Abstract
The attitudes toward emerging COVID-19 vaccines have been of great interest worldwide, especially among vulnerable populations such as patients with rheumatic and musculoskeletal diseases (RMDs). The aim of this study was to analyze the relationship between the nationwide number of COVID-19 cases and deaths, and vaccine acceptance or hesitancy of patients with RMDs from four patient care centers in Mexico. Furthermore, we explored differences in acceptance according to specific diagnoses: rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). This ecological study was a secondary analysis of a cross-sectional study using a validated questionnaire to measure vaccine acceptance. We generated a global Likert scale to evaluate overall attitudes toward the COVID-19 vaccine. We analyzed data from 1336 patients from March to September 2021: 85.13% (1169) were women, with a mean age of 47.87 (SD 14.14) years. The most frequent diagnoses were RA (42.85%, 559) and SLE (27.08%, 393). 635(47.52%) patients were unvaccinated, 253(18.93%) had one dose and 478(35.77%) had two doses. Of all participating patients, 94% were accepting toward the COVID-19 vaccine. Vaccine acceptance remained consistently high throughout the study. However, differences in vaccine acceptance are identified when comparing diagnoses. The peak of the national epidemic curve coincided with an increase in hesitancy among patients with RA. Contrastingly, patients with SLE became more accepting as the epidemic curve peaked. Mexican patients show high acceptance of the COVID-19 vaccine, influenced in part by a patient’s specific diagnosis. Furthermore, vaccine acceptance increased mirroring the curve of COVID-19 cases and deaths in the country. This should be taken into consideration when updating recommendations for clinical practice.
Similar content being viewed by others
Introduction
The COVID-19 pandemic caused by the SARS-CoV-2 virus has mobilized efforts at an unprecedented scale to develop vaccines and to implement vaccination programs in record time [1,2,3]. In Mexico, the COVID-19 vaccine allocation strategy has prioritized those most vulnerable to infection, including older adults, persons with associated comorbidities, and immunosuppressed individuals such as patients with rheumatic and musculoskeletal diseases (RMDs). These patients are made vulnerable to infection both by their underlying conditions and, in some cases, by immunomodulatory treatments [3].
The impact of COVID-19 on patients with RMDs is currently being assessed by the international rheumatology community through the COVID-19 Global Rheumatology Alliance [4]. Similarly, expert groups such as the European Alliance of Associations for Rheumatology (EULAR) have published recommendations for the vaccination of patients with RMDs. Generally, vaccination is encouraged for all patients regardless of their rheumatic disease or type of vaccine. There are no general recommendations to change or pause medications when patients receive a vaccine, and modifications should only be considered on a case-by-case basis [5].
In 2019, the World Health Organization declared vaccine hesitancy one of the top 10 threats to global health [6]. This phenomenon is defined as a “delay in acceptance or refusal of vaccination despite availability of vaccination services” [7]. In Mexico, different types and brands of COVID-19 vaccines have been made available throughout the pandemic depending on availability given global vaccine nationalism. Figure 1 illustrates the timeline of the vaccination campaign in Mexico, starting on December 2020, distributed by public health entities. The campaign prioritized frontline health workers and education staff, followed by older adults (60 and over). Afterwards, vaccines were made available in stages, according to descending age ranges and existing vulnerabilities (i.e., pregnancy, comorbidities). The market share was occupied as follows: PfizerBioNTech (December 2020), CanSinoBio (January 2021), Sputnik V (February 2021), AstraZeneca (March 2021), Sinovac (April 2022), Johnson & Johnson (June 2021), Moderna (August 2021), and Abdala (November 2022). This heterogeneity in vaccines has caused some uncertainty about the efficacy and safety of specific vaccines among the general population, potentially generating vaccine hesitancy.
To evaluate the phenomenon of COVID-19 vaccine hesitancy, acceptance, or refusal among the general population in Mexico, researchers at the National Institute of Public Health conducted the nationwide study Ensanut 2020 Covid-19 from August to November 2020, prior to the start of the national vaccination campaign. The study included 10,796 participants: 62.3% reported potential acceptance of the vaccines, 28.2% reported refusal, and 9.5% reported a state of hesitancy [8]. Similar surveys have been conducted in other countries, finding that acceptance rates of the COVID-19 vaccination ranged from 54.9% to 90% in the general population globally [9]. Further studies tried to describe this phenomenon among patients with RMDs specifically. In Mexico, for example, a recent study reported 72.2% acceptance among patients [10]. Meanwhile, vaccine acceptance is 85% for patients in the United States and Italy [11, 12], 65% in Australia [13], 63% in Arab countries [14], 54% in Western India [15] 29.2% in Turkey [16].
In this study, the main objective was to analyze the relationship between vaccine attitudes (acceptance or hesitancy) and the nationwide number of COVID-19 cases and deaths as a proxy for risk perception. Additionally, we aimed to analyze differences in acceptance between patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) as the two most frequent rheumatic diagnoses, and between patients with RMDs from different hospitals in the country.
Materials and methods
An ecological study was conducted as a secondary analysis of a cross-sectional study integrated by aggregate measures of COVID-19 vaccine acceptance among patients with RMDs, as well as the inference of the contextual effect of the national COVID-19 epidemic curve [17].
Population
Patients with RMDs were included from four patient care centers in Mexico: Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” (INCMNSZ) and Hospital General de México “Dr. Eduardo Liceaga” (HGM) in Central Mexico, and Hospital Universitario “Dr. José Eleuterio Gonzalez” (HU) and Hospital General “Dr. Salvador Zubirán” (HGCh) in Northern Mexico.
We invited patients with RMDs (rheumatoid arthritis, systemic lupus erythematosus, osteoarthritis, primary Sjögren syndrome, systemic sclerosis, etc.) that were at least 18 years old, and that attended routine follow-up visits at one of the participating hospitals between March and September 2021. Patients gave their informed consent prior to the assessments. Rheumatologists completed complementary questionnaires for each patient, regarding their perception of disease activity control and treatment. Patients with RMDs completed questionnaires related to sociodemographic information and a validated instrument of COVID-19 vaccine hesitancy (COVID-19VHQ). Further details about the study population can be found in the primary study [10, 18].
Data collection
The primary information was captured digitally directly on the LimeSurvey® platform or collected on paper and then transcribed and uploaded. The information was analyzed with the SSEM program [19].
Statistical analysis
The primary study variables included: sociodemographic variables (age, sex, schooling, occupation, marital status, healthcare coverage), diagnosis, treatment, rheumatic disease activity control, comorbidities, COVID-19 vaccine acceptance, number of COVID-19 vaccine doses received, and risk perception of COVID-19 infection.
COVID-19 vaccine acceptance was measured using the “COVID-19 Vaccine Hesitancy Among Patients with Rheumatic Diseases Questionnaire” (C19VHQ). C19VHQ was adapted for and validated in Mexican patients with RMDs [10], from a scale designed for the UK general population [20]. C19VHQ consists of a seven-item scale that evaluates: vaccine acceptance, desire to receive the vaccine, attitude toward the vaccine, willingness to vaccinate, encouragement for others to vaccinate, and impact of receiving the vaccine. Response options were coded from 1 to 5 using a Likert scale [21]: 1 denoted high acceptance, 2 acceptance, 3 neutral position, 4 hesitancy and 5 high hesitancy. A “Don’t know” option was also provided, which was excluded from scoring (score of 0).
The national number of COVID-19 cases and deaths for the same period of the study was obtained from the Mexican Secretariat of Health and was also included in the analysis [22].
Descriptive statistics were used to analyze each of the seven items in the questionnaire, by date, by hospital, and by rheumatic disease. Comparisons between hospitals were made using the Chi-squared test for categorical variables and the Kruskal–Wallis test for continuous variables.
The seven items were grouped into a global Likert scale to measure vaccine acceptance. Patients were classified as accepting when they endorsed four or more of the seven items with a clear positive response (rating of 1–2).
Two types of heatmaps were generated: (a) to show patient-reported number of COVID-19 vaccine doses, and (b) to show national number of COVID-19 cases and deaths during the questionnaire administration. The correlation of these datapoints with C19VHQ responses was evaluated in 17-day intervals.
The analysis was done for all diagnoses, and for RA and SLE specifically. Incomplete questionnaires were excluded from the analysis.
Results
A total of 1500 patients with RMDs participated in the survey; 1,366 were selected according to the criteria for completeness. Of these, 85.13% (1,169) were women, with a mean age of 47.87 years (SD 14.14), and a mean of 11.93 years of schooling (SD 4.52). The most frequent diagnoses were RA (42.85%, 559) and SLE (27.08%, 393), and the most frequent comorbidity was High Blood Pressure (HBP) in 18.20% (275) of patients.
Table 1 shows the comparison between hospitals of sociodemographic, clinical and vaccine-related variables. With the exception of sex and glucocorticoid (≤ 10 mg) use, all evaluated variables were statistically significant (p < 0.001, highlighted in bold) between hospitals.
Comparisons between patients according to how many COVID-19 doses they had received are shown in Table 2. Statistically significant differences (p < 0.001, highlighted in bold) were found for the following variables: hospital, age, HBP as a comorbidity, and RA or SLE diagnosis.
In Table 3, we describe the responses to C19VHQ between hospitals, as well as risk perception of COVID-19 infection. Items 2, 3, 6 and 7 of the questionnaire were the only ones with statistically significant differences (p < 0.001, highlighted in bold). Risk perception was low for patients from any hospital, when patients were asked if they believed they would catch a COVID-19 infection.
Considering the number of patients who expressed a positive response (scores of 1–2) in at least four of the seven items in C19VHQ, we determined that 94% of patients with RMDs accept the COVID-19 vaccine. It’s worth noting that 90% of patients who had had the opportunity to receive a vaccine at the time of their participation in the study, had chosen to get vaccinated.
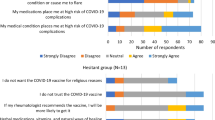
Figure 2 depicts the responses of three groups: total population of patients with RMDs (2A), and patients with RA (2B) and SLE (2C) specifically. For visualization purposes, responses are graphed in 17-day intervals. Graphs illustrate the global Likert scale generated to measure vaccine acceptance (high acceptance, acceptance, neutral, hesitancy, and high hesitancy). Figure 2D depicts heatmaps showing the average number of COVID-19 cases (column 1) and deaths (column 2) in Mexico during the study. The peak of the national epidemic curve (higher numbers of cases and deaths) occurred from end of July to September, as highlighted with bolded text.
COVID-19 vaccine acceptance in patients with rheumatic diseases, and with rheumatoid arthritis and systemic lupus erythematosus specifically. Diverging bar charts illustrate the global Likert scale used to measure vaccine acceptance (left of zero), and hesitancy (right of zero). Heatmaps in A–C show the number of vaccinated patients with one or two doses at each 17-day interval. Heatmap D shows national epidemiological trends by number of COVID-19 cases and deaths in Mexico. Bolded dates denote the pandemic peak
Irrespective of the diagnosis (2A), we observed that vaccine acceptance remained constant throughout the study, regardless of the course of the pandemic. However, patterns of acceptance differed for specific diagnoses. The peak of the national epidemic curve coincided with an increase in the endorsement of hesitancy responses by patients with RA, who had higher acceptance on previous dates (2B). In contrast, patients with SLE were initially more hesitant and became more accepting as the national epidemic curve peaked (2C).
Heatmaps in 2A-2C depict the number of participants who had received one or two COVID-19 vaccine doses in each period. The largest number of vaccinated patients coincided with the national epidemic curve peak (July to September) for total patients with RMDs and for specific diagnoses, despite the different patterns of vaccine acceptance described above.
Discussion
In this study, we found a higher vaccine acceptance rate among Mexican patients with RMDs using a Likert scale (94%), compared to previous reports for both the global and national general population and patients with RMDs in other countries [11,12,13,14,15,16]. High acceptance coincided with the high number of participants who had chosen to receive one or two doses of a COVID-19 vaccine at the time of the study. Contrastingly, other studies have reported lower numbers of vaccinated RMD patients (61.8%), coinciding with higher hesitancy measured by similar questionnaires [23].
This phenomenon of acceptance could be influenced by different factors. First, the “vaccination culture” and confidence toward vaccines that have been historically promoted to the general population by the health sector in Mexico [24]. Second, for patients with RMDs specifically, during the initial phases of the vaccination campaigns, both patients and rheumatologists made a strategic effort to spread information and recommendations about receiving the COVID-19 vaccine [25], including through digital communication and telemedicine [26], taking into consideration previous guidance about the annual influenza vaccine established by rheumatology associations and colleges in the country [27]. The effect of these cultural and historical factors on vaccine attitudes should be further studied in future. The higher acceptance rates observed in patients with SDRs in Mexico compared to other countries could also be explained by the different timing at which these studies were conducted, considering the preliminary data about vaccine efficacy and safety available at said moments.
Discrepancies in vaccination rates can be partly explained by availability according to the different stages of the national vaccination campaign (as shown in Fig. 1) when the questionnaire was administered at each institution. For example, patients with RMDs from INCMNSZ were invited to participate in this study when the campaign was just starting with older adults. Importantly, most of the participating patients with RMDs were in their 40 s, and therefore were not eligible for vaccination at the beginning of the campaign.
It must be noted that complete refusal of the vaccine was low in the study population, and the majority of those who expressed hesitancy did not consistently endorse the strongest negative responses throughout the C19VHQ. These observations of the high variability of responses demonstrate that selecting a cut-off point to binarize the population between “accepting” and “refusing” can be misleading. Alternatively, a global Likert scale like the one developed in this study, which considers the different categories, can more accurately represent the data without over-segmenting the population or overestimating vaccine refusal.
Interestingly, though we found a constant pattern of high vaccine acceptance when evaluating all patients together, differences can be identified when disaggregating diagnoses. Patients with SLE reported more hesitancy at the beginning stages of the study, coinciding with the start of the national vaccination campaign, but became more accepting in times when the number of cases and deaths were highest. In contrast, patients with RA displayed an opposite trend of acceptance, with the pandemic peak leading to more hesitancy. A possible explanation of these discrepancies could be the different nature of the conditions and the patients’ understanding of them. For example, patients with SLE have been highlighted as a group with reservations and caution, possibly due to general doubts about vaccine safety considering their immunocompromised state and the involvement of the immune system in vaccination [28]. These doubts may then become less pressing when risk perception is higher, leading to a change in attitude toward the need for a vaccine. Acceptance regardless of fear is consistent with other studies, which have reported a decrease in hesitancy from 2021 to 2022 despite increase in concerns over long-term safety of vaccines [29].
One of the major strengths of this study is that it was conducted using a validated instrument in person during the medical consultation, unlike studies in other countries where vaccine acceptance was evaluated through open virtual/digital questionnaires in social networks or online survey platforms [11, 13, 30, 31], or which have focused solely on physician-reported registries [32]. This has allowed us to describe the phenomenon of acceptance more precisely and accurately in Mexican patients with RMDs, taking into consideration information reported by both the patients and their rheumatologists [33].
One potential limitation is the discrepancy between when the study was conducted in each participating institution, and the development of the national vaccination campaign. Furthermore, given the cross-sectional nature of this study, we are unable to establish a causal association between the measured variables.
In conclusion, patients’ attitudes relating to vaccine acceptance or hesitancy vary according to the national COVID-19 epidemic curve, represented by number of deaths and cases. Though patients with RMDs as a group show high levels of vaccine acceptance, different attitudes are identified when comparing diagnoses, possibly due to the specific nature of each condition, the patients’ understanding of their disease and the risk perception of each particular group. Finally, this study shows high acceptance of an emergency use vaccine among patients with RMDs, which is consistent with historical acceptance of fully approved vaccines.
Additionally, this study has clinical relevance and actionability since the results can be used to update the Mexican guides and recommendations for the management of rheumatic diseases. Similar updates have been previously made by the EULAR [5, 33], which to our knowledge are the only existing guides which include patient participation and preference in their elaboration. Updated guides should consider the fluctuating nature of the pandemic, the appearance of endemic variants, and the need for boosters for the general population and for vulnerable patients. Importantly, the discrepancies shown between diagnoses highlights the importance of specific guidance for different patient populations, taking into consideration their unique concerns and needs, and how their understanding of their conditions affects patients’ views of vaccines in general. Improved guides that include the patients’ perspectives will be helpful for the rheumatologists’ daily clinical practice.
Data availability
The data that support the findings of this study are accessible through the corresponding author, registered at Open Science Framework at http://doi.org/10.17605/OSF.IO/UAZ2P. (Accessed on 30 May 2021).
References
González-Melado FJ, Di Pietro ML (2020) The vaccine against COVID-19 and institutional trust. Enfermedades Infecc Microbiol Clin Engl Ed 39:510–515. https://doi.org/10.1016/j.eimc.2020.08.001
Koch T, Fathi A, Addo MM (2021) The COVID-19 vaccine landscape. Adv Exp Med Biol 1318:549–573. https://doi.org/10.1007/978-3-030-63761-3_31
Xu C, Yi Z, Cai R, Chen R, Thong BY-H, Mu R (2021) Clinical outcomes of COVID-19 in patients with rheumatic diseases: a systematic review and meta-analysis of global data. Autoimmun Rev 20:102778. https://doi.org/10.1016/j.autrev.2021
Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, Mateus EF, Richez C, Santos MJ, Schmajuk G, Scirè CA et al (2021) Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 80:930–942. https://doi.org/10.1136/annrheumdis-2020-219498
Bijlsma JW, EULAR COVID-19 Task Force (2022) EULAR 2021 updated viewpoints on SARS-CoV-2 vaccination in patients with RMDs: a guidance to answer patients’ questions. Ann Rheum Dis 81:786–788. https://doi.org/10.1136/annrheumdis-2021-221965
PAHO/WHO (2019) Ten threats to global health in 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed 21 June 2022
MacDonald NE, SAGE Working Group on Vaccine Hesitancy (2015) Vaccine hesitancy: definition, scope and determinants. Vaccine 33:4161–4164. https://doi.org/10.1016/j.vaccine.2015.04.036
Carnalla M, Basto-Abreu A, Stern D, Bautista-Arredondo S, Shamah-Levy T, Alpuche-Aranda CM, Rivera-Dommarco J, Barrientos-Gutiérrez T (2021) Acceptance, refusal and hesitancy of Covid-19 vaccination in Mexico: Ensanut 2020 Covid-19. Salud Publica Mex 63:598–606. https://doi.org/10.21149/12696
Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, Kimball S, El-Mohandes A (2021) A global survey of potential acceptance of a COVID-19 vaccine. Nat Med 27:225–228. https://doi.org/10.1038/s41591-020-1124-9
Guaracha-Basañez GA, Contreras-Yáñez I, Álvarez-Hernández E, Reyes-Cordero G, Flores-Alvarado DE, González-Chávez SA, Galarza-Delgado DÁ, Martínez-Leyva PR, Moctezuma-Ríos JF, García-García C, Medrano-Ramírez G, Gastelum-Strozzi A, Pacheco-Tena C, Peláez-Ballestas I, Pascual-Ramos V (2022) Factors associated to COVID-19 vaccine acceptance in Mexican patients with rheumatic diseases: A cross-sectional and multicenter study. Hum Vaccines Immunother 18:2049131. https://doi.org/10.1080/21645515.2022.2049131
Tedeschi SK, Ellrodt J, Stratton J, Santacroce L, Chandler PD, Gravallese EM, Solomon DH (2022) Acceptability of vaccines against preventable infections including Coronavirus Disease 2019 among patients with Rheumatic Disease. ACR Open Rheumatol 4:3–7. https://doi.org/10.1002/acr2.11351
Priori R, Pellegrino G, Colafrancesco S, Alessandri C, Ceccarelli F, Di Franco M, Riccieri V, Scrivo R, SiliScavalli A, Spinelli FR, Conti F (2021) SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis 80:953–954. https://doi.org/10.1136/annrheumdis-2021-220059
Ko T, Dendle C, Woolley I, Morand E, Antony A (2021) SARS-COV-2 vaccine acceptance in patients with rheumatic diseases: a cross-sectional study. Hum Vaccines Immunother 17:4048–4056. https://doi.org/10.1080/21645515.2021.1958611
Gaur P, Agrawat H, Shukla A (2021) COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview-based survey. Rheumatol Int 41:1601–1605. https://doi.org/10.1007/s00296-021-04938-9
El Kibbi L, Metawee M, Hmamouchi I, Abdulateef N, Halabi H, Eissa M, El Rakawi M, Masri B, Abutiban F, Hamdi W, Adnan A, Najm AA, Felten R, Arnaud L, Ziadé N (2022) Acceptability of the COVID-19 vaccine among patients with chronic rheumatic diseases and health-care professionals: a cross-sectional study in 19 Arab countries. Lancet Rheumatol 4:e160–e163. https://doi.org/10.1016/S2665-9913(21)00368-4
Yurttas B, Poyraz BC, Sut N, Ozdede A, Oztas M, Uğurlu S, Tabak F, Hamuryudan V, Seyahi E (2021) Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web-based survey. Rheumatol Int 41:1105–1114. https://doi.org/10.1007/s00296-021-04841-3
Morgenstern H (1995) Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health 16:61–81. https://doi.org/10.1146/annurev.pu.16.050195.000425
Guaracha-Basáñez G, Contreras-Yáñez I, Álvarez-Hernández E, Román-Montes CM, Meza-López Y, Olguín G, Morales-Graciano MJ, Valverde-Hernández SS, Peláez-Ballestas I, Pascual-Ramos V (2021) COVID-19 vaccine hesitancy among Mexican outpatients with rheumatic diseases. Hum Vaccines Immunother. 17:5038–5047. https://doi.org/10.1080/21645515.2021.2003649
Gastelum-Strozzi A, Peláez-Ballestas I (2021) BIOCOMLAB—SSEM. http://www.biocomlab.com/apps/SSEM.html. Accessed 21 June 2022.
Freeman D, Loe BS, Chadwick A, Vaccari C, Waite F, Rosebrock L, Jenner L, Petit A, Lewandowsky S, Vanderslott S, Innocenti S, Larkin M, Giubilini A, Yu LM, McShane H, Pollard AJ, Lambe S (2020) COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol Med 11:1–15. https://doi.org/10.1017/S0033291720005188
Heiberger Richard M, Naomi Robbins B (2014) Design of diverging stacked bar charts for likert scales and other applications. J Stat Softw 57:1–32. https://doi.org/10.18637/jss.v057.i05
Secretaría de Salud (2022) Datos Abiertos Dirección General de Epidemiología. https://www.gob.mx/salud/documentos/datos-abiertos-152127. Accessed 30 September 2021.
Mohanasundaram K, Santhanam S, Natarajan R, Murugesan H, Nambi T, Chilikuri B, Nallasivan S (2022) Covid-19 vaccination in autoimmune rheumatic diseases: a multi-center survey from southern India. Int J Rheum Dis 25:1046–1052. https://doi.org/10.1111/1756-185X.14378
Santos JI (2014) La vacunación en México en el marco de las “décadas de las vacunas”: logros y desafíos. Gac Médica México. 150:180–188
Singh JA, Richards JS, Chang E, Joseph A, Ng B (2021) Management of rheumatic diseases during the COVID-19 pandemic: a national veterans affairs survey of rheumatologists. Arthritis Care Res 73:998–1003. https://doi.org/10.1002/acr.24487
Hammam N, Tharwat S, Shereef RRE, Elsaman AM, Khalil NM, Fathi HM, Salem MN, El-Saadany HM, Samy N, El-Bahnasawy AS, Abdel-Fattah YH, Amer MA, ElShebini E, El-Shanawany AT, El-Hammady DH, Noor RA, ElKhalifa M, Ismail F, Fawzy RM, El-Najjar AR, Selim ZI, Abaza NM (2021) Rheumatology university faculty opinion on coronavirus disease-19 (COVID-19) vaccines: the vaXurvey study from Egypt. Rheumatol Int 41:1607–1616. https://doi.org/10.1007/s00296-021-04941-0
Cardiel MH, Díaz-Borjón A, del Mercado Espinosa MV, Gámez-Nava JI, BarileFabris LA, Tena CP, Silveira Torre LH, Ramos VP, Goycochea Robles MV, Aguilar Arreola JE, Díaz VG, Nemegyei JÁ, del Carmen González-López L et al (2014) Actualización de la Guía Mexicana para el Tratamiento Farmacológico de la Artritis Reumatoide del Colegio Mexicano de Reumatología. Reumatol Clínica 10:227–240. https://doi.org/10.1016/j.reuma.2013.10.006
Colmenares-Roa T, ManriquedeLara A, Pascual Ramos V, MOCTEZUMA RIOS J, Contreras-Ibañez I, Alvarez-Hernandez E, Guaracha Basañez G, Meza-LópezyOlguin G, Pelaez-Ballestas I (2022) Sociocultural and Moral Factors Influencing the Decision to Vaccinate Among Rheumatic Patients: A Qualitative Study [abstract]. Arthritis Rheumatol 74 (suppl 9). Accessed 9 April 2023.
Sen P, Naveen R, Houshmand N, Moghadam Kia S, Joshi M, Saha S, Jagtap K, Agarwal V, Nune A, Nikiphorou E, Tan AL, Shinjo SK, Ziade N, Velikova T, Milchert M, Parodis I, Edgar Gracia-Ramos A, Cavagna L, Kuwana M, Knitza J, Makol A, Patel A, Pauling JD, Wincup C, Barman B, Zamora Tehozol EA, Rojas Serrano J, La Torre IG, Colunga-Pedraza IJ, Merayo-Chalico J, Chibuzo OC, Katchamart W et al (2023) Vaccine hesitancy decreases, long term concerns remain in myositis, rheumatic disease patients: a comparative analysis of the COVAD surveys. Rheumatology 3:kead057. https://doi.org/10.1093/rheumatology/kead057
Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, Costecalde C, Pijnenburg L, Fort J, Chatelus E, Sordet C, Javier RM, Gottenberg JE, Sibilia J, Fuentes-Silva Y, Arnaud L (2021) Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol 3:e243–e245. https://doi.org/10.1016/S2665-9913(21)00039-4
Fathi HM, Gazzar IIE, Elazeem MIA, AboulKheir E, Gamal NM, Ismail F, Shereef RRE, Tharwat S, Elwan S, Samy N, Baki NA, Elsaid NY, El-Bahnasawy AS, Moshrif A, Fattah YA, Amer MA, Ibrahim ME, Khalil NM, El-Dessouki S, Abaza N, El-Shanawany AT, Mohamed EF, El-Ghobashy N, Ayoub N, Hammam O, Fawzy S, Sayed S, Gheita TA, Hammam N (2022) Rheumatologists’ knowledge and perception of COVID-19 and related vaccines: the vaXurvey2 online survey. Rheumatol Int 42:989–998. https://doi.org/10.1007/s00296-022-05130-3
Machado PM, Lawson-Tovey S, Strangfeld A, Mateus EF, Hyrich KL, Gossec L, Carmona L, Rodrigues A, Raffeiner B, Duarte C, Hachulla E, Veillard E, Strakova E, Burmester GR, Yardımcı GK, Gomez-Puerta JA, Zepa J, Kearsley-Fleet L, Trefond L, Cunha M, Mosca M, Cornalba M, Soubrier M, Roux N et al (2022) Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis 81:695–709. https://doi.org/10.1136/annrheumdis-2021-221490
Landewé RBM, Kroon FPB, Alunno A, Najm A, Bijlsma JW, Burmester GR, Caporali R, Combe B, Conway R, Curtis JR, Elkayam O, Gossec L, Heijstek MW, Haupt L, Iagnocco A, Isaacs JD, Juhász IÁ, Makri S, Mariette X et al (2022) EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis 81:1628–1639. https://doi.org/10.1136/annrheumdis-2021-222006
Acknowledgements
We extend our gratitude to Julio Casasola Vargas, Graciela Meza López y Olguin and Gabriela Huerta Sil for their valuable participation. To the project PAPIIT IA103922 for the server resources provided to acquiree the data.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Study conception and design: AG-S, VP-R, AMdL, IP-B, GCR-C. Acquisition of data: AG-S, DEF-A, VP-R, EA-H, CFP-T, GAG-B, CGG, SAG-C, JFM-R, AMdL, JAE-V, IC-Y, DAG-D, JV-M, IP-B, GCR-C. Analysis and interpretation of data: AG-S, DEF-A, VP-R, EA-H, CFP-T, GAG-B, CGG, SAG-C, JFM-R, AMdL, JAE-V, IC-Y, DAG-D, JV-M, IP-B, GCR-C. All authors were involved in drafting the article or revising it critically for important intellectual content. All authors declare that they have approved the final version of this manuscript and take full responsibility for the integrity and accuracy of all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board
This study was approved by the Research Ethics Committees in each of the participating institutions: INCMNSZ IRE-3467, HGM DI/21/404-D/03/21, HU RE21-00005, and HGCh CI-033-21. The dates of approval were 16 February 2021 (INCMNSZ), 17 May 2021 (HGM), 02 July 2022 (HU) and 16 August 2021 (HGCh).
Informed consent
Informed consent was obtained from all subjects involved in the study. All participants signed informed consent forms and data privacy policies, given the digital nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gastelum-Strozzi, A., Flores-Alvarado, D.E., Pascual-Ramos, V. et al. The COVID-19 epidemic curve and vaccine acceptance among patients with rheumatic diseases: an ecological study. Rheumatol Int 43, 1253–1264 (2023). https://doi.org/10.1007/s00296-023-05334-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-023-05334-1