HD. Sporadic inclusion body myositis affects type 1 and type 2 myofibres differently in terms of morphology, regeneration and inflammation. Proc. 4th Global Conference on Myositis (GCOM) (Eds: Vencovský J, Klein M, Mann H, Kubínová K, Kryštůfková O, Šmucrová H), p.498, 2022 (Abstract). https://doi.org/https://doi.org/10.55563/clinexprheumatol/zka1xq.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jensen, K.Y., Nielsen, J.L., Aagaard, P. et al. Effects of sporadic inclusion body myositis on skeletal muscle fibre type specific morphology and markers of regeneration and inflammation. Rheumatol Int 44, 1077–1087 (2024). https://doi.org/10.1007/s00296-024-05567-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-024-05567-8