Abstract
Patients with rheumatoid arthritis (RA) are at an increased risk of cardiovascular disease and vascular morbidity. The association between peripheral arterial disease (PAD) and RA has not been previously investigated within the scope of a review. Conjoined disease manifestations may impact patient well-being, perpetuating increased mortality and quality of life deficits. To investigate the association between RA and PAD, along with RA and the ankle-brachial pressure index (ABPI), the impact of disease concomitance on health-related quality of life (HRQOL) and functional capacity (FC) was also investigated. Individual study appraisal was completed using the Crowe Critical Appraisal Tool (CCAT). A level of evidence analysis was conducted using the American Society of Plastic Surgeons (ASPS) Evidence Rating Scale for Prognostic/Risk Studies. AMED®, CINAHL®, Health Source: Nursing/Academic Edition, MEDLINE®, AHFS®, Scopus, Web of Science, Cochrane Library and Google scholar. Ten studies produced a CCAT rating of ≥ 30 (75%) and were deemed high quality, while a single study demonstrated a score of 26 (65%) suggesting moderate quality. A grade “II” levels of evidence was awarded to positive association between RA and PAD. A gradation of “I” was awarded to the association between ABPI and RA. The impact of concomitant manifestations on HRQOL and FC did not qualify for a level of evidence analysis. The systematic inflammatory nature of RA likely contributes to the increased incidence of PAD within the population. Further investigations are required to ascertain the impact of conjoined disease manifestations on HRQOL and FC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an autoimmune inflammatory disorder of the synovial tissue [1]. It is associated with increases in patient morbidity and mortality [2, 3]. RA affects 1% of the general population, with early intervention significantly mediating a favorable prognosis [4]. Ongoing medical innovations in biologic agents and disease-modifying drugs (DMARDs) have facilitated improved clinical outcomes [5]. In recent decades, RA has been denoted as an independent cardiovascular disease (CVD) risk factor [5]. This risk is perpetuated by the use of steroid-derived medication in the management of acute RA exacerbations, atherosclerotic changes inherent to the systemic inflammatory nature of RA, and the prevalence of vascular morbidities, including peripheral arterial disease (PAD) [2].
The underlying atherosclerotic mechanisms responsible for CVD within RA have also been suggested to impact the incidence of PAD [6]. Despite reported decreases in CVD risk secondary to the implementation of DMARDs and biologics, their impact on symptomatic or asymptomatic PAD development in the RA population is unknown [7, 8]. PAD is often associated with medium- and large-sized blood vessels of the lower limb, commonly the infra-inguinal or aortoiliac arteries [9]. Diagnosis of PAD is established through the ankle-brachial systolic pressure index (ABPI), with a score of ≤ 0.90 typically acting as the diagnostic threshold [10]. Secondary to PAD, patients may present with intermittent claudication (IC), manifesting as pain on movement of the lower limbs; or critical limb ischemia (ABPI ≤ 0.3) marked by rest pains, ulcerations and gangrene development [9]. In 2% of cases, amputation becomes a necessary clinical consideration [11]. Some patients describe the incidence of IC as an inconvenience, while others denote significant lifestyle detriments, social isolation and unemployment [11]. Management of symptoms thus becomes paramount in mitigating detriments to patient functional capacity (FC) and improving health-related quality of life (HRQOL).
As an independent disease entity, PAD correlates with premature mortality [12]. It also acts to perpetuate cardiovascular and cerebrovascular events at a two- to six-fold increased risk [12, 13]. The former remains valid irrespective of an asymptomatic or symptomatic disease manifestation [14]. McDermott et al. [15] concluded that individuals diagnosed with the asymptomatic variant of PAD present with poorer quality of life (QOL), FC and adverse calf muscle characteristics when compared to non-PAD controls. Treatment is carried out to reduce the risk of CVD events and manage lower extremity symptoms including activity limitations they impose [11]. The potential association between RA and PAD has not previously been formally investigated by means of a systematic review.
Makismovic et al. [16] suggested that patients with PAD demonstrate significantly lower physical functioning, social functioning and mental health when compared to healthy controls. The former deficits are magnified in patients with PAD presenting with IC or gangrene formation. Similarly, Tothova et al. [17] demonstrated a statistically significant difference between healthy individuals and patients with RA concerning physical health, level of independence, spirituality/religion/personal belief and the environmental domains pertinent to the WHOQOL-100 questionnaire. Considering FC alone, the literature correlates low ABPI scores negatively with 6-min walk times [18]. This functional decline is more pronounced in females [18]. Likewise, disease activity of patients with RA tends to negatively correlate with FC. Patients with RA in a state of remission as defined by ACR/EULAR criteria pose similar FC to healthy age–sex-matched controls, while patients presenting with elevated disease activity tend to demonstrate greater functional limitations and diminished HRQOL [19]. Despite independent RA and PAD manifestations’ impact on FC and HRQOL, reviews discussing their concomitant impact on the noted outcomes are largely absent.
RA and PAD manifestations may have a deleterious influence on a patient’s activity levels and concomitantly impact patient morbidity and mortality. Both RA and PAD are associated with suboptimal exercise levels, despite the established benefits exercise elicits to HRQOL, FC and complication risk [15, 20]. In states of elevated RA disease activity, severe disease manifestations, and multi-morbid presentations, symptomatic PAD progression may become accentuated. Moreover, symptomatic PAD progression may act to exacerbate the concomitant presentation’s impact on HRQOL and FC. In RA and PAD manifestations, IC presents a prevalence of 29% [15]. The incidence of symptomatic PAD in RA may cyclically act to further reduce activity levels, manifesting in further HRQOL and FC detriments. Understanding the association between the two disorders may facilitate early detection of PAD in patients with RA, enabling early intervention and prevention of subsequent complications. Currently, SIGN [21] and NICE [22] RA guidelines do not directly take into consideration the vascular detriments that RA poses. Exercise emphasis in these publications as it pertains to vascular health is largely absent.
This systematic review attempted to (1a) investigate the association between RA and PAD. As a subsect of the former, (1b) The association between RA and ABPI scores was examined. This review further aimed to (2) denote the significance of RA and PAD concomitance on HRQOL and FC.
Methods
Search strategy
The following electronic databases were examined: AMED® (The Allied and Complementary Medicine Database), CINAHL® (Cumulative Index of Nursing and Allied Health Literature), Health Source: Nursing/Academic Edition, Web of Science (WoS), Scopus, MEDLINE®, AHFS®—Consumer Medication Information and the Cochrane Library. Google Scholar was the only search engine employed to supplement the examined databases. These databases were considered appropriate due to their inclusion of literature pertinent to the biomedical sciences, arthritis and rheumatology, and the global allied health professional and medical disciplines. The search strategy comprised two searches implemented to address the primary and secondary aims of this review. The search strategy was completed between March 3, 2019 and October 1, 2022. In addition, reference lists of pertinent articles were investigated for relevant literature.
Publication dates were set at the maximum possible afforded by the relative databases. Table 1 provides an overview of the search terms, MeSh terms and Boolean operators relevant to the first and second literature searches conducted. Abbreviations and truncations of search terms were used as appropriate to ensure comprehensiveness.
Study selection
Cohort, case–control and cross-sectional studies were included. Randomized control trials (RCT) were considered as well if relevant prevalence or association data could be derived. Hospital audits and case reviews were omitted given their inability to garner sufficient data suggestive of a potential association [23]. Other inclusion criteria included the following:
-
A clinically established RA diagnosis garnered by a rheumatologist using 1987 ACR or 2010 ACR/EULAR criteria.
-
A clinically justified PAD diagnosis. PAD is typically considered with an ABPI ≤ 0.90 [9]. Diagnostic interpretations of ≤ 1.0 were still included if appropriate justification was provided. An ABPI of ≤ 1.0 is considered indicative of atherosclerotic changes and has been previously used as a PAD diagnostic threshold [24].
-
Studies discussing an association between RA and PAD.
-
Studies investigating the association between RA and ABPI independent of a PAD diagnosis.
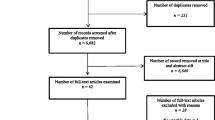
Research investigating HRQOL, FC and the impact of IC on the former in conjoined disease manifestation is scarce. The noted outcome measures were not implemented as inclusion criteria due to this. The retrieval and filtering process is summarized in Fig. 1.
Risk of bias assessment
The methodological rigor and quality of included studies were appraised using the Crowe Critical Appraisal Tool (CCAT) (Table 2) [24]. This tool was developed to encompass most study designs, including the ones pertinent to this review [25]. It consists of eight groupings: preliminaries, introduction, design, sampling, data collection, ethical matters, results and discussion [25]. Each grouping contains 22 items, carrying 98 item descriptors [25]. Groupings are individually scored between 0 and 5, determined by a piece of literature’s perceived adherence to the group items listed. A score of 0 denotes no evidence of item use, while integers 1, 2, 3, 4 and 5 suggest growing tiers of item use respectively (Table 2) [26]. A total score is then derived. A CCAT score of ≥ 30 (75%) denotes a high-quality design, a score between 22 (55%) and 26 (65%) suggests a moderate-quality design, and a score of ≤ 22 (55%) is deemed poor. This tool is accompanied by a comprehensive user guide in ensuring the attainment of valid scores when one conducts an appraisal [26]. Both the complete checklist and guide can be accessed through Crowe [27]. Crowe and Sheppard [27] demonstrated that the tool presents a good degree of construct validity when compared to the Physiotherapy Evidence Database (PEDro) scale, Cho and Bero scale, Single-Case Experimental Design (SCED) scale, and the Assessment of Multiple Systematic Reviews (AMSTAR) scale. A second study suggested the CCAT generates significant absolute agreement ICCs in the individual research designs tested [28]. Sufficient G scores were demonstrated by the CCAT as well, accentuating its reliability in appraising multiple study designs. Crow, Sheppard and Campbell [25] verified the CCAT’s capacity to diminish the influence of researcher experience on the validity and reliability of scoring.
Results
Figure 1 outlines the strategy used in acquiring relevant studies. The search strategy resulted in 324 articles. 311 remained following duplicate removal. Following the process of title and abstract screening, 19 studies were eligible and included for full-text analysis. The most common reason for exclusion involved investigations of RA, PAD or ABPI independently, rather than conjoined manifestations or impact on relevant outcomes. A total of eleven studies met the inclusion criteria and were deemed appropriate for this review. Tables 3, 4 summarize the relevant literature found.
Six studies were of a cross-sectional design [24, 29,30,31,32,33]. Four studies involved a cohort design, three of which were of a retrospective nature [34,35,36,37] and one implemented a prospective design [38]. Eight studies discussed the association between PAD and RA [24, 30, 31, 33,34,35,36,37] while the remaining three queried an association between ABPI and RA [29, 32, 38]. Three cross-sectional studies investigated concomitant disease impact on HRQOL and FC [24, 33, 39]. Outcome measures employed by relevant studies to ascertain HRQOL and FC involved the Modified Health Assessment Questionnaire (M-HAQ), the Health Assessment Questionnaire (HAQ) and the 6-m walk test [24, 29, 33]. None of the studies investigated the incidence of symptomatic IC in conjoined RA and PAD manifestations or discussed its role in perpetuating HRQOL and FC detriments.
Table 5 outlines the results of the CCAP study quality assessment. All studies pertinent to this review presented with a total % CCAP score of ≥ 60%. Bacani et al. [34], Chuang et al. [36], Grech et al. [31] and Turesson et al. [37] produced the highest totals, 90%, 93%, 90% and 92.5% respectively. The majority of studies produced a group item score of ≥ 3, adhering to most sub-item CCAT classifications [26]. Some articles neglected to discuss potential bias involved in their design [24, 29, 31, 32, 36]. The impact of potential selection bias, detection bias and categorical bias on result generalizability was discussed in five articles [30, 34, 35, 37, 38]. Discussion pertinent to conflict of interest and sources of funding was omitted by a single article [29].
Evidence pertaining to the positive association between RA and PAD produced a “II” level of evidence grading, due to the retrospective cohort studies found. The positive association between ABPI and RA was awarded a level of evidence rating of “I,” in adherence to the prospective single-catered design ASPS scale criterion. Lastly, the concomitant disease manifestation's effect on disease burden and poor health, defined through HRQOL and FC, did not qualify for a level of evidence classification due to the cross-sectional designs implemented [24, 29, 33].
RA/PAD association
Five studies discussed the role of PAD in RA [24, 30, 31, 36, 37]. One of which was a retrospective cohort querying the incidence of PAD and its association with RA [36]. The authors demonstrated an overall incidence of 6.27 per 1000 person-years, posing an adjusted hazard ratio (HR) of 1.73 (95% CI 1.21–1.83) [36]. Disease development risk peaked during the first follow-up year (adjusted HR 2.42, 95% CI 1.92–3.05), and declined during the 4–5-year follow-up period (adjusted HR 1.45, 95% CI 1.17–1.81) [36]. The sex-specific PAD development risk was higher in women (adjusted HR 1.80, 85% CI 1.61–2.02) than in men (adjusted HR 1.49, 95% CI 1.21–1.83) [36]. Moreover, the relative increase in the number of comorbidities a patient presented with, resulted in higher HR values [36]. Relevant comorbidities included diabetes mellitus (DM), hypertension, hyperlipidemia, heart failure, coronary artery disease (CAD) and stroke [36]. Three studies of a cross-sectional nature were in agreement with an increased PAD prevalence in RA, while one was not [33]. The study in disagreement recruited participants in RA remission, demonstrating no differences in vascular outcomes compared to controls [33]. Al-Kaabi et al. [24] reported a 25% prevalence of PAD in patients with RA and a 2.5% prevalence among healthy controls (P = 0.007), in the unadjusted model used. PAD was defined as an ABPI ≤ 1.0. The systolic blood pressure was significantly higher in patients with RA when compared to healthy controls (mean 137 vs. 126 mmHg; P = 0.01). Grech et al. [31] noted a 4% prevalence of PAD, derived from ABPI, in the RA population examined. Vascular obstruction indicative of PAD was noted in 33.3% of the population when investigated using Doppler Spectral Waveform Sonography. No significant differences between groups were found regarding smoking [23, 31], diabetes mellitus [24], blood glucose [24, 31], serum cholesterol [24] and serum triglycerides [24, 31]. Kim et al. [30] denoted a PAD prevalence of 1.5% in the RA population studied.
RA/ABPI association
Two of the studies reported a notable association between ABPI and RA [29, 38]. A single study presented RA as an independent risk factor for reduced ABPI scores but rejected the role inflammatory markers play in facilitating the latter [32]. Del-Rincon et al. [38] demonstrated that patients with RA presented with an adjusted odds ratio (OR) of 3.33 (95% CI 0.79–13.96, P = 0.02) in developing arterial obstruction (ABPI ≤ 0.90) when compared to healthy controls. The authors stratified for age, sex, diabetes mellitus (DM), hypercholesterolemia, systolic blood pressure and BMI. In considering the former confounding variables on arterial incompressibility (ABPI ≥ 1.3), an OR of 9.50 (2.03–44.5, P = 0.004) was found. The highest degree of obstruction was demonstrated among patients with RA carrying the greatest joint deformity tertials (≥ 20), producing a Relative-Risk Ratio (RR) of 7.6 (95% CI 1.02–1.06, P < 0.0001). Fan et al. [32] demonstrated no correlation between ABPI and plasma markers, inflammatory markers or endothelial markers among patients with RA. Investigated markers included CRP, Interleukin (IL)—1β, IL-6, tumor necrosis factor (TNF)–α, macrophage migration inhibitory factor (MIF) and von Willebrand factor (vWF). Kumeda et al. [29] reported a significantly lower ABPI in patients with RA when compared to healthy controls (P = 0.0206). No significant difference was noted with respect to age, sex, BMI, smoking index, serum lipid levels and systolic/diastolic BP between groups.
Disease burden and poor health
Three cross-sectional studies considered the impact of concomitant PAD and RA on HRQOL and FC [24, 33, 39]. Patients with RA diagnosed with PAD elicited higher HAQ scores compared to patients with normal ABPI measures; mean S.E 1.7 (0.2) versus 0.78 (0.14), P = 0.01 respectively [24]. Al-Kaabi et al. [24] noted an IC prevalence of 7.5% in the conjoined RA and PAD population studied. The authors did not ascertain HRQOL and FC detriments imposed by IC in RA. No association was found in the RA group between inflammatory markers, disease duration or HAQ scores. Kumeda et al. [29] demonstrated reduced M-HAQ scores in the RA group when compared to healthy controls. This reduction best correlated with carotid intima-media thickness (0.305, P ≤ 0.05). No comparison between M-HAQ scores and ABPI ensued. These findings were independent of age, sex, smoking index, blood pressure, total cholesterol and blood triglyceride count of the examined populations. Tehan et al. [33] denoted reduced gait speed as a measure of FC in the RA populations with reduced ABPI scores. This correlation was not significant likely due to limited RA disease activity in the population studied.
Discussion
This review considers the association between RA and PAD, coupled with their concomitant impact on HRQOL and FC. Four retrospective cohort studies investigating the association between RA and PAD were of a high-quality design [24, 34, 35, 37]. Moreover, four articles were cross-sectional, three of which demonstrated a high-quality design [24, 29, 31, 33], while a single article was of moderate quality [30]. Investigated articles isolating PAD in their analysis were in agreement with an increased PAD incidence or prevalence among patients with RA [24, 35,36,37]. One exception to the former persisted denoting no increase in prevalence between the RA and controls [33]. Grech et al. [31] questioned the validity of ABPI as a PAD diagnostic method in RA, suggesting that PAD remains substantially underdiagnosed. The examined literature determined that PAD incidence is associated with either extra-articular RA disease manifestations, the prevalence of comorbidities, or RA disease severity marked by CRP or ESR concentrations and joint deformity counts [35,36,37,38]. These findings suggested that disease manifestations of greater severity and chronicity would increase an RA patient’s susceptibility to PAD development. CVD risk factors at the time of RA diagnosis, including smoking history, are significantly associated with PAD development but are not independently responsible for its incidence [35]. The former ideation was echoed by other meta-analyses and systematic reviews concerning the association between RA and vascular morbidities [40,41,42]. These included venous thromboembolism (VTEs) [41] and CAD [40,41,42].
A grade “I” level of evidence was demonstrated concerning the positive association between ABPI and RA, suggesting RA as an independent risk factor for arterial obstruction or incompressibility [38]. One high-quality study presented the necessary “prospective cohort” and “single-centered” criterion proposed by the ASPS to award the noted gradation [38]. Two studies of a high-quality cross-sectional design noted an increased prevalence of reduced ABPI in patients with RA [29, 32]. This remained true when individuals diagnosed with PAD were excluded [32]. ABPI acts as a valid marker of generalized atherosclerotic changes concerning the peripheral [10, 21], carotid [41] and coronary [10] arterial beds. The association noted in this review may further validate ABPI as a marker of atherosclerosis in RA.
Articles addressing the impact of conjoint RA and PAD manifestations on HRQOL and FC measures were not eligible for a level of evidence gradation, despite a robust high-quality design [24, 29, 33]. Evidence appraised suggested diminished HRQOL and FC in concomitant disease manifestations [24]. This finding is independent of the reduced FC demonstrated by RA alone [39]. Detriments to HRQOL and FC more accurately correlated with RA severity and joint deformity, rather than the prevalence of PAD in concomitant manifestations [24]. The concomitance of RA and reduced ABPI was correlated with reduced FC in an RA population with non-significant disease activity [33]. No discussion ensued of the potential impact of symptomatic PAD on FC, HRQOL or perceived disease burden, despite denoting a 7.5% prevalence of IC among the RA population examined in one study [24]. Symptomatic PAD may reduce a patient’s HRQOL and FC as it often presents with pain on movement. Previously symptomatic patients with PAD demonstrated greater detriments to their HRQOL and FC when compared to non-symptomatic PAD counterparts, and were at risk of disease progression-related mobility loss [18, 43]. Investigating the impact of symptomatic PAD in RA remains paramount in improving patients’ prognostic outlook.
Individual study appraisal scores were determined using the CCAT, with all included studies demonstrating a robust design, carrying minimal potential for bias. Some studies, however, were subject to selection bias, limiting validity [30, 32]. Kim et al. [30] and Tehan et al. [33] for instance recruited RA patients with mild disease severity. Previous studies have denoted RA disease severity as a propagator of atherosclerotic changes[24, 31, 32, 34,35,36,37], suggesting the findings of Kim et al. [30] and Tehan et al. [33] lack generalizability. Fan et al. [32] recruited healthy controls from the risk evaluation clinic of Baker IDI Heart and Diabetes Institute, rather than from the community. This may have reduced the significance of their findings given that data derived from the control group may be subject to Berkson’s Bias [44]. Other studies derived their samples from a predominately ethnically homogenous population, reducing the potential implication of their findings on ethnically diverse groups [29, 34, 36]. Despite a degree of methodological homogeneity, evident heterogeneity was present regarding ABPI acquisition. Notably, studies either implemented manual Doppler [24, 30, 33, 38], sphygmomanometric methods [29], or automated oscillometry [30, 31] in deriving ABPI scores. Manual methodological approaches in ascertaining ABPI posit a degree of observer bias [45]. ABPI score inaccuracies may have been present since the observer must measure ankle and brachial pressures simultaneously rather than successively [45]. Despite reducing the risk of observer bias, no evidence was found validating automated oscillometry in an RA population.
RA has been previously documented as a novel CVD risk factor, with disease-associated cardiovascular event incidence similar to coronary heart disease (CHD) [46]. The presence of other CVD risk factors in RA has been demonstrated to accentuate vascular event risk [46]. The former is validated by the studies included in this review, where-by inclusion of CVD risk factors in individual studies perpetuated PAD incidence risk [24, 34,35,36]. All pertinent studies controlled for most noted CVD risk factors through exclusion or adjustment. However, 90% of reviewed studies neglected to control for physical activity levels, further limiting their respective internal validity [24, 30,31,32, 34,35,36,37,38]. The evidence suggests that patients with RA tend to exhibit more sedentary behavior when compared with population-based controls [47]. Despite this being likely due to pain, joint deformity and the prevalence of comorbidities, it may independently act to exaggerate PAD incidence within the population, affecting the internal validity and generalizability of appraised studies. This view is validated by Kumeda et al. [29], denoting that a lack of physical activity in the population perpetuates the multi-factorial incidence of arterial wall thickening, propagating atherosclerotic change.
This review has some limitations that warrant consideration. Available Journals were restricted to free-access organizations and those provided by Glasgow Caledonian University (GCU) access. This may have diminished the search strategy’s validity. Included studies consisted of peer-reviewed literature written in English exclusively, which may have acted to omit pertinent non-English literature. The appraisal and levels of evidence processes were conducted by a single researcher. CCAT and ASPS results may have been either attenuated or accentuated due to human error, limiting the validity of the results.
The implementation of exercise in the management of RA has been demonstrated to increase patient self-efficacy and reduce joint morning stiffness [21]. This benefit was best enabled when mild to moderate aerobic exercise was implemented [21]. The noted exercise intensity has been shown to combat disease-related muscle wasting and diminished fitness levels, which may improve HRQOL and act to attenuate morbidity [21]. Considering PAD, exercise therapy acts as a gold-standard first-line approach to treatment [22]. It is paramount in alleviating the risk of symptomatic disease or reducing the impact of symptomatic manifestations [22]. Exercise may be prominent in combating the HRQOL and FC hindrances that are likely present in conjoined RA and symptomatic/asymptomatic PAD manifestations [48]. Individualized exercise regimes have demonstrated improvements in endothelial function among patients with RA [49].
Future research would benefit from stratifying for physical activity levels and investigating the impact of symptomatic PAD on the relevant outcomes. Studies appraised did not implement exercise as a risk factor due to logistic difficulty, the subjectivity of self-reported measures and the inadequacy of medical databases used. No cohort or case–control studies produced by the search strategy implemented discussed the impact of conjoined RA and PAD on HRQOL and FC. Undertaking prospective cohort studies to improve the level of evidence pertaining to the subject is necessary for perpetuating the appropriate guideline revisions.
To conclude, the evidence presented in this review demonstrated an association between RA and PAD. Multi-morbid patient presentations act to accentuate the incidence risk of PAD in RA. Little evidence was found articulating the impact of conjoined disease manifestations on HRQOL and FC. Future studies will benefit from controlling for exercise as a prominent PAD risk factor and establishing the impact of symptomatic PAD on HRQOL and FC in the RA population.
References
Holmdahl R, Malmstrom V, Burkhardt H (2014) Autoimmune priming, tissue attack and chronic inflammation - the three stages of rheumatoid arthritis. Eur J Immunol 44(6):1593–1599. https://doi.org/10.1002/eji.201444486
Gkaliagkousi E, Gavriilaki E, Doumas M, Petidis K, Aslanidis S, Stella D (2012) Cardiovascular risk in rheumatoid arthritis: pathogenesis, diagnosis and management. J Clin Rheumatol 18(8):422–430. https://doi.org/10.1097/RHU.0b013e31827846b1
El Miedany Y (2017) Comorbidity in Rheumatic Diseases. Springer, Cham
Weisman MH (2011) Rheumatoid Arthritis. Oxford University Press, New York
Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E, Fautrel B, van Vollenhoven R (2016) Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis 75(8):1428–1437. https://doi.org/10.1136/annrheumdis-2016-209201
Kuo D, Crowson CS, Gabriel SE, Matteson EL (2014) Hyperuricemia and incident cardiovascular disease and noncardiac vascular events in patients with rheumatoid arthritis. Int J Rheumatol 2014:523897. https://doi.org/10.1155/2014/523897
Chatterjee S, Sarkate P, Ghosh S, Biswas M, Ghosh A, Chatterjee S (2013) Early, structured disease modifying anti-rheumatic drug (DMARD) therapy reduces cardiovascular risk in rheumatoid arthritis–A single centre study using non-biologic drugs. J Assoc Physicians India 61(8):531–534
Greenberg JD, Kremer JM, Curtis JR, Hochberg MC, Reed G, Tsao P, Farkouh ME, Nasir A, Setoguchi S, Solomon DH (2011) Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis 70:576
Lawrence M, Stephenson J, Al-taan O, McCarthy MJ (2011) Peripheral vascular disease. Innovait 4(7):399–407. https://doi.org/10.1093/innovait/inq164
Schahab N, Fimmers R, Mahn T, Schaefer C, Tiyerili V, Nickenig G, Sinning JM, Stundl A (2019) Comparative study of pressure (ankle-brachial pressure index) and flow (strain gauge plethysmography and reactive hyperaemia) measurements in diagnosis of peripheral arterial disease in patients with severe aortic stenosis. PLoS ONE 14(7):e0220510. https://doi.org/10.1371/journal.pone.0220510
Harwood AE, Broadbent E, Totty JP, Smith GE, Chetter IC (2017) “Intermittent claudication a real pain in the calf”-patient experience of diagnosis and treatment with a supervised exercise program. J Vasc Nurs 35(3):131–135. https://doi.org/10.1016/j.jvn.2017.03.001
Agnelli G, Belch JJF, Baumgartner I, Giovas P, Hoffmann U (2020) Morbidity and mortality associated with atherosclerotic peripheral artery disease: a systematic review. Atherosclerosis 293:94–100. https://doi.org/10.1016/j.atherosclerosis.2019.09.012
Ali F, Carman T (2012) Medical management for chronic atherosclerotic peripheral arterial disease. Drugs 72(16):2073–2085. https://doi.org/10.2165/11640810-000000000-00000
Aponte J (2011) The prevalence of asymptomatic and symptomatic peripheral arterial disease and peripheral arterial disease risk factors in the US population. Holist Nurs Pract 25(3):147–161. https://doi.org/10.1097/HNP.0b013e3182157c4a
Daoud EM (2011) Associations of symptomatic or asymptomatic peripheral arterial disease with all-cause mortality and cardiovascular mortality. Egypt Heart J 63(1):7–12. https://doi.org/10.1016/j.ehj.2011.08.022
Maksimovic M, Vlajinac H, Marinkovic J, Kocev N, Voskresenski T, Radak D (2014) Health-related quality of life among patients with peripheral arterial disease. Angiology 65(6):501–506. https://doi.org/10.1177/0003319713488640
Tóthová V, Bártlová S, Dolák F, Kaas J, Kimmer D, Maňhalová J, Martinek L, Olišarová V (2014) Quality of life in patients with chronic diseases. Neuro Endocrinol Lett 35(Suppl 1):11
Campia U, Liao Y, McDermott MM (2012) Decline in the Ankle Brachial Index and Functional Outcome in Patients with Peripheral Arterial Disease. Circulation, 126(21):A17874
Listing J, Strangfeld A, Kekow J, Wassenberg S, Klopsch T, Kohlmann T, Zink A (2011) Patients with rheumatoid arthritis who are in remission according to the new ACR/EULAR criteria have a very high functional capacity which is comparable to healthy subjects. Arthr Rheum 63(10):S53–S54
Larkin L, Kennedy N, Gallagher S (2015) Promoting physical activity in rheumatoid arthritis: a narrative review of behaviour change theories. Disabil Rehabilit 37(25):2359–2366. https://doi.org/10.3109/09638288.2015.1019011
(SIGN) SIGN (2011) (123) Management of Early Rheumatoid Arthritis—A National Clinical Guideline. https://www.sign.ac.uk/assets/sign123.pdf. Accessed 3 June 2022, 2019
Excellence NIfHaC (2012) Recommendations | Peripheral arterial disease: diagnosis and management | Guidance | NICE. https://www.nice.org.uk/guidance/cg147/chapter/Recommendations#diagnosis. Accessed 3 May 2019
Hackshaw AK (2015) A concise guide to observational studies in healthcare. Wiley, Chichester, West Sussex, UK
Alkaabi JK, Ho M, Levison R, Pullar T, Belch JJF (2003) Rheumatoid arthritis and macrovascular disease. Rheumatology (Oxford) 42(2):292–297
Crowe M, Sheppard L, Campbell A (2011) Comparison of the effects of using the crowe critical appraisal tool versus informal appraisal in assessing health research: a randomised trial. Int J Evid Based Healthc 9(4):444–449. https://doi.org/10.1111/j.1744-1609.2011.00237.x
Crowe M, Sheppard L (2011) A general critical appraisal tool: an evaluation of construct validity. Int J Nurs Stud 48(12):1505–1516. https://doi.org/10.1016/j.ijnurstu.2011.06.004
Crowe M (2015) Crowe Critical Appraisal Tool (v1.4). https://conchra.com.au/2015/12/08/crowe-critical-appraisal-tool-v1-4/. Accessed 5 June 2019
Crowe M, Sheppard L, Campbell A (2012) Reliability analysis for a proposed critical appraisal tool demonstrated value for diverse research designs. J Clin Epidemiol 65(4):375–383. https://doi.org/10.1016/j.jclinepi.2011.08.006
Kumeda Y, Inaba M, Goto H, Nagata M, Henmi Y, Furumitsu Y, Ishimura E, Inui K, Yutani Y, Miki T, Shoji T, Nishizawa Y (2002) Increased thickness of the arterial intima-media detected by ultrasonography in patients with rheumatoid arthritis. Arthr Rheum 46(6):1489–1497. https://doi.org/10.1002/art.10269
Kim Y-S, Sung Y-K, Choi C-B, Uhm W-S, Kim T-H, Shin J-H, Jun J-B (2012) The major determinants of arterial stiffness in Korean patients with rheumatoid arthritis are age and systolic blood pressure, not disease-related factors. Rheumatol Int 32(11):3455–3461. https://doi.org/10.1007/s00296-011-2198-y
Grech AC, Gatt A, Borg AA, Formosa C (2017) Determining the presence of peripheral arterial disease in patients with rheumatoid arthritis. Mediterr J Rheumatol 28(2):86–93. https://doi.org/10.31138/mjr.28.2.86
Fan F, Galvin A, Fang L, White DA, Moore X-L, Sparrow M, Cicuttini F, Dart AM (2014) Comparison of inflammation, arterial stiffness and traditional cardiovascular risk factors between rheumatoid arthritis and inflammatory bowel disease. J Inflamm (London, England) 11(1):29. https://doi.org/10.1186/s12950-014-0029-0
Tehan PE, Stewart S, Chuter VH, Carroll M, Rutherfurd KJ, Brenton-Rule A (2019) Relationship between lower limb vascular characteristics, peripheral arterial disease and gait in rheumatoid arthritis. Int J Rheum Dis 22(11):2017–2024. https://doi.org/10.1111/1756-185X.13717
Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL (2012) Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthr Rheum 64(1):53–61. https://doi.org/10.1002/art.33322
Liang KP, Liang KV, Matteson EL, McClelland RL, Christianson TJH, Turesson C (2006) Incidence of noncardiac vascular disease in rheumatoid arthritis and relationship to extraarticular disease manifestations. Arthr Rheum 54(2):642–648. https://doi.org/10.1002/art.21628
Chuang Y-W, Yu M-C, Lin C-L, Yu T-M, Shu K-H, Huang S-T, Kao C-H (2016) Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost 115(2):439–445. https://doi.org/10.1160/TH15-07-0600
Turesson C, McClelland RL, Christianson TJH, Matteson EL (2007) Severe extra-articular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis 66:70. https://doi.org/10.1136/ard.2006.052506
Del Rincón I, Haas RW, Pogosian S, Escalante A (2005) Lower limb arterial incompressibility and obstruction in rheumatoid arthritis. Ann Rheum Dis 64:425–432
Burns PB, Rohrich RJ, Chung KC (2011) The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 128(1):305–310. https://doi.org/10.1097/PRS.0b013e318219c171
Meyer-Olesen C, Nielsen SF, Nordestgaard B (2015) Increased rheumatoid factor and deep venous thrombosis: 2 cohort studies of 54628 individuals from the general population. Clin Chem 61(2):349–359. https://doi.org/10.1373/clinchem.2014.233296
Tyrrell NP, Beyene MJ, Feldman WB, McCrindle DB, Silverman JE, Bradley JT (2010) Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 30(5):1014–1026. https://doi.org/10.1161/ATVBAHA.109.198424
Lee JJ, Pope JE (2014) A meta-analysis of the risk of venous thromboembolism in inflammatory rheumatic diseases. Arthr Res Ther 16(5):435. https://doi.org/10.1186/s13075-014-0435-y
McDermott MM, Ferrucci L, Liu K, Guralnik JM, Tian L, Kibbe M, Liao Y, Tao H, Criqui MH (2011) Women with peripheral arterial disease experience faster functional decline than men with peripheral arterial disease. J Am Coll Cardiol 57(6):707–714. https://doi.org/10.1016/j.jacc.2010.09.042
Westreich D (2012) Berksonʼs bias, selection bias, and missing data. Epidemiology 23(1):159–164. https://doi.org/10.1097/EDE.0b013e31823b6296
Chesbro SB, Asongwed ET, John EB, Haile N (2013) Reliability of ankle-brachial index measurements. Top Geriatr Rehabilit 29(3):195–202. https://doi.org/10.1097/TGR.0b013e31828aee0d
Peters MJL, Symmons DPM, McCarey D, Dijkmans BAC, Nicola P, Kvien TK, McInnes IB, Haentzschel H, Gonzalez-Gay M, Provan S, Semb A, Sidiropoulos P, Kitas G, Smulders YM, Soubrier M, Szekanecz Z, Sattar N, Nurmohamed MT (2010) EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 69:325. https://doi.org/10.1136/ard.2009.113696
Liao KP, Solomon DH (2013) Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology 52(1):45–52. https://doi.org/10.1093/rheumatology/kes243
Lemmey A (2012) Exercise for rheumatoid arthritis patients. Rheumatology 51:3
Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen vZ, Nightingale P, Sandoo A, Dimitroulas T, Kitas GD, Koutedakis Y (2014) Individualised exercise improves endothelial function in patients with rheumatoid arthritis. Ann Rheum Dis 73:748. https://doi.org/10.1136/annrheumdis-2013-203291
Acknowledgements
Without the assistance of Dr Gordon Hendry the successful completion of this paper would not have been possible.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zoubi, T., Gordon, H. Systematic review of associations between concomitant rheumatoid arthritis and peripheral arterial disease, health-related quality of life and functional capacity. Rheumatol Int 43, 221–232 (2023). https://doi.org/10.1007/s00296-022-05245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05245-7