Abstract
This study assessed quality of life, direct and indirect healthcare costs related to ankylosing spondylitis (AS). This study included 650 prevalent AS patients visiting seven centers at tertiary healthcare institutions in Turkey who were interviewed using a standard questionnaire to determine annual direct and indirect healthcare costs. Eligible patients were age ≥18 years with AS for at least 12 months. Direct costs were categorized as inpatient, outpatient and pharmacy, and AS-related consultation. Indirect costs were categorized as workday loss, additional AS-related costs, and caregiver costs. Clinical outcome measures were obtained, including Patients’ Global Disease Activity (Pt-GDA); visual analog scale (Pain-VAS) for pain; Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Functional Index (BASFI), and Metrology Index (BASMI) scores, and EuroQoL 5 dimension (EQ-5D) health status survey scores. Mean (€4,335.20) and median (€5,671.00) annual costs per patient were calculated. Pharmacy costs (€4,032.73) were highest among overall expenditures, followed by additional AS-related consultation (€2,480.38), outpatient (€225.02), and inpatient costs (€29.98). Over half of AS patients (54.8 %) experienced work loss. Related average annual costs were €414.16, based on income level. 10.3 % of AS patients incurred an additional €2,008.07 in 1 year. 6.8 % of patients required caregivers and incurred €778.70 in average annual patient paid costs. Mean Pt-GDA, Pain-VAS, EQ-5D, BASDAI, BASFI, and BASMI scores were 4.4, 40.5, 62.7, 3.6, 3.1, and 2.9, respectively. Direct and indirect AS-related costs are high and represent a considerable economic burden on Turkish AS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic, systematic, and inflammatory rheumatic disease that primarily affects the axial skeleton and can lead to structural and functional impairments, low productivity, decrease in life quality, and substantial healthcare resource use [1]. Clinical features of AS include back pain, stiffness, asymmetrical peripheral oligoarthritis, and specific organ involvement such as anterior uveitis, psoriasis, and chronic inflammatory bowel disease [2].
Approximately, 80 % of patients develop their first symptoms before age 30 and less than 5 % develop AS when they are over age 45. Men are more likely to be affected compared to women at an approximate 2:1 ratio [3]. Also, juvenile-onset AS is usually associated with worse outcomes than adult-onset AS [4]. Reported AS prevalence ranges between 0.1 and 0.8 %, and recent studies suggest that prevalence is actually closer to 0.5 % in European populations, especially in Northern Europe [5]. Surveys have estimated AS prevalence in the Turkish adult population at 0.49 % [6].
Traditionally, the “gold standard” of AS treatment is non-steroidal anti-inflammatory drugs (NSAIDs), which improve inflammatory symptoms of AS including the inflammatory back pain. Furthermore, gastrointestinal complications are a common side effect of NSAIDs [7]. Disease-modifying antirheumatic drugs (DMARDs), including sulfasalazine, methotrexate, and leflunomide, though not proven effective, are widely used in some countries as a secondary treatment approach for patients who are refractory to NSAID treatment.
In recent years, anti-tumor necrosis factor alpha (TNFα) use has been studied extensively and has been shown to provide significant clinical benefits for AS patients. Available TNFα inhibitors are infliximab, adalimumab, etanercept, golimumab, and certolizumab. These drugs strongly suppress inflammation [8].
Prior to development of efficacious biologics, overall treatment costs were low in AS patients. Annual estimates included €2,335 in the Netherlands, €2,064 in France, €1,572 in Belgium, and €1,750 in the United States [7, 9]. However, availability of improved but expensive treatment options required the need for information concerning the current disease burden. Many recent studies have been implemented around the world to estimate the economic burden of AS per patient in different countries. In the United Kingdom, total annual direct costs per patient were calculated at £15,973 [10]. In Spain, the total mean annual cost per patient was estimated at €20,328, with direct costs accounting for 22.8 % [11]. In Canada, the mean annual cost per patient was $9,008 Canadian dollars (€7,383), of which direct healthcare costs represented 62.0 %, or $5,585 (€4,578) [12]. In Hong Kong, total annual costs were calculated at €7,045, of which direct costs accounted for 38.0 % [13].
The economic burden of AS in Turkey is not well documented. This study aimed to examine AS patient characteristics and the economic impact of the disease. In this respect, the study objectives were to evaluate AS patient demographic and clinical characteristics, examine patient work environment and prescribed medication types, estimate direct and indirect costs of AS, and determine the association between cost and disease activity score. The present study also aimed to highlight the significance of the economic burden of AS inform policy decision makers to better accommodate Turkish employees diagnosed with AS.
Methods
In the present noninterventional, cross-sectional study, information was collected directly from patients through a questionnaire distributed to seven university hospitals, tertiary healthcare centers in Turkey. All subjects were at least age 18 years, with an AS diagnosis for a minimum of 12 months. Patients were interviewed in a single visit during the 1-year study period from May 2011 through August 2012. Patients were interviewed regarding demographic information (age, gender, and region), AS-related healthcare services including rheumatologic care, prescription medications, rehabilitative therapies, diagnostic and therapeutic procedures (including complementary therapies), inpatient hospitalization, and medications used during hospitalization, comorbid conditions (heart disease, diabetes), medication use (DMARDs, non-selective Cox inhibitors) during the 3-month pre-index period, as well as expenditures for medical devices, caregiver needs, and work capacity (sick leave days).
Clinical outcome measures were collected for Patients’ Global Disease Activity (Pt-GDA) assessment, EuroQoL 5 dimension (EQ-5D) health status survey, pain score on VAS, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [14], Bath Ankylosing Spondylitis Functional Index (BASFI) [15], and the Bath Ankylosing Spondylitis Metrology Index (BASMI) [16]. Pt-GDA, BASDAI, BASFI, and BASMI results are measured on a scale of 0–10, where higher scores indicate higher disease activity. EQ-5D and pain scores were measured on a VAS of 0–100 mm. High pain VAS, BASDAI, and BASFI scores indicate higher pain severity, and higher EQ-5D scores indicate better quality of life.
Total direct medical costs were calculated as the summation of inpatient, outpatient, pharmacy costs, and other AS-related interventions (e.g., acupuncture, homeopathic, other). Total indirect costs were calculated as the sum of those due to work loss, caregiver, and other expenses such as new car, apartment, or special equipment purchases due to AS. Annual costs for outpatient care and pharmacy, work loss, caregiver, and any consultation expenses that are not covered by social insurance were calculated by multiplying 3-month data by four, and data for additional AS-related investments were collected for 1-year period. For work loss, income ranges were taken into account, and the human capital approach was used. The direct and indirect costs were expressed per year. All costs were expressed in 2011 euros (€1.00 = 2.3 Turkish Liras, without inflation adjustment).
Univariate statistics for demographic and clinical variables were created for descriptive analysis. Continuous variables were summarized by providing the number of observations, mean and standard deviation (SD), and median (minimum–maximum). Numbers and percentages are provided for dichotomous and polychotomous variables. Chi-square test was used to compare categorical variables. All statistical analyzes were conducted using SAS v.9.3 and STATA v11 software.
Results
After applying all inclusion criteria, the analytic sample included 648 patients. Mean patient age was 40.5 years, and 98.6 % of them were under age 65 years. Almost one-third of the patients were women. Average disease duration was 7.7 years. Nearly, 8 % of the study subjects were diagnosed with at least one of the following comorbid conditions: heart disease, diabetes, respiratory disease, allergies, Crohn’s disease, uveitis, or rheumatoid arthritis. Of the patients, 7.5 % were hospitalized, 3.5 % were in rehabilitation services, 2.1 % underwent surgery, and 1.2 % had prosthesis (Table 1).
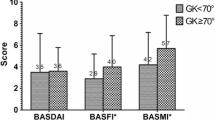
Interestingly, the percentage of female patients who were prescribed biologic agents was lower than for male patients. In addition, average Pt-GDA, Pain-VAS, BASDAI, and BASFI scores were found to be significantly lower in males than in females, whereas EQ-5D and BASMI scores were similar (Table 2).
Most patients were prescribed biologic agents, DMARDs, and NSAIDs (66.5, 50.5, and 37.7 %), followed by gastrointestinal-related medications such as proton pump inhibitors, histamine h2-receptors (28.9 %), and glucocorticoids (10.34 %) (Table 3).
Table 4 shows the direct and indirect costs related with AS. Average annual healthcare costs for AS patients were calculated at €4,335.20. The most significant share of overall expenditures consisted of pharmacy costs (€4,032.73), followed by AS-related consultations (€2,480.38), outpatient (€225.02), and inpatient costs (€29.98) (Table 4). A minority of patients (2 %) had other AS-related therapies not covered by social insurance (acupuncture, homeopathy, other), bringing their average annual burden to €2,480.38. Indirect AS-related costs due to work loss, additional AS-related costs, and caregiver costs were evaluated (Table 4). Nearly, 55 % of AS patients were employed, of which 59.4 % had sick leave with the permission of the employer, costing an average of €414.16 annually due to workday loss. Nearly, one-tenth of AS patients incurred additional AS-related costs (e.g., need for new car, apartment, special equipment) at €2,008.07 in 1 year. The utilization of health caregivers and the average annual out-of-pocket cost for caregivers was €778.70 (Table 4).
Discussion
A variety of studies in the United States and Europe exist evaluating the economic burden and prevalence of AS. Epidemiologic studies have indicated that AS is more prevalent than previously thought [17]. Prevalence of AS as well as its economic burden have been studied only in a limited number of studies in Turkey [6, 18, 19]. Thus, the current study is designed to thoroughly examine AS patient characteristics and the economic impact of the disease in Turkey.
The most significant strength of this study is that it was performed using real-world data. No previously published studies include such data. A recent study by Malhan et al. [20] examined direct and indirect costs of AS patients in Turkey, which was limited because the data were gathered from an expert panel. Although an expert panel is a relatively inexpensive and rapid tool to produce a synthetic judgment based on qualitative and quantitative data, it has some limitations. Comparing opinions often leads to under evaluation of lesser known or underreported points of view. It represents the consensus of panel attendants rather than real-world outcomes [21, 22]. Total direct medical costs per patient differ significantly between the study by Malhan et al. [20] and this study (€3,566 and €4,335.20, respectively).
In addition to direct medical costs, AS patients in Turkey experienced work productivity loss, contributing to the total economic burden. Average annual workday losses in our study (€414.16) exceeded the estimate by Malhan et al. [20] (€245.50). According to Boonen et al. [23], patients in France (n = 53) had adjusted productivity losses (using the friction method) totaling €428 per year, and patients in Belgium (n = 26) and the Netherlands (n = 130) experienced adjusted productivity losses of €476 and €1,257, respectively. Adjusted work disability was 23 % in France, 9 % in Belgium, and 41 % in the Netherlands. In a more recent study conducted by Kvamme et al. [24], annual adjusted productivity loss, using the friction method, for hospitalized patients in the Netherlands reached €7,686 for synthetic DMARD use (n = 49) and €8,186 for biologic DMARD use (n = 137). The variation in work status and productivity costs observed in European countries suggests that economic generalizability may be limited. This underscores the importance of conducting health economic studies specific to the country of interest for the effective allocation of resources and other healthcare applications. Other indirect cost measures in our research included AS-related complementary therapies and additional costs, as well as out-of-pocket costs for caregiver utilization. These variables were not assessed in the previous studies from Turkey.
The present study is unique in that it uses real-world data, assesses variables not previously examined in other studies and includes a thorough analysis of nationally representative direct and indirect costs of AS in Turkey. It should be noted that all patients were diagnosed at tertiary medical centers, which may explain the unexpectedly high proportion of AS patients receiving biologic therapy, which may be considered as a limitation of the study. It should be noted that use of biologic therapy was less prevalent among female patients than among male patients. That is most probably due to the relatively common presence of fibromyalgia accompanying AS in female patients, which negatively affects disease activity and functional scores as previously reported [25]. It might have been also due to differences in socioeconomic status. It has been shown that groups with lower socioeconomic status seem to have less access to biologic medications, in terms of lower availability, affordability, and acceptability. More barriers that may have resulted in worse disease course in female patients [26]. More hospitalized patients were administered infliximab, possibly because the medication was dispensed via percutaneous infusion, which is a possible weakness of the present study.
Our results also indicated higher disease activity scores among female patients compared to males. Previous studies have also shown that there are gender differences regarding autoimmune disease characteristics, with women scoring higher on pain and quality of life measurement scales [27, 28]. However, these scores were considered higher subjectively and not objectively, suggesting that disease activity may have been discounted in the treatment decision.
AS has significant economic implications for individuals and society. Yet for Turkey, the cost of care has not been analyzed using real-world clinical data. This study also demonstrated that indirect AS-related costs in Turkey are significant. The young age of patients who reported sick leave reveals the negative effect of AS on national economy. For this reason, indirect cost should be taken into consideration during Payor’s decision making process. Moreover, future comparative effectiveness studies concerning AS treatment should include direct and indirect costs.
References
Russell A (1998) Ankylosing spondylitis: history. In: Rheumatology, 2nd edn. Mosby, London, p 11–12
Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369(9570):1379–1390
Feldtkeller E, Khan MA, van der Heijde D et al (2003) Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 23(2):61–66
Stone M, Warren RW, Bruckel J et al (2005) Juvenile-onset ankylosing spondylitis is associated with worse functional outcomes than adult-onset ankylosing spondylitis. Arthritis Rheum 53(3):445–451
Akkoc N, Khan MA (2006) Epidemiology of ankylosing spondylitis and related spondyloarthropathies. In: Weisman MH, Reveille JD, van der Heijde (eds) Ankylosing spondylitis and the spondyloarthropathies. Mosby, St. Louis, pp 117–131
Onen F, Akar S, Birlik M et al (2008) Prevalence of ankylosing spondylitis and related spondyloarthritides in an urban area of Izmir, Turkey. J Rheumatol 35(2):305–309
Ward MM (2002) Functional disability predicts total costs in patients with ankylosing spondylitis. Arthritis Rheum 46(1):223–231
Chang J (2007) Clinical use of anti-TNF-alpha biological agents. Aust Fam Physician 36(12):1035–1038
Boonen A, Van der Heijde D, Landewe R et al (2003) Direct costs of ankylosing spondylitis and its determinants: an analysis among three European countries. Ann Rheum Dis 62(8):732–740
Ara RM, Packham JC, Haywood KL (2008) The direct healthcare costs associated with ankylosing spondylitis patients attending a UK secondary rheumatology unit. Rheumatology (Oxford) 47(1):68–71
Kobelt G, Sobocki P, Mulero J et al (2008) The burden of ankylosing spondylitis in Spain. Value Health 11(3):408–415
Kobelt G, Andlin-Sobocki P, Maksymowych WP (2006) Cost and quality of life of patients with ankylosing spondylitis in Canada. J Rhematol 33(2):289–295
Zhu T, Tam LS, Lee VWY et al (2008) Costs and quality of life of patients with ankylosing spondylitis in Hong Kong. Rheumatology 47(9):1422–1425
Akkoc Y, Karatepe AG, Akar S et al (2005) A Turkish version of the bath ankylosing spondylitis disease activity index: reliability and validity. Rheumatol Int 25:280–284
Karatepe AG, Akkoc Y, Akar S et al (2005) The Turkish versions of the bath ankylosing spondylitis and dougados functional indices: reliability and validity. Rheumatol Int 25:612–618
Jenkinson TR, Mallorie PA, Whitelock HC et al (1994) Defining spinal mobility in ankylosing spondylitis (AS). The bath AS metrology index. J Rheumatol 21:1694–1698
Bakland G, Nossent HC, Gran JT (2005) Incidence and prevalence of ankylosing spondylitis in Northern Norway. Arthritis Care Res 53(6):850–855
Akkoc N (2008) Are spondyloarthropathies as common as rheumatoid arthritis worldwide? A review. Curr Rheumatol Rep 10(5):371–378
Beslek A, Onen F, Birlik M et al (2009) Prevalence of spondyloarthritis in Turkish patients with inflammatory bowel disease. Rheumatol Int 29(8):955–957
Malhan S, Pay S, Ataman S et al (2012) The cost of care of rheumatoid arthritis and ankylosing spondylitis in tertiary care rheumatology units in Turkey. Clin Exp Rhematol 30(2):202–207
Witkin BR, Altschuld JW (1995) Planning and conducting needs assessments: A practical guide. Sage Publications, Incorporated
Cozzens SE (1987) Expert review in evaluating programs. Sci Public Policy 14(2):71–81
Boonen A, van der Heijde D, Landewe R et al (2002) Work status and productivity costs due to ankylosing spondylitis: comparison of three European countries. Ann Rheum Dis 61(5):429–437
Kvamme MK, Lie E, Kvien TK, Kristiansen IS (2012) Two year direct and indirect costs for patients with inflammatory rheumatic joint diseases: data from real-life follow-up of patients in the NOR-DMARD registry. Rheumatology (Oxford) 51(9):1618–1627
Aloush V, Ablin JN, Reitblat T et al (2007) Fibromyalgia in women with ankylosing spondylitis. Rheumatol Int 27(9):865–868
Sokka T, Kautianinen H, Pincus T et al (2009) Disparities in rheumatoid arthritis disease activity according to gross domestic product in 25 countries in the QUEST-RA database. Ann Rheum Dis 68(11):1666–1672
Arkema EV, Neovius M, Joelsson JK et al (2012) Is there a sex bias in prescribing anti-tumour necrosis factor medications to patients with rheumatoid arthritis? A nationwide cross-sectional study. Ann Rheum Dis 71(7):1203–1206
Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF (2012) Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med 12:82
Acknowledgments
This study was sponsored by Pfizer Pharmaceutical Co. Ltd.
Conflict of interest
D. Balkan Tezer and B. Hacıbedel are employees of Pfizer Pharmaceutical Co. Ltd. N. Akkoç, H. Direskeneli, H. Erdem, A. Gül, Y. Kabasakal, S. Kiraz, and V. Hamuryudan received honoraria from Pfizer Pharmaceutical Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Akkoç, N., Direskeneli, H., Erdem, H. et al. Direct and indirect costs associated with ankylosing spondylitis and related disease activity scores in Turkey. Rheumatol Int 35, 1473–1478 (2015). https://doi.org/10.1007/s00296-015-3236-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-015-3236-y